Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
See Box 28-1 .
Follicular adenoma and variants
Follicular carcinoma and variants
Papillary carcinoma and variants
Poorly differentiated carcinoma
Undifferentiated (anaplastic) carcinoma
Others:
Squamous cell carcinoma
Mucoepidermoid carcinoma
Tumors with thymic-like differentiation
Medullary thyroid carcinoma
Mixed medullary and follicular carcinoma
Mixed medullary and papillary carcinoma
Paraganglioma
Teratoma
Mesenchymal tumors:
Benign peripheral nerve sheath tumor
Granular cell tumor
Hemangioma
Leiomyoma
Solitary fibrous tumor
Others
Malignant lymphoproliferative lesions:
Non-Hodgkin malignant lymphoma
Plasmacytoma
Hodgkin lymphoma
Others
Sarcomas:
Angiosarcoma
Follicular dendritic cell sarcoma
Leiomyosarcoma
Others
Secondary Tumors
Thyroid cancer is the most common endocrine malignancy but represents 1.4% to 4.6% of all new human cancers diagnosed in the United States.
Incidence of thyroid carcinoma in United States has increased rapidly since 1980:
More refined/sophisticated detection methods including radiologic imaging (ultrasound, CT, MRI) contribute to increase in incidence.
Sharpest increase is in diagnosis of papillary thyroid carcinoma, particularly papillary microcarcinoma but also follicular variant of papillary thyroid carcinoma.
Percentage of cancer deaths (mortality rate) due to thyroid cancer is low (less than 0.4% in women and in men):
In contrast to increase in incidence, changes in mortality rates have been much smaller, likely resulting from:
Improved treatment
Increased incidence primarily in early stage papillary carcinoma
Clinically apparent thyroid nodules occur in fairly large percentage of the population (up to 10%):
Many of these nodules are probably benign.
Differential diagnosis of any thyroid nodule includes a malignant thyroid neoplasm.
Demographics:
In general, thyroid tumors are more common in women than in men and occur in all ages ranging from the young (children in the first and second decades of life) to elderly adults.
Risk factors for development of thyroid cancer include:
Radiation exposure:
Causes thyroid carcinoma primarily by direct effects on DNA
Primarily associated with external radiation
Internal ( 131 iodine) radiation therapy used safely for diagnostic and therapeutic purposes associated with small increase risk of thyroid carcinoma incidence
Risk factors associated with radiation-induced thyroid tumors include:
Amount of radiation exposure
Young age at exposure to radiation
High serum thyroglobulin levels
Genetic predisposition:
Only 5% of follicular cell-derived thyroid carcinomas are a component of a familial cancer syndrome.
Familial follicular cell-derived tumors or nonmedullary thyroid carcinoma encompass a heterogeneous group of diseases classified into two distinct groups:
Syndromic-associated tumors occur in syndromes in which nonmedullary thyroid carcinomas are predominant tumor encountered, including:
Phosphase and tensin (PTEN)-hamartoma tumor syndrome/Cowden syndrome
Familial adenomatous polyposis/Gardner syndrome
Carney complex type 1, Werner syndrome, Pendred syndrome
Other syndromes, such as McCune-Albright syndrome, Peutz-Jeghers syndrome, and ataxia-telangiectasia syndrome, may be associated with development of follicular cell-derived tumors but link is less established than above syndromes.
Nonsyndromic tumors occur in tumor syndromes in which thyroid involvement is minor component, including:
Familial follicular cell-derived tumor syndromes or nonsyndromic tumors:
Non-medullary thyroid carcinomas are major findings, including:
Pure familial papillary thyroid carcinoma with or without oncocytic cytoplasmic change; familial papillary thyroid carcinoma with papillary renal cell carcinoma; familial papillary thyroid carcinoma with multinodular goiter; familial nonmedullary (papillary) thyroid carcinoma type 1
Familial adenomatous polyposis (FAP) syndrome, including its subtype Gardner syndrome:
Autosomal dominant inheritance with mutations in APC gene (5q21)
Characterized by multiple adenomatous polyps of the large intestine, multiple osteomas of the skull and mandible, cutaneous keratinous cysts, and soft tissue tumors (e.g., fibromatosis)
Associated with an increased risk of papillary thyroid carcinoma (PTC):
Thyroid carcinoma occurs in up to 12% of patients
Tend to occur at early age
Histologically, include PTC, cribriform-morular variant, and solid variant
Phosphatase and tensin homolog (PTEN)-hamartoma tumor syndrome, including Cowden disease (multiple hamartoma syndrome):
Autosomal dominant inheritance
Germline mutation of PTEN gene (10q23.2)
Characterized by multiple hamartomas, mucocutaneous lesions, including trichilemmomas, acral keratoses, and oral mucosal papillomas
Associated with increased risk of follicular epithelial cell lesions/tumors, including:
Adenomatoid nodules accounting for 75% of thyroid abnormalities in this setting
PTC and follicular carcinomas may also occur.
Other familial disorders with specific gene mutations associated with thyroid carcinoma include:
Carney complex:
Germline mutation PRKR1α (17q22-24)
Follicular carcinoma and papillary carcinoma
Werner syndrome:
Germline mutation WRN (8p11-22)
Follicular carcinoma, papillary carcinoma, undifferentiated (anaplastic) thyroid carcinoma
Pendred syndrome
Mutation of Pendred syndrome (PDS) gene ( SLC26A4 gene on chromosome 7q31) encodes amino acid protein pendrin shown to function as iodide/chloride transporter
Goitrous thyroid
Familial nonmedullary thyroid carcinoma (FNMTC):
Originate from follicular cells of thyroid gland accounting for more than 90% of all thyroid cancers
Approximately 3% to 10% of FNMTCs are of familial origin defined as two or more affected first-degree relatives with NMTC in the absence of other known familial syndromes.
Greater than 85% of thyroid tumors in nonsyndromic FNMTC are papillary thyroid carcinoma followed by follicular thyroid carcinoma (approximately 10%); poorly differentiated and undifferentiated (anaplastic) thyroid carcinomas represent approximately 5%.
Compared to sporadic NMTC, FNMTC:
Presents at younger age
Associated with higher incidence of multifocal disease, extrathyroidal extension, and nodal metastasis
Multiple endocrine neoplasia syndrome:
Linked to development of C-cell–related lesions/neoplasms
See Section 10.
Dietary factors:
Iodine:
In iodine-deficient diets (endemic goiter areas), follicular thyroid carcinoma more common
In iodine-sufficient diets, papillary thyroid carcinoma more common
Pre-existing thyroid diseases:
Thyroid carcinoma often preceded by other thyroid abnormalities, including:
Adenomatoid nodules
Lymphocytic thyroiditis
Graves disease
Remains uncertain whether patients with above abnormalities are at increased risk for developing thyroid carcinoma
Hormonal and reproductive factors:
Thyroid carcinoma occurs more commonly in women than in men.
Differences between genders in thyroid cancer incidence declines with age.
Increasing parity may increase risk of thyroid carcinoma.
Thyroid carcinoma reported to occur more often among women who are older when they first give birth.
Small increase in risk of thyroid carcinoma with increasing age at menopause
Other suggested risk factors for thyroid carcinoma in women are:
Exogenous estrogens, including oral contraceptives
Lactation-suppressant drugs
Postmenopausal estrogen therapy
Fertility drugs (e.g., clomiphene, progesterone)
Clinical findings:
Most patients with benign thyroid nodules/tumor and carcinomas present with asymptomatic thyroid nodule.
Clinical findings related to risk of carcinoma in thyroid nodule include:
Age:
Overall most patients with thyroid carcinoma are middle age.
Thyroid nodule is more likely to be carcinoma in patients under 20 years and in patients over 65 years of age.
Gender:
Men are more apt to have malignant thyroid tumors than women.
Family history of thyroid cancer or syndrome associated with thyroid cancer
History of childhood head and neck therapeutic irradiation, total body irradiation, or exposure to ionizing radiation
History of other cancer:
Kidney, breast, lung, and melanoma
Sudden or rapid enlargement of thyroid or of long-standing thyroid nodule(s) with or without pain:
Most likely represents hemorrhage into a cystic nodule:
Occurs in benign and malignant nodules
Hallmark presentation for some thyroid malignant tumors, including undifferentiated (anaplastic) carcinoma and malignant lymphoma
Large nodule with distortion of structures of upper neck and mediastinum and/or tracheal compression with difficulty breathing:
Can occur in association with benign thyroid nodules but presence of these symptoms raises concern for a malignant thyroid lesion, although most malignant thyroid tumors are asymptomatic
Hoarseness and/or vocal cord paralysis:
Most commonly related to laryngeal-based disease (benign or malignant) but can be associated with infiltration of recurrent laryngeal nerve by thyroid cancer
Physical examination:
Number of nodules:
Although not always true, multiple nodules more likely to be benign, whereas solitary nodules more likely to be malignant
Hard and fixed thyroid mass:
More likely to be malignant
Ipsilateral cervical adenopathy:
May indicate metastasis from an (ipsilateral) thyroid malignant tumor clinically overt or occult
Laboratory testing:
Most patients with thyroid cancer are euthyroid.
Serum thyrogloublin:
Often elevated in patients with papillary and follicular carcinoma:
May be elevated in association with follicular adenoma so does not necessarily allow for distinguishing benign and malignant thyroid follicular neoplasms.
Not typically elevated in medullary thyroid carcinoma and undifferentiated (anaplastic) thyroid carcinoma
Useful in monitoring patients following treatment (surgery and radioactive iodine) for papillary or follicular carcinoma as elevated levels may indicate recurrence or metastasis.
Serum calcitonin:
Elevated levels represent diagnostic feature that may occur in medullary thyroid carcinoma
Germline RET proto-oncogene mutation testing:
Indicated in patients with thyroid nodule and family history of medullary thyroid carcinoma or potential diagnosis of multiple endocrine neoplasia (MEN) type II syndrome
Radiologic imaging:
Ultrasonography:
Thyroid ultrasound used in patients with palpable nodules (with or without elevated TSH) to:
Determine presence of single discrete nodule or multiple nodules
Demonstrate anatomic location of nodule in thyroid (anterior versus posterior) as well as composition (cystic versus solid)
Assess nodule(s) size
Thyroid malignancies tend to:
Be hypoechogenic (particularly marked hypoechogenicity)
Be solid
Have infiltrative or microlobulated margins
Show presence of microcalcifications
Be taller than wide shaped when measured in transverse view
However, no single feature or combination of sonographic features is sufficiently sensitive to be the sole screening test for thyroid cancer.
Thyroid nodule may be considered benign when:
Purely cystic
Spongiform appearing (>50% of nodule volume occupied by microcystic spaces)
On basis of thyroid sonographic imaging features, nodule appearance can be classified as:
Low risk for malignancy:
Tend to be purely cystic or spongiform
Noninfiltrative borders
Intermediate risk for malignancy:
Predominantly solid, hypo- or iso- to hyperechoic
Noncalcified
Regular margins
High risk for malignancy:
Hypoechoic
Microcalcifications
Infiltrative or microlobulated margins
Radionuclide imaging
Thyroid scintigraphy using 123 I or 99m Tc pertechnetate
Incidence of carcinoma higher for hypofunctioning “cold” nodules than for hyperfunctioning (“hot”) nodules
“Hot” nodules are almost always benign
Fine-needle aspiration biopsy (FNAB)
Represents an extremely useful initial approach in diagnosis of thyroid mass
Quick and inexpensive with minimal complications
Thyroid nodules are one of most common indications for neck FNAB.
Presurgical diagnosis via FNAB prevents unneeded surgery for benign, nonprogressive lesions and helps to triage patients with a neoplasm for appropriate procedure.
Diagnostic sensitivity and specificity reported to be high, greater than 90%
Standardization and interpretation of thyroid cytology greatly improved by widespread adoption of Bethesda reporting system ( Table 28-1 ), which classifies biopsies in six-tiered system as well as including implied risk of malignancy and associated usual management ( Table 28-2 )
| Category | Diagnoses | Findings | Frequency of Diagnosis |
|---|---|---|---|
| I | Nondiagnostic or unsatisfactory | Cyst fluid only; virtually acellular specimen; other (obscuring blood, clotting artifact, etc.) | <15% |
| II | Benign | c/w:
|
30% to 75% |
| III | Atypia of undetermined significance or follicular lesion of undetermined significance | Contain cells with architectural and/or nuclear atypia not sufficient to be classified as suspicious for follicular neoplasm, suspicious for malignancy, or malignant but show atypia more marked than benign lesions | <10% |
| IV | Follicular neoplasm or suspicious for follicular neoplasm | Specify if oncocytic (Hürthle) cell type | ~5% |
| V | Suspicious for malignancy | Suspicious for: PTC; MTC; metastatic carcinoma; lymphoma |
<5% |
| VI | Malignant | PTC; PDTC; MTC; UC; SCC; carcinoma with mixed features; metastatic carcinoma; NHL | ~5% |
| Bethesda Classification | Risk of Malignancy | Recommended Management |
|---|---|---|
| Nondiagnostic or unsatisfactory (Bethesda I) | N/A | Repeat FNA with ultrasound guidance |
| Benign (Bethesda II) | 0 to 3% | Clinical follow-up |
| Atypia of undetermined significance, follicular lesion of uncertain significance (Bethesda III) | 5% to 15% | Repeat FNA |
| Follicular neoplasm, suspicious for follicular neoplasm (Bethesda IV) | 15% to 30% | Surgical lobectomy |
| Suspicious for malignancy (Bethesda V) | 60% to 75% | Near total thyroidectomy or surgical lobectomy |
| Malignant (Bethesda VI) | >95% | Near total thyroidectomy |
Post-FNAB histologic changes may create diagnostic problems in the evaluation of tissue sections, including presence of features raising concern for malignancy, including:
Pseudoinvasion, necrosis, papillary architecture, cytologic atypia
See Chapter 29 .
Intraoperative consultation (frozen section diagnosis):
Use of intraoperative frozen sections in diagnosis of thyroid tumors has decreased with increasing use of FNAB.
See Chapter 29 .
Histologic types of thyroid neoplasms:
Majority of thyroid tumors are of follicular epithelial cell origin and include:
Follicular adenoma and variants thereof
Follicular carcinoma and variants thereof
Papillary carcinoma and variants thereof
Less common are C-cell–derived medullary thyroid carcinoma
Nonepithelial neoplasms of thyroid are uncommon:
Most common nonepithelial thyroid neoplasm is malignant lymphoma.
Rarely, primary mesenchymal tumors as well as metastases to thyroid gland occur.
Frequency of malignant thyroid neoplasms include:
Papillary carcinoma: approximately 80% to 85%
Follicular carcinoma: approximately 10% to 15%
Medullary carcinoma: <5%
Poorly differentiated thyroid carcinoma: <2%
Undifferentiated (anaplastic) thyroid carcinoma: <2%
Malignant lymphoma: <5%
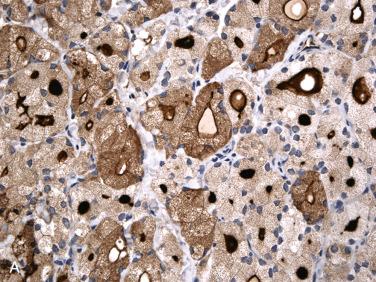
Immunohistochemistry of thyroid neoplasms
Majority of thyroid cancers are differentiated carcinomas of follicular epithelial cell origin diagnosed on morphologic grounds.
Immunohistochemical staining can be important and at times essential in confirming specific diagnosis.
Table 28-3 includes an overview of more common antibodies that may be used in diagnosis of the more common thyroid neoplasms.
| Antibody | FA | FTC | PTC | MTC | UTC | PDTC |
|---|---|---|---|---|---|---|
| Thyroglobulin | + | + | + | − | − | + * |
| TTF-1 (N) | + | + | + | + | − | + |
| PAX8 (N) | + | + | + | + | + | + |
| Calcitonin | − | − | − | + | − | − |
| NE markers | − | − | − | + | − | − |
| CD56 | + | + | R/A | + | − | − |
| CK (AE1/AE3) | + | + | + | + | ± | + |
* Typically very focal limited to abortive or small follicles containing colloid or limited to isolated cells as paranuclear globules or vacuoles.
Molecular genetics of thyroid tumors ( Table 28-4 ):
Papillary thyroid carcinomas have activating mutations of genes coding for proteins, which signal along the mitogen-activated protein kinase pathway (MAPK).
Papillary thyroid carcinomas commonly have three genetic alterations, including:
RET/PTC rearrangements
BRAF point mutations
RAS point mutations
Mutations in RET, BRAF, and RAS genes found in approximately 70% of all papillary thyroid carcinomas but rarely overlap in same tumor
RET (rearranged during transfection) protooncogene:
Rearrangements of RET gene, known as RET/PTC rearrangements, occur in papillary thyroid carcinoma (PTC).
RET/PTC rearrangement implicated in early stages of PTC representing early event in development of PTC
Several types of RET/PTC identified differing according to 5′ partner gene involved in rearrangement
Two most common rearrangement types are:
RET/PTC1: typically found in classic PTC, papillary microcarcinomas
RET/PTC3: more common than the other fusion proteins in solid and radiation-induced PTCs, especially in children exposed to the radiation fallout from the Chernobyl accident
Uncommon to rare in follicular variant of PTC
Germline mutations present in virtually all patients with familial forms of medullary thyroid carcinoma, including:
MEN-2A
MEN-2B
Familial medullary thyroid carcinoma
Somatic mutations of RET codon 918 occur in approximately 50% of sporadic medullary thyroid carcinomas.
B-RAF proto-oncogene, serine/threonine kinase (BRAF) mutation:
Belongs to RAF family of protein kinases important components of the mitogen-activated protein kinase (MAPK) signaling pathway mediating cell growth, differentiation, and survival
Spectrum of mutations include point mutations, small in-frame deletions or insertions, and chromosomal rearrangement.
V600E is most common mutation (98% to 99%) and involves nucleotide 1799, resulting in valine-to-glutamate substitution at residue 600 (V600E).
Mutations of BRAF gene found in 40% to 45% of PTCs
Occur early in development of PTC based on presence in papillary microcarcinomas
Among thyroid tumors BRAF mutations restricted to:
Papillary thyroid carcinoma:
Classic type
Papillary microcarcinomas
Tall cell variant
Poorly differentiated thyroid carcinomas
Undifferentiated (anaplastic) thyroid carcinoma arising from thyroid papillary carcinoma.
Uncommon to rare in follicular variant of PTC
Not found in follicular carcinoma and benign thyroid nodules
Reported to serve as prognostic marker for PTC associated with more aggressive features including:
Tumor recurrence
Extrathyroidal extension
Metastatic disease (regional, distant)
Advanced tumor stage (AJCC III/IV)
Purported aggressive behavior of BRAF -associated PTCs not universally reported
Rat sarcoma (RAS) oncogene mutations:
Found in benign and malignant thyroid neoplasms, suggesting RAS activation may be an early step in thyroid tumor development
3 RAS genes prominent in cancer pathogenesis, including:
Neuroblastoma RAS viral oncogene homolog (N-RAS)
Kirsten rat sarcoma viral oncogene homolog (K-RAS)
Harvey rat sarcoma viral oncogene homolog (H-RAS)
Found in all types of follicular cell-derived tumors:
Prevalence includes N-RAS > H-RAS > K-RAS
RAS mutations found in:
40% to 50% follicular carcinomas:
Lower incidence of oncocytic variant
20% to 40% follicular adenomas:
Lower incidence of oncocytic variant
10% to 20% PTCs:
Virtually all follicular variants of PTC
Also found in 20% to 50% of poorly differentiated thyroid carcinoma and 10% to 50% of undifferentiated (anaplastic) thyroid carcinoma
Other molecular genetic findings in thyroid neoplasms:
Peroxisome proliferator-activated receptor γ (PAX8/PPARγ) gene rearrangement:
Nuclear receptors that bind DNA as heterodimers with retinoid X receptors
Shown to play important role in regulating genes involved in adipocytic differentiation and lipid metabolism
Result in recurrent translocation t(2;3)(q13;p25) leading to fusion of thyroid transcription factor PAX8 and PPARγ genes
PAX8/PPARγ rearrangements involved in thyroid cancer found in:
20% to 50% of conventional FTC: ~5% of oncocytic carcinomas
5% to 20% of follicular adenoma s
1% to 5% follicular variant of PTC
0 to 1% of classic PTC
Thyroid tumors with PAX8/PPARγ rearrangement do not usually carry any RAS mutation, suggesting that the development of FTC involves two independent pathways associated with either PAX8/PPARγ translocation or RAS mutation
Tend to be present in:
Younger-age patients
Smaller tumors
Tumors more likely to be angioinvasive
Telomerase reverse transcriptase (TERT) gene mutations:
Telomeres are the terminal portion of chromosomes that protect chromosome integrity by preventing their degradation and uncontrolled fusion and breaking cycles with other chromosomes
Telomerase (telomere terminal trnasferase) is an RNA-dependent reverse transcriptase complex that maintains telomere length allowing for cell replication and extending the life span of the cell
TERT is the enzyme protein core encoded by the TERT gene and is expressed in thyroid tumors (but is not normally expressed in thyroid tissue).
Found in 12% of papillary thyroid cancers and 14% of follicular thyroid cancers
Found to significantly correlate with older age at diagnosis
Demonstrated in thyroid cancers particularly prevalent in aggressive, less differentiated thyroid cancers, including:
Tall cell variant-PTC, poorly differentiated thyroid cancer, undifferentiated (anaplastic) thyroid cancer, and BRAF V600E mutation-positive PTC
Shown to strongly correlate with poorer outcome in differentiated thyroid tumors
Prognostic value of TERT mutations reported to be significantly stronger than that of BRAF (V600E)
TERT protein by immunohistochemical staining found to be more expressed in neoplastic than in normal tissues and to display different cellular localization, suggesting it could contribute to thyroid cancer progression by mechanisms taking place in cytoplasm
Phosphatidylinositol 3-kinase (PI3K)/AKT (AKT) signaling pathway
Plays important role in regulation of cell survival, proliferation, and migration
Activating mutations in RAS, PIK3CA, and AKT genes or inactivating mutations in PTEN tumor suppressor gene can inappropriately stimulate signaling pathway.
Mutations in signaling pathway occurs in:
5% to 10% of (differentiated) follicular carcinomas
More common in poorly differentiated thyroid carcinoma and undifferentiated (anaplastic) thyroid carcinoma harboring: PIK3CA mutation (10% to 20%) and AKT1 mutation (5% to 10%)
PIK3CA and AKT1 mutations frequently present in undifferentiated (anaplastic) thyroid carcinoma coexisting with BRAF and RAS mutations
Late event in thyroid carcinogenesis:
Higher prevalence in advanced thyroid cancer and undifferentiated (anaplastic) thyroid carcinoma
β-catenin:
Ubiquitously expressed cytoplasmic protein
Inappropriate stabilization followed by nuclear translocation proposed as important step in oncogenesis
CTNNB1 gene encodes β-catenin
Somatic mutations in exon 3 reported in:
25% of poorly differentiated thyroid carcinoma
60% of undifferentiated (anaplastic) thyroid carcinoma
Rare in (well) differentiated thyroid cancers
Oncogenic signaling through β-catenin occurs in the cribriform morular variant of PTC in the setting of familial adenomatous polyposis with germline APC mutations and in sporadic cases.
Tumor protein 53 (TP53) tumor suppressor gene
Most commonly mutated tumor suppressor gene in human cancer
Transcriptional activator involved in cell cycle progression:
Ability to arrest cell cycle and activate program cell death confers significant to TP53 in determining cell survival
Inactivating point mutations of TP53 tumor suppressor gene highly prevalent in poorly differentiated thyroid carcinoma and undifferentiated (anaplastic) thyroid carcinoma
Not present in (well) differentiated thyroid cancers
Represent late event in progression and dedifferentiation of thyroid cancer
Micro-RNAs (miRNAs or miRs):
Belong to class of small noncoding messenger RNA that have emerged as potent regulators of a variety of biologic processes, including oncogenesis
Act as posttranscriptional regulators of gene expression and are constantly deregulated in human cancer; found downregulated in lung, colon, and prostate cancer
Increasingly implicated in regulating malignant progression of cancer
Involved in thyroid cell proliferation and migration validating role in downregulation in thyroid carcinogenesis
MiR-221 and miR-222 found to be deregulated in human papillary thyroid carcinomas; involved in cell proliferation through inhibition of cell cycle regulator, p27kip1, in human papillary carcinomas
miRs may regulate fundamental aspects of the PTC phenotype, i.e., signaling, differentiation, invasion and metastasis, by fine tuning gene expression
Increased role in determining thyroid cancer phenotype serving as important diagnostic tool and useful as class identifiers especially in context of follicular thyroid carcinoma, papillary thyroid carcinoma, and anaplastic thyroid carcinoma
Cancer-relevant miRs include oncomiRs (miR-21 and miR-146b) and tumor suppressor miRs (let-7 family, miR-204, and miR-375).
Increased expression of miR-21 associated with known aggressive form of PTC (tall cell variant) and may be a critical event in pathogenesis
OncomiRs miR-221 and miR-222 reported to play role in PTC aggressiveness; associated with less differentiated tumors
miRNAs and their target genes could be targeted for novel therapeutics
| Tumor Type | Affected Genes | Prevalence |
|---|---|---|
| Follicular adenoma | RAS | 20% to 40% |
| PAX8/PPARγ translocation | 5% to 20% | |
| Follicular carcinoma | RAS | 30% to 50% |
| PAX8/PPARγ translocation | 20% to 50% | |
| PIK3CA mutation | 5% to 10% | |
| PTEN | 5% to 10% | |
| Papillary carcinoma, classical | BRAF | 30% to 70% |
| RET/PTC translocation | 20% to 40% | |
| RAS | 0 to 10% | |
| TRK | 0 to 10% | |
| Papillary carcinoma, follicular variant | RAS | 25% to 45% |
| PAX8/PPARγ translocation | 0 to 30% | |
| RET/PTC translocation | 5% to 10% | |
| BRAF | 5% to 10% | |
| Poorly differentiated carcinoma | RAS | 20% to 50% |
| TP53 | 15% to 40% | |
| B-catenin (CTNNB1) | 0 to 25% | |
| BRAF | 5% to 15% | |
| PIK3CA mutation | 10% to 20% | |
| AKT1 mutation | 5% to 10% | |
| Undifferentiated (anaplastic) carcinoma | TP53 | 50% to 80% |
| B-catenin (CTNNB1) | 5% to 65% | |
| RAS | 10% to 50% | |
| BRAF | 10% to 40% | |
| PIK3CA mutation | 5% to 25% | |
| PTEN mutation | 5% to 20% | |
| Medullary carcinoma Sporadic Familial | RET, somatic | 50% |
| RET, germline mutation | >95% |
Molecular classification
The Cancer Genome Atlas (TCGA) project:
Comprehensive multiplatform analysis of nearly 500 PTCs (excluding clinically aggressive thyroid cancers including poorly and undifferentiated carcinomas)
Integrated genomic characterization of papillary thyroid carcinoma (PTC)
Refine classification of PTC into molecular subtypes and associate them with clinically relevant parameters
PTC is MAPK-driven cancer that has two mutually exclusive drivers with distinct signaling consequences, including:
BRAF V600E–like tumors include classic PTC with papillary architecture and characteristic nuclear features (whether invasive or noninvasive); gene expression profile shows relatively less evidence of thyroid differentiation with lower expression levels of thyroid differentiation scores (TDS) genes; used for all infiltrative lesions despite follicular architecture that may predominate
RAS- like tumors include encapsulated or circumscribed (noninvasive) tumors with follicular architecture and nuclear atypia (hallmarks characteristic of follicular variant of PTC); gene expression profile that resembles normal thyroid (high TDS scores); genetics similar to follicular adenoma and follicular carcinoma (not part of the TCGA project analysis).
Reclassification of follicular variant (FV) of PTC is inevitable with recent recommended by panel of expert thyroid pathologists to replace FVPTC with alternative nomenclature such as “Noninvasive follicular tumor [NIFT] with papillary-like features.” See later under FVPTC.
Treatment for thyroid cancer:
Surgery is preferred treatment for vast majority of thyroid tumors:
Extent of surgery varies to include:
Lumpectomy:
Removal of a nodule/mass alone with minimal surrounding thyroid tissue
Generally not recommended for removal of thyroid tumors
Partial thyroidectomy:
Removal of nodule/mass with larger margin of surrounding thyroid tissue
Unacceptable in management of thyroid cancer
Isthmusectomy:
Removal of tumor within isthmus to include a margin of surrounding thyroid tissue
Lobectomy or hemithyroidectomy:
Removal of thyroid lobe with or without isthmus
Subtotal thyroidectomy:
Complete resection of one lobe and isthmus and partial removal of contralateral lobe
Bilateral subtotal thyroidectomy:
Significant portion of both lobes removed
Near total thyroidectomy:
Removal of all thyroid tissue except for 1 gram remnant on one side typically preserved to protect adjacent parathyroid tissue or to avoid distal recurrent laryngeal nerve dissection; such a remnant must be anodular and away from cancer focus
Total thyroidectomy:
Removal of entire thyroid gland; advantages of total thyroidectomy include association with higher survival rate for larger lesions (greater than 1.5 cm); association with lowest recurrence rate; improved sensitivity of serum thyroglobulin as marker for persistent or recurrent disease; allows for utilization of radioactive iodine to treat persistent or recurrent disease; reduction in (unlikely) possibility of residual tumor in contralateral lobe transforming to an anaplastic carcinoma
Surgical complications may include:
Recurrent laryngeal nerve paralysis
In hands of expert thyroid surgeon risk is low (e.g., 1% to 2%)
Risk increases (e.g., 6% to 8%) in nonexpert thyroid surgeons
Hypoparathyroidism:
May be a significant problem in patients following bilateral or total thyroidectomy
Rates of temporary hypoparathyroidism (calcium levels <8.0 mg/dl within 6 months after thyroidectomy) reported to be 17% to 40%
Rates of permanent hypoparathyroidism range from 1.2% to 6.5%.
Risk of hypoparathyroidism increases with invasive cancers; when lymph node dissection is performed with thyroidectomy; linked to experience of surgeon
Lymph node dissection:
Cervical lymph nodes divided into levels I through VI and grouped into central and lateral compartments
See Section 4 for more detailed discussion of anatomy of cervical neck lymph nodes.
Surgical treatment of cervical lymph nodes in thyroid carcinoma remains subject of debate:
Node dissection indicated when there are palpable nodes and/or radiographically suspicious or positive lymph nodes
Selective neck dissection designed to remove regional lymph nodes involved by tumor or at risk for metastasis
Central compartment neck dissection includes tissue from hyoid bone to mediastinum and from one jugular vein to the other jugular vein:
Lateral deep jugular area is carefully examined (palpated) for disease.
In presence of palpable lymph nodes laterally lymphadenectomy to include levels II, III, IV is performed.
Further, evaluation of the lymph nodes accompanying the inferior thyroid artery into the posterior triangle of the neck is indicated in the presence of palpable nodes, dissection of level V nodes is performed.
Submandibular triangle usually is not included in the dissection because thyroid cancer rarely metastasizes to the submandibular triangle.
In presence of cervical nodal metastasis and in patients with aggressive disease, imaging to include the parapharyngeal space and base of skull is indicated; because standard neck dissections do not allow for dissection of lymph nodes in these areas, if significant adenopathy of these regions is visualized, then appropriate techniques for nodal dissection of these sites should be planned and performed in addition to the standard cervical node dissection.
Radioactive iodine therapy ( 131 I):
Thyroid ablation with 131 I used in treatment of differentiated thyroid carcinoma:
131 I destroys residual microscopic (metastatic) thyroid cancer.
Facilitates identification of metastatic foci by radioactive iodine scanning
Used in treatment of distant metastasis with best results seen in patients who are under 40 years of age at time of their metastasis and whose metastatic foci concentrate 131 I
Poorer outcomes seen in patients over 40 years of age at time of their metastasis, have extensive metastatic disease, poorly differentiated tumors, and/or tumors that do not take up 131 I
Utility of radioactive iodine therapy is negated in presence of a normally situated thyroid gland proper because the latter would concentrate radioactive iodine rather than the planned intent for this therapy to destroy residual cancer outside the confines of thyroid gland proper.
Reasonably safe procedure
Potential complications may include:
Usually involve organs known to concentrate iodine, including salivary glands causing sialadenitis, loss or change in taste, xerostomia; eyes causing xerophthalmia, epiphora; gonads causing transient reductions in fertility; serious life-threatening complications are rare but may include acute bone marrow failure, reduced pulmonary function, second primary malignancy (e.g., leukemia, solid tumors)
External irradiation:
May be used postoperatively in patients with differentiated thyroid carcinoma with or without metastasis
Chemotherapy:
Generally has limited role in treatment of thyroid cancers; most often used in conjunction with other modes of therapy (surgery and radiation) in treatment of poorly differentiated or undifferentiated (anaplastic) carcinomas
Thyroid hormone therapy:
Differentiated thyroid carcinomas contain functional thyroid stimulating hormone (TSH) receptors that are more abundant in follicular carcinoma than papillary carcinoma.
TSH stimulates growth of differentiated thyroid carcinoma.
In theory, suppression of TSH receptors with suppressive doses of thyroxine may result in tumor regression.
Prognosis:
Prognosis with more common types of thyroid cancers is good with best overall survival rates associated with papillary carcinoma.
Important prognostic factors include:
Presence or absence of extrathyroidal extension
Presence or absence of metastatic disease: in lymph nodes, presence or absence of extranodal extension
Age and gender of patient
Pathologic features including histology, tumor size, presence or absence of encapsulation
Follow-up:
There is no standard protocol in follow-up of patients with thyroid cancer.
In general, scintiscan should be done within 3 months following initial therapy with reexamination at 1 year.
Disease-free patients then have whole-body radioactive iodine scans at 3 and 5 years.
Recurrent tumor is treated with radioactive iodine.
Serum thyroglobulin measurement
Reliable biomarker for well-differentiated thyroid follicular epithelial cell–derived cancer recurrence
Specific and sensitive marker
Shown to have poor accuracy for predicting malignancy in follicular neoplasms with oncocytic cells
Serum calcitonin measurement:
Used to follow patients with residual or metastatic medullary thyroid carcinoma
Clinical staging of thyroid tumors is determined by physical examination, thyroid imaging, or endoscopic examination:
Inspection and palpation of thyroid gland and cervical neck
Imaging studies include radioisotope thyroid scans, ultrasonography, CT scan, MRI scan.
Endoscopic evaluation includes indirect laryngoscopy to evaluate vocal cord motion.
TNM classification of thyroid gland cancers ( Table 28-5 ) must include all information obtained in clinical staging plus patient demographics (i.e., age and gender) and information gathered on pathologic evaluation (i.e., fine-needle aspiration biopsy, gross and microscopic evaluation) including:
Cancer type (e.g., papillary or follicular carcinoma versus medullary carcinoma versus undifferentiated [anaplastic] carcinoma)
Primary tumor size (T)
Regional lymph node involvement (N)
Distant metastasis (M)
| Primary Tumor (T) | |
| Note : all categories may be subdivided: (s) solitary tumor and (m) multifocal tumor (the largest determines the classification). | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor 2 cm or less in greatest dimension limited to thyroid |
| T1a | Tumor 1 cm or less, limited to the thyroid |
| T1b | Tumor more than 1 cm but not more than 2 cm in greatest dimension, limited to the thyroid |
| T2 | Tumor more than 2 cm but not more than 4 cm in greatest dimension, limited to the thyroid |
| T3 | Tumor more than 4 cm in greatest dimension limited to the thyroid or any tumor with minimal extrathyroid extension (e.g., extension to sternothyroid muscle or perithyroid soft tissues) |
| T4a | Moderately advanced disease Tumor of any size extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve |
| T4b | Very advanced disease Tumor invades prevertebral fascia or encases carotid artery or mediastinal vessels |
| All anaplastic carcinomas are considered T4 tumors. | |
| T4a | Intrathyroidal anaplastic carcinoma |
| T4b | Extrathyroidal anaplastic carcinoma with gross extrathyroid extension |
| Regional Lymph Nodes (N) | |
| Regional lymph nodes are the central compartment, lateral cervical, and upper mediastinal lymph nodes. | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| N1a | Metastasis to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian lymph nodes) |
| N1b | Metastasis to unilateral, bilateral, or contralateral cervical (Levels I, II, III, IV, or V) or retropharyngeal or superior mediastinal lymph node (Level VII) |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
NOTE: Pathologic staging (p): correspond to the T (pT), N (pN), and M (pM) categories
Clinical staging of thyroid gland cancers, including papillary or follicular carcinoma, medullary carcinoma, and undifferentiated (anaplastic) carcinoma is detailed in Table 28-6 .
| Separate stage groupings are recommended for papillary or follicular (differentiated), medullary, and undifferentiated (anaplastic) carcinoma. | |||
| Papillary or Follicular (Differentiated) | |||
| Under 45 years | |||
| Stage I | Any T | Any N | M0 |
| Stage II | Any T | Any N | M1 |
| 45 Years and Older | |||
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1 | N1a | M0 | |
| T2 | N1a | M0 | |
| T3 | N1a | M0 | |
| Stage IVA | T4a | N0 | M0 |
| T4a | N1a | M0 | |
| T1 | N1b | M0 | |
| T2 | N1b | M0 | |
| T3 | N1b | M0 | |
| T4a | N1b | M0 | |
| Stage IVB | T4b | Any N | M0 |
| Stage IVC | Any T | Any N | M1 |
| Medullary Carcinoma (All Age Groups) | |||
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| T3 | N0 | M0 | |
| Stage III | T1 | N1a | M0 |
| T2 | N1a | M0 | |
| T3 | N1a | M0 | |
| Stage IVA | T4a | N0 | M0 |
| T4a | N1a | M0 | |
| T1 | N1b | M0 | |
| T2 | N1b | M0 | |
| T3 | N1b | M0 | |
| T4a | N1b | M0 | |
| Stage IVB | T4b | Any N | M0 |
| Stage IVC | Any T | Any N | M1 |
| Anaplastic Carcinoma | |||
| All anaplastic carcinomas are considered Stage IV. | |||
| Stage IVA | T4a | Any N | M0 |
| Stage IVB | T4b | Any N | M0 |
| Stage IVC | Any T | Any N | M1 |
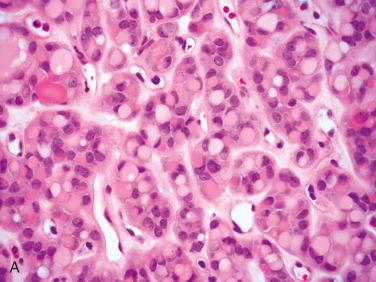
Definition: Benign encapsulated tumor with evidence of follicular cell differentiation showing growth pattern and cytomorphology different from surrounding thyroid parenchyma, but lacking features of papillary thyroid carcinoma.
Whether clonality is a part of definition of an adenoma in contrast to adenomatoid nodules is controversial because clonality has been shown to be present in a large percentage (70%) of dominant adenomatoid nodules in setting of nodular goiter.
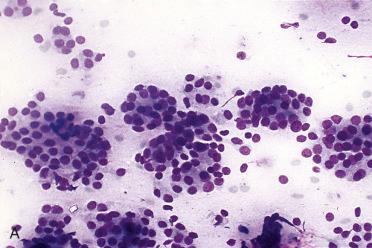

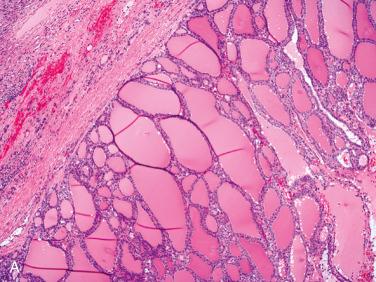
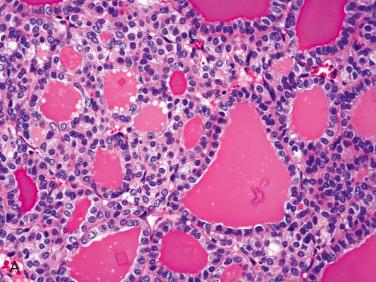
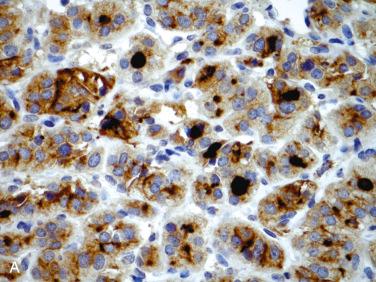
Affects women more than men; occurs over a wide age range but is most common in the fifth to sixth decades of life
Clinical presentation is usually that of a painless neck (thyroid) mass; duration of symptoms varies from months to years.
Most often solitary and limited to one part of the thyroid lobe but may involve the entire lobe:
Uncommonly, multiple adenomas may be present in a single gland.
Patients are usually euthyroid; serum thyroglobulin may be raised but clinical evidence of hyperthyroidism is rarely seen.
Radiology:
Thyroid imaging ( 123 I or 99m technetium):
Most often “cold” or hypofunctional or poorly functional nodule
May be hyperfunctioning (“hot”) nodule:
Tend to be more cellular than hypofunctioning (“cold”) adenoma
Sometimes associated with hyperthyroidism (toxic or Plummer adenoma)
No specific etiologic factors associated with development of FA
Often FNAB diagnosis is “follicular neoplasm, suspicious for follicular neoplasm (Bethesda IV),” which informs treating physician that a neoplasm is present requiring surgical removal (e.g., lobectomy)
Features associated with a follicular neoplasm that contrast with those of a (cellular) adenomatoid nodule or other lesions include:
Syncytial groups with or without distinct microfollicles; microfollicular or trabecular growth
Cellular smears
Increased cellularity
Scanty colloid, which is usually dense and in follicular lumina
Uniform cells with round nuclei, inconspicuous nucleoli, and ill-defined cell borders
Nuclear chromatin is opaque to coarsely granular and usually evenly distributed
Cytoplasmic features vary from scant to oxyphilic.
Absence of nuclear features diagnostic for papillary thyroid carcinoma
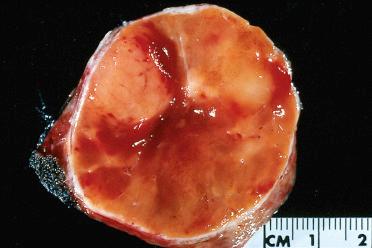
Solitary encapsulated mass:
Capsule varies in thickness but usually is thin.
Generally completely envelops tumor
Vary in size but generally measure <3 cm; larger tumors measuring more than 10 cm can be seen
Pale tan to brown to orange (oncocytic)
Solid with a rubbery to firm consistency and a homogeneous appearance except in the presence of retrogressive changes, which may include:
Hemorrhage, fibrosis, cyst formation, calcification, and infarction
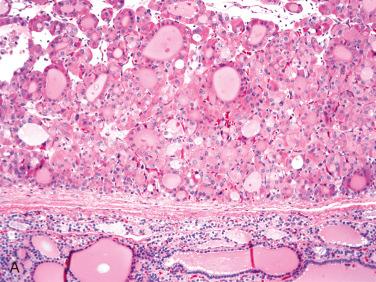
Encapsulated tumors without evidence of capsular and/or vascular invasion:
Generally fibrous caspule completely envelops tumor
Capsule is composed of fibrous tissue, within which small to medium-size vascular spaces and smooth muscle bundles may be seen.
Capsule is generally thin and clearly demarcated from neoplasm on one side and uninvolved thyroid tissue on the other side, which is usually compressed and may be atrophic.
Capsule may vary in thickness from thin and regular to thick and irregular but tends to retain relative uniformity in thickness, enveloping the lesion without wide variations in thickness.
Typically envelops lesion, remaining peripheral to and not within lesion
Markedly thickened capsule should engender ample sampling to include tumor-to-capsule-to-parenchymal interface to exclude possible presence of a invasion that may confer a diagnosis of carcinoma.
Composed of relatively uniform-appearing colloid-filled follicles with varying growth patterns that may include:
Normofollicular (simple)
Macrofollicular (colloid)
Microfollicular (fetal)
Solid (embryonal)
Trabecular (embryonal)
Organoid (cell nests) and/or insular:
Presence of insular growth pattern does not convey a diagnosis of poorly differentiated (“insular”) thyroid carcinoma:
Requisite criteria for poorly differentiated thyroid carcinoma includes presence of increased mitotic activity and necrosis with or without invasion; see later in chapter for discussion on poorly differentiated thyroid carcinoma
In general, follicular adenomas usually show single architectural pattern but may show an admixture of patterns:
Although not pathognomonic, a neoplasm with a variety of growth patterns should raise suspicion for papillary thyroid carcinoma.
Cellularity and cytologic appearance of follicular adenoma varies from tumor to tumor and even within the same tumor:
Neoplastic cells are generally uniform with defined cell borders.
Nuclei tend to:
Be round to oval with coarse to hyperchromatic nuclear chromatin, smooth (rounded) nuclear contours, absent to inconspicuous nucleoli, and a variable amount of amphophilic to eosinophilic cytoplasm:
Granular eosinophilic cytoplasm may be present not as brightly eosinophilic as present in oncocytic (Hürthle) cells
In association with granular eosinophilic cytoplasm nuclei may be enlarged with vesicular-appearing nuclei and some irregularities in size and shape, but nuclear chromatin tends to remain coarse and overall findings do not reach level of papillary thyroid carcinoma.
Align along basal aspect of cell.
Eosinophilic nuclear (pseudo)inclusions may rarely be present.
Colloid-filled follicles are generally readily apparent but in some instances may be difficult to identify.
Periodic acid Schiff (PAS) stains assist in identifying presence of colloid.
Follicular adenomas are well vascularized, and stromal component includes small to large vascular spaces:
Neoplastic cells can be seen within stromal vascular spaces but any neoplastic foci in vascular spaces within the tumor itself do not qualify as a carcinoma.
Rare mitotic figures can be seen:
Presence of increased mitotic activity (>3-5 mitoses per 10 high power fields) and necrosis should be of concern and raise suspicion for a carcinoma.
Degenerative stromal changes may uncommonly be seen and not as frequently found as in adenomatoid nodules:
Papillary or pseudopapillary architecture may be present but cytomorphologic (i.e., nuclear) findings associated with papillary thyroid carcinoma are not present.
Term papillary adenoma has been used for such lesions but the use of this designation should be avoided.
Immunohistochemistry:
Thyroglobulin, thyroid transcription factor-1 (TTF-1, nuclear), and PAX8 (nuclear) positive
Cytokeratins (AE1/AE3, CK7, CK8, CK18) positive
CK19 negative
CD56 positive (membranous)
Calcitonin, synaptophysin, chromogranin negative
Cytogenetics and molecular genetics:
RAS mutation in 20% to 40%:
Lower incidence in follicular adenoma, oncocytic type
PAX-PPARγ translocation in 5% to 20%
Absence of:
RET/PTC translocation
BRAF mutation
PTEN mutation
PIK3CA/AKT pathway mutation
Adenomatoid nodule ( Table 28-7 )
| Feature | Follicular Adenoma | Adenomatoid Nodules |
|---|---|---|
| Number | Solitary | Multiple |
| Capsule | Well-developed, completely surrounding mass; appears rather uniform in thickness and appearance | Poor encapsulation; a capsule may be present but it does not completely encapsulate mass; in association with retrogressive changes fibrosis may envelop lesion but appears irregular of variable thickness, including marked thickening within and around lesion(s) |
| Adjacent thyroid gland | Compression of surrounding gland | No compression of surrounding gland |
| Growth as compared to the rest of the thyroid gland | Different growth pattern in adjacent gland | Comparable growth pattern in adjacent gland |
| Appearance as compared to the rest of the thyroid gland | Uniform cellularity dissimilar to remainder of gland | Variable cellularity similar to those outside nodules |
| Degenerative changes | May be present but not common | Frequently present |
| Clonality | Monoclonal cell population | Polyclonal cell population; studies have shown that at least some adenomatoid nodules are monoclonal with cytogenetic abnormalities, aneuploidy, and oncogenic mutations to support a neoplastic origin |
Follicular carcinoma:
Differentiating features separating follicular adenoma from follicular carcinoma is presence of invasion, including capsular invasion and/or vascular invasion:
See Follicular Carcinoma later in chapter for detailed discussion of vascular and capsular invasion.
Features in an adenoma raising concern for a possible diagnosis of carcinoma include:
Thickened fibrous capsule
Increased mitotic activity and/or necrosis
Diffuse nuclear atypia
No known molecular test at present assisting in differentiating follicular adenoma from follicular carcinoma
Papillary thyroid carcinoma
Medullary thyroid carcinoma
Conservative surgery (lobectomy) is preferred treatment.
No recurrences or metastases
Generally, histologic variants of follicular adenoma do not confer on a given neoplasm any difference in clinical parameters or biologic behavior as compared to conventional types of follicular adenomas.
Follicular adenoma, oncocytic (Hürthle cell)
Hyalinizing trabecular adenoma (paraganglioma-like)
Follicular adenoma with atypical features
Follicular adenoma, clear cell
Follicular adenoma, signet ring cell
Follicular adenoma with intracellular fat droplets
Mucinous follicular adenoma (follicular adenoma with mucinous stroma)
Follicular adenoma with spindle cell metaplasia
Follicular adenoma with papillary architecture
Follicular adenoma with bizarre nuclei
Follicular adenoma with mesenchymal cell components
Hyperfunctioning adenoma (toxic or “hot” adenoma)
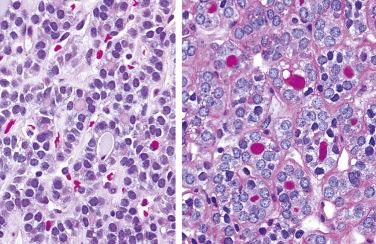
Definition: Follicular epithelial cell-derived neoplasm dominated by presence of mitochondria-rich cells (i.e., oncocytes, oxyphilic cells) without evidence of invasion or metastasis.
Terminology derived from the Greek word meaning “swollen”
Results from increase in mitochondrial content of a cell
By light microscopy, an oncocytic cell is one that has a prominent granular eosinophilic-appearing cytoplasm.
Should be distinguished from cells with cytoplasmic eosinophilia, which:
Has eosinophilic appearance of cytoplasm in hematoxylin and eosin stain (H&E)-stained sections but lacks granularity and bright pink appearance of oncocytic cells
Oncocytic change in thyroid gland not restricted to follicular cells as it can also occur in:
Neoplastic C-cells (i.e., oncocytic medullary thyroid carcinoma [MTC])
Synonyms: Hürthle cell adenoma; oncocytic adenoma; oxyphilic cell adenoma; Askanazy cell adenoma
Hürthle originally described the cell that is now felt to represent parafollicular cell or C-cell of ultimobranchial derivation and not the oncocyte; description of oncocyte attributed to Askanazy
Use of designation oncocytic (Hürthle, oxyphilic) is purely descriptive, indicative of a type of change in a cell and NOT indicative of any specified biologic behavior in a thyroid tumor
Too often, assumption made that diagnosis of “Hürthle cell neoplasm” qualifies tumor as malignant, essentially being synonymous with a follicular carcinoma:
Erroneous assumption as oncocytic cell changes can be seen in non-neoplastic and neoplastic (benign and malignant) thyroid lesions ( Box 28-3 ).
Nodular goiter (adenomatoid nodules)
Chronic lymphocytic (Hashimoto) thyroiditis
Graves disease
Postradiation
Aging
Follicular adenoma variants
Follicular carcinoma variants
Papillary carcinoma variants
Medullary carcinoma
Undifferentiated (anaplastic) thyroid carcinoma
Similar demographics, clinical presentation, treatment, and biology to “conventional” follicular adenoma
Serum thyroglobulin measurement reliable biomarker for thyroid cancer recurrence but shown to have poor accuracy for predicting malignancy in follicular neoplasms with oncocytic cells
FNAB diagnosis is “follicular neoplasm or suspicious for a follicular neoplasm, oncocytic (Hürthle) cell type (Bethesda IV),” which informs treating physician that a neoplasm is present requiring surgical removal (e.g., lobectomy)
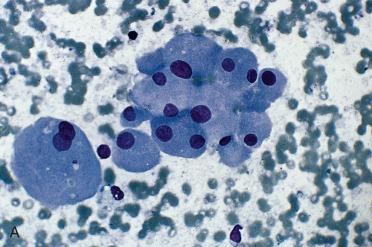
Smears or aspirates dominated by enlarged oval to polygonal cells, often in sheets, that have abundant, granular-appearing cytoplasm:
Nuclei tend to be enlarged, round to oval, eccentrically located with prominent eosinophilic nucleoli.
Binucleated cells can be seen.
Nuclear chromatin tends to be course.
Colloid is minimal to absent.
Similar to “conventional” follicular adenoma except that oncocytic cell change imparts distinct bright orange to brown coloration
Vary in size and may be quite large (>4 cm):
Large size may correlate to presence of malignant neoplasm (follicular carcinoma, oncocytic type) but does not uniformly correlate to malignancy.
Similar histologic features as “conventional” follicular adenoma:
Encapsulated tumors
Capsule may vary in thickness.
No capsular and/or vascular invasion
Growth patterns may include:
Follicular
Microfollicular
Solid
Trabecular
Organoid
Papillary architecture
May be focal or extensive
Prominent/extensive papillary growth may be seen as a retrogressive phenomenon occurring spontaneously or following fine-needle aspiration biopsy
Nuclear characteristics diagnostic for papillary carcinoma absent
Characterized by presence of cells with abundant eosinophilic granular-appearing cytoplasm
Cytoplasmic margins often distinctly seen
Presence of oncocytic cytoplasm often causes nuclear enlargement but in spite of nuclear enlargement nuclei tend to remain rather uniform-appearing round to oval with coarse to granular to vesicular-appearing chromatin and presence of distinct (centrally located) eosinophilic nucleoli
Nuclear grooves and nuclear (pseudo)inclusion may be identified.
Presence of such features not diagnostic for papillary thyroid carcinoma and can be seen in benign thyroid lesions/neoplasms
Clear cell change may be seen:
May be juxtaposed or intermixed with oncocytic cells
Caused by swelling of mitochondria
Colloid:
May be readily apparent or limited in extent
May calcify including concentric laminations, suggesting presence of psammoma bodies and a possible diagnosis of papillary thyroid carcinoma:
Typically located within lumens, not usual location in papillary thyroid carcinomas
Cytomorphologic (i.e., nuclear) features not those of papillary thyroid carcinoma
Oncocytic cells owing to oxygen-sensitive nature of mitochondria more readily undergo retrogressive changes either spontaneously or following traumatic event such as post-fine-needle aspiration biopsy; such alterations may include:
Infarction
Necrosis
Hemorrhage (recent and remote in form of hemosiderin-laden macrophages)
Papillary architecture
Cyst formation
Fibrosis
Calcifications
Term Hürthle cell neoplasm of uncertain malignant potential has been ascribed to those tumors showing worrisome but inconclusive features of malignancy, including:
Smaller cells with high nuclear-to-cytoplasmic ratio
Increased mitotic activity
Because these tumors have uniformly followed a benign clinical course this terminology is not advocated and designation of follicular adenoma, oncocytic type is preferred.
Histochemistry:
Stains for mitochondria may be positive and include:
Phosphotungstic acid hematoxylin (PTAH): red staining
Novelli stain: dark purple staining
Immunohistochemistry:
Cytokeratins, thyroglobulin, and TTF-1 positive:
Thyroglobulin reactivity less intense than in nononcocytic follicular cells
Chromogranin, synaptophysin, and calcitonin negative
Low proliferation indices (less than 5%) by KI67 (MIB-1) staining
Electron microscopy:
Oncocytic cells are packed with mitochondria.
Mitochondrial abnormalities can be seen, including quantitative and qualitative (size, shape, content) changes.
Cytogenetics and molecular genetics:
RET/PTC rearrangements may be present:
Questionable relevance relative to diagnosis and classification
May reflect very low rearrangement level using highly sensitive detection method or tumor heterogeneity
Additional findings reported include:
Large deletions of mitochondrial DNA (mtDNA), mutations of mtDNA genes coding for oxidative phosphorylation (OXPHOS) proteins, and mutations of nuclear genes coding also for mitochondrial OXPHOS proteins
Such mitochondrial alterations lead to energy production defects in Hürthle cell tumors:
Increased proliferation of mitochondria may reflect compensatory mechanism for such defects and is associated with overexpression of factors involved in mitochondrial biogenesis
Mitochondrial abnormalities also thought to play a major role in predisposition for necrosis instead of apoptosis, which seems to be blocked in most Hürthle cell tumors
Follicular carcinoma, oncocytic type:
Diagnosis of follicular carcinoma, oncocytic type made in presence of capsular or vascular invasion
In absence of invasive growth, features found in an encapsulated follicular neoplasm (including the oncocytic follicular adenoma) that may increase concern for malignancy include:
Increased cellularity in particular at the tumor-to-capsule-to-stromal interface
Smaller cells with increased nuclear-to-cytoplasmic ratio
Increased mitotic activity
Necrosis either individual cell or confluent foci
Papillary thyroid carcinoma (PTC):
Usual type:
Does not have oncocytic cytoplasm but nuclear enlargement and to some degree nuclear clearing seen in follicular adenoma, oncocytic type may be mistaken for PTC
Tall cell type:
Some authorities consider tall cells to be variant of oncocyte.
Medullary thyroid carcinoma, oncocytic type:
Oncocytic change in thyroid gland not restricted to follicular cells because it can also occur in neoplastic C-cells (i.e., oncocytic medullary thyroid carcinoma [MTC]):
Immunohistochemical staining for calcitonin, neuroendocrine markers present in MTC and absent in follicular epithelial cell tumors
Immunohistochemical staining for thyroglobulin and TTF1 present in oncocytic follicular adenoma and absent in MTC
Conservative surgery (lobectomy) is preferred treatment and considered curative.
Definition: Encapsulated follicular epithelial-derived tumor with trabecular growth pattern and presence of prominent intratrabecular hyalinization located in cytoplasm of lesional cells and in extracellular space.
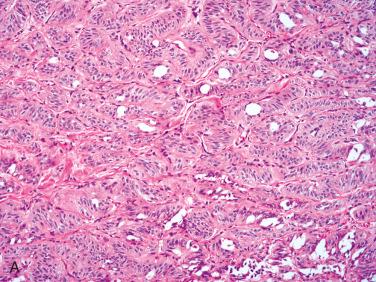
Synonym: Paraganglioma-like adenoma of thyroid (PLAT)
Controversy among authorities whether hyalinizing trabecular adenoma should be considered variant of papillary thyroid carcinoma (PTC) based on:
Occurrence of hyalinizing trabecular adenoma in the setting of lymphocytic thyroiditis or in patients with a history of radiation therapy, two settings more typically seen in association with papillary thyroid carcinoma
Co-existence with typical thyroid papillary carcinoma
Presence of HTA-like foci in typical thyroid papillary carcinoma
Overlapping nuclear features shared with PTC
Demonstration of RET/PTC translocation in percentage of cases
Occasional presence of nodal metastasis showing pattern of growth similar to hyalinizing trabecular adenoma
Weighing in favor for categorization as adenoma includes:
Overwhelming majority of solitary encapsulated thyroid lesions showing feature of hyalinizing trabecular adenoma behave as benign neoplasms
BRAF mutations a finding present in PTCs not reported in hyalinizing trabecular adenoma
Micro (mi)-RNAs known to be upregulated in PTC were downregulated in hyalinizing trabecular adenoma
At present, most authorities view hyalinizing trabecular adenoma as a benign neoplasm but given similarities to papillary carcinoma designation hyalinizing trabecular tumor recommended
Affects women more than men; occurs over a wide age range from the third through eighth decades of life
Clinical presentation is that of an asymptomatic neck mass.
May occur in any portion of the thyroid gland
Radiology:
Thyroid imaging ( 123 I or 99m technetium): “cold” nodule; may appear as a “hot” nodule
Etiology unknown:
Several cases reported following radiation exposure
Cohesive aggregates radially oriented around hyaline material
Cells with abundant cytoplasm and low nuclear-to-cytoplasmic ratio
Intranuclear cytoplasmic inclusions, nuclear grooves, and nuclear overlapping are common (best seen with the Papanicolaou method):
Cytoplasmic yellow bodies useful cytomorphologic indicator of hyalinizing trabecular adenoma; see details under microscopic description below
May be present but uncommon in papillary thyroid carcinoma and in follicular adenomas (including oncocytic variant)
Diff-Quik–stained smears highlighted the hyaline material (metachromatic), perinucleolar clearing, and cytoplasmic bodies.
Overall features may suggest papillary thyroid carcinoma or even medullary thyroid carcinoma but combination of a bloody background, radially oriented cohesive cells, cells with abundant cytoplasm, nuclei with very frequent cytoplasmic inclusions and grooves, and presence of hyalin should suggest hyalinizing trabecular adenoma.
Well-circumscribed, single, solid, encapsulated or circumscribed tumor measuring from 1 to 5 cm in diameter but usually less than 2.5 cm
Cut surface shows tendency to appear lobulated.
Circumscribed to encapsulated tumor characterized by trabecular to organoid growth with fibrovascular stroma:
Trabeculae may appear coiled.
Lobulated growth can be seen.
Presence of prominent extracellular and intracellular hyalinization
Follicle formation is minimal or absent:
Tumor has little colloid formation.
Characteristically, microcysts and larger cystic spaces are seen:
Possibly represent abortive follicle formation
Cells are elongated and sharply outlined oriented perpendicular to fibrovascular stroma.
Nuclei are round, oval, or elongated with
Nuclear grooves
Eosinophilic nuclear (pseudo)inclusions
Perinucleolar halos:
Represent giant lysosomes as seen by electron microscopy
Cytoplasm is finely granular with acidophilic, amphophilic, or clear appearance.
Cytoplasmic yellow bodies common and frequent finding representing very useful finding in FNAB or in surgical pathology material in diagnosis:
On H&E staining, appear as solid, round, pale yellow cytoplasmic inclusions surrounded by clear halo
Are refractile with a microvacuolated or granular substructure
Located close to the nucleus (paranuclear) sometimes indenting the nucleus resulting in semilunar deformity of nucleus (nuclear molding); rarely, may be intranuclear
Positive staining with PAS, GMS, alcian blue-PAS, Sudan black B
Ultrastructurally, cytoplasmic intermediate filaments, myelinosomes with parallel whorled and stacked membranes (“fingerprint” bodies), swollen mitochondria and huge membrane-limited organelles composed of disrupted membranes and microtubules, vesicles, and myelinosomes with “fingerprint” bodies; the organelles may occupy a semilunar depression in the nucleus.
Yellow bodies are consistent with giant lysosomes.
Yellow bodies are uncommon but may be identified in other thyroid neoplasms, including papillary carcinoma, follicular carcinoma, follicular adenoma or carcinoma with oncocytic cells
Calcifications including calcified colloid and/or psammoma body-like formations can be seen.
Chronic lymphocytic thyroiditis is often found in surrounding thyroid gland.
Occasional mitotic figure may be identified
Histochemistry:
Hyalinized foci may suggest presence of amyloid but staining for amyloid including Congo red is negative.
Immunohistochemistry:
Thyroglobulin and TTF-1:
Thyroglobulin may be limited to follicles and microcysts.
TTF-1 (nuclear staining) present in lesional cells
Unique cell membrane and cytoplasmic granular immunoreactivity for MIB-1 (Ki67):
Present with some monoclonal antibodies but not polyclonal antibodies suggesting this staining is artifactual
Calcitonin, chromogranin, synaptophysin negative
Galectin-3 expression may be present:
Variable expression; seen in some but not all cases and when present is never as intense as might be present in papillary thyroid carcinoma
Until recently HBME-1 negative but immunoreactivity reported with staining of lesional cells and intratrabecular hyaline matrix material identified
CD56 reactivity (membranous) may be present.
Discrepant results reported for high-molecular-weight cytokeratins and CK19
Cytogenetics and molecular genetics:
Presence of RET/PTC somatic translocations with similar (or greater) frequency to that of thyroid papillary carcinoma.
Absence of BRAF mutation
Micro (mi)-RNAs known to be upregulated in PTC not found in hyalinizing trabecular adenoma
Other thyroid lesions/neoplasms with trabecular growth and hyalinization:
Adenomatoid nodules
Lymphocytic thyroiditis
Papillary thyroid carcinoma
Medullary thyroid carcinoma
Paraganglioma
Conservative surgery (lobectomy or subtotal thyroidectomy)
Excellent prognosis following surgical removal
Hyalinizing trabecular neoplasms with minimally invasive growth have been identified and termed.
Considered to be malignant counterparts of hyalinizing trabecular adenoma:
Extremely rare:
Some cases reported may in fact represent cribriform-morular variant of papillary thyroid carcinoma that may be associated with familial-adenomatous polyposis (FAP)
Measure from 2.5 to 4 cm
Histology is identical to that hyalinizing trabecular adenoma except there is capsular and/or vascular invasion.
These minimally invasive tumors are biologically low grade.
Conservative surgical removal is indicated with close follow-up.
Definition: Any encapsulated follicular neoplasm that lacks features for papillary thyroid carcinoma but shows histologic features suspicious for a more aggressive neoplasm (i.e., carcinoma) without definitive evidence of either capsular or vascular space invasion .
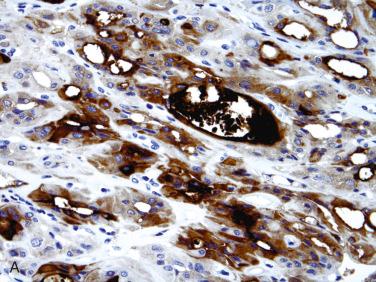
Synonyms: Atypical follicular adenoma; follicular tumor/neoplasm of uncertain malignant potential; well-differentiated (follicular) tumor of uncertain malignant potential
Demographics and clinical presentation similar to those of (typical) follicular adenoma
Macroscopic and FNAB features similar to those of (typical) follicular adenoma
Histologic features that raise possibility of a potentially more aggressive follicular neoplasm (i.e., carcinoma) and that may be considered as “atypical” include:
Increased mitotic activity in absence of necrosis:
Benign endocrine organ neoplasms generally are amitotic, and presence of mitoses raises concern for possibility of a malignant tumor.
However, as an isolated finding increased mitotic activity is not indicative of malignancy.
In presence of necrosis consideration for a possible diagnosis of poorly differentiated thyroid carcinoma should be entertained (see under Malignant Neoplasms)
Necrosis (in absence of previous FNAB):
Presence of coagulative type necrosis considered potentially worrisome features for carcinoma (even more so than mitotic activity)
Pronounced cellular proliferation with diffuse nuclear atypia
Categorization of a tumor as being atypical or of uncertain biologic behavior may include encapsulated follicular tumors in which tumor extends only to inner half of its capsule but falling short for definitive evidence of capsular invasion, which would allow diagnosis of follicular carcinoma
Classification of an encapsulated follicular tumor showing equivocal cytomorphologic features for papillary thyroid carcinoma or limited foci diagnostic for papillary thyroid carcinoma remains controversial:
If extent of change is significant/widespread (to date there is no clear definition of what constitutes “significant” or “widespread”) then diagnosis of encapsulated (noninvasive) papillary thyroid carcinoma, follicular variant, can be made although recent recommendation is to replace the term “follicular variant of PTC” with “noninvasive follicular tumor with papillary-like features.” (See under PTC in malignant neoplasms.)
If nuclear features are equivocal and there is no invasion, then such a tumor can be termed as an atypical adenoma.
If features are equivocal but there is definitive evidence of invasion, then tumor can be diagnosed as carcinoma:
In such circumstances specific designation of type of carcinoma (i.e., papillary versus follicular) is academic because treatment should be similar.
Depending on one's level of confidence following designations can be used for a neoplasm with invasive growth but equivocal cytomorphologic features:
Carcinoma, favor papillary thyroid carcinoma, follicular variant
Carcinoma, favor follicular carcinoma, minimally invasive
Well-differentiated carcinoma, not otherwise specified
In follicular neoplasm considered to be atypical (or in any encapsulated thyroid neoplasm with questionable features for malignancy) most critical issue is adequate and appropriate sectioning to evaluate tumor-capsule-thyroid parenchymal interface for evidence of invasive growth:
Guideline to number of sections considered adequate to exclude the presence of invasion include:
For a tumor measuring <6 cm = submit entire tumor
For a tumor measuring 6 cm = submit at least 10 blocks
For a tumor measuring >6 cm = submit one additional block per centimeter of tumor
Cytogenetics and molecular genetics:
Telomerase reverse transcriptase (TERT) promoter mutations (C228T and C250T) found in many malignancies, including in thyroid carcinomas, may be present in atypical follicular adenoma (and less commonly in “usual” follicular adenoma).
May occur as early genetic event in thyroid follicular tumors that have not developed malignant features on routine histopathologic workup
Follicular carcinoma, minimally invasive
Adenomatoid nodules
Papillary thyroid carcinoma, follicular variant
Treatment is surgical removal (similar to usual types of follicular adenomas):
Surgery is conservative in extent limited to the affected portion(s) of the thyroid gland (lobectomy or subtotal thyroidectomy).
Prognosis is excellent.
Long-term follow-up shows atypical follicular adenomas to behave in a benign course.
All have similar demographics, clinical presentation, treatment, and biology as “conventional” follicular adenomas.
Predominantly or exclusively composed of cells with clear (empty) to finely granular-appearing cytoplasm:
Clear-appearing cytoplasm may be due to:
Massively dilated mitochondria appearing as intracytoplasmic vesicles on electron microscopy
Intracytoplasmic glycogen, lipid, or mucin accumulation
Intracytoplasmic thyroglobulin deposition:
Intracellular thyroglobulin accumulation may be related to effects of thyroid stimulating hormone (TSH) causing increased thyroglobulin deposition within cell cytoplasm due to inability of cell to release or excrete it.
Clear cell change may be closely linked to oncocytic cell change representing “end-stage” cytoplasmic changes of a tumor that once was predominantly oncocytic.
Nuclei are centrally situated, are small, round, and regular with hyperchromasia with or without sharp cell outlines.
Growth pattern is usually follicular but may include trabecular or solid growth.
Colloid-containing follicles may be absent or only focally identified; periodic acid Schiff (PAS) is helpful in identifying the presence of colloid.
Histochemistry:
Diastase-sensitive, PAS-positive intracytoplasmic material
Immunohistochemistry:
Thyroglobulin, TTF-1 (nuclear), and PAX8 (nuclear) positive but may be focal and of limited intensity
Calcitonin and neuroendocrine cell markers (chromogranin and synaptophysin) negative
CD10, renal cell carcinoma (RCC) antibody, carbonic anhydrase IX (CAIX), PAX2, PAX8 negative
Metastatic renal cell carcinoma ( Table 28-8 )
| Features | Follicular Adenoma/Carcinoma with Clear Cells | Metastatic Renal Cell Carcinoma |
|---|---|---|
| Luminal secretion | Colloid; will be PAS positive | No colloid; red blood cells; pseudofollicles |
| Nested growth with fibrovascular stroma | Present | Present |
| Cytoplasm | Foamy appearing | Clear with distinct cell borders |
| Nuclear | Round; dispersed or coarse chromatin | Small, round, hyperchromatic |
| Glycogen (diastase-sensitive, PAS-positive) | Yes, but colloid will be diastase resistant | Yes; intensely positive |
| IHC findings | Thyroglobulin, TTF-1, PAX8 positive; CD10, RCC, CAIX, PAX2 negative | Thyroglobulin, TTF-1 negative CD10, RCC, CAIX, PAX2, PAX8 positive |
Follicular carcinoma with clear cells
Papillary thyroid carcinoma
Parathyroid lesions
Medullary thyroid carcinoma with clear cells
Synonym: Signet ring cell adenoma
Signet ring cells are round to oval, with large cytoplasmic vacuoles and hyperchromatic, eccentric nuclei.
Colloid in the background may be scanty.
Characterized by cells that have large intracytoplasmic vacuoles that result in eccentric displacement of cell nucleus creating a signet ring appearance:
Cytoplasm appears clear to acidophilic to finely granular.
Nuclei are hyperchromatic and flattened or semilunar in appearance but may retain a rounder appearance.
Growth pattern is microfollicular and nested; colloid is readily apparent.
Histochemistry:
Diastase-resistant, PAS-positive intracytoplasmic material
Mucicarmine and alcian blue may be positive.
Thyroglobulin is a sialic acid–containing glycoprotein, and therefore thyroglobulin will stain with periodic acid Schiff (PAS) and acid mucin stains (e.g., alcian blue at pH 2.5, sulfomucin).
Mucicarmine, a stain for neutral mucins, is positive but usually weakly positive:
Mucicarminophilia seen in the signet ring cells is attributed to intracytoplasmic thyroglobulin accumulation.
Immunohistochemistry:
Strong thyroglobulin reactivity is present:
Intense thyroglobulin immunoreactivity correlates to intracytoplasmic thyroglobulin deposition that, in turn, gives cell its signet ring appearance.
Intracellular thyroglobulin accumulation may be related to effects of thyroid-stimulating hormone (TSH), causing increased thyroglobulin deposition within cell cytoplasm due to inability of cell to release or excrete it.
Weak thyroglobulin staining may be present in a given example.
TTF-1 and PAX8 (nuclear) immunoreactive
Calcitonin and neuroendocrine cell markers (chromogranin and synaptophysin) are negative.
Metastatic adenocarcinoma (lung, gastrointestinal tract)
Synonym: Lipid-rich adenoma
Follicular adenoma with fat is extremely rare.
Fat in follicular epithelial cells may occur as a result of aging.
Characterized by cells that have small to medium-sized intracytoplasmic vesicles that result in indentation of centrally situated nucleus.
Histochemistry:
Oil red O or other fat stains will be positive.
Immunohistochemistry:
Thyroglobulin and TTF-1 positive
Thyrolipoma
Characterized by presence of abundant extracellular basophilic-appearing mucin
Growth patterns include microcystic, reticular, or multicystic
Mucinous pools may be present.
Signet ring cells may be identified.
Extracellular mucin stains with alcian blue and colloidal iron but not with mucicarmine and DPAS
Tumor cells positive for thyroglobulin and negative for calcitonin, CEA, galectin-3, HBME-1, and CK19
Considered a degenerative phenomenon
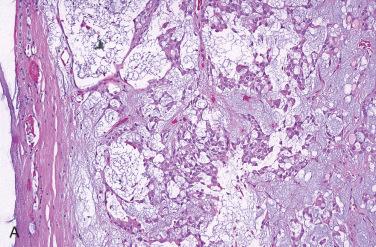
Follicular adenoma with spindle cell metaplasia ( Fig. 28-18 )
Variably seen in a given tumor:
May be focal or more diffuse
Short fascicles, storiform, or whorling growth
Bland cytomorphology lacking significant pleomorphism, mitotic activity, necrosis, or invasive growth
No cytomorphologic features diagnostic of papillary thyroid carcinoma
Immunoreactivity for:
Thyroglobulin, TTF-1, PAX8, and cytokeratin
Low proliferation indices (MIB1 or Ki67)
Spindle cell metaplasia can also be seen in adenomatoid nodules, follicular carcinoma, papillary thyroid carcinoma, and medullary thyroid carcinoma.
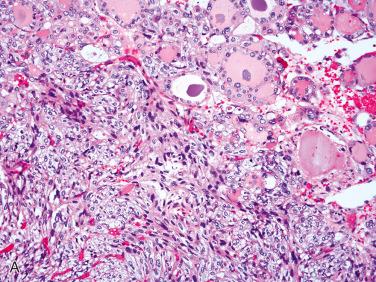
Follicular adenoma with papillary architecture ( Fig. 28-19 )
Referred to as follicular adenoma with papillary hyperplasia or papillary adenoma
These lesions:
Have a tendency to occur in children and adolescents
Have papillae that often are wider as compared to the more narrower-appearing papillae seen in papillary thyroid carcinoma
Despite papillary architecture lack cytomorphology of papillary thyroid carcinoma
Have a benign biologic course
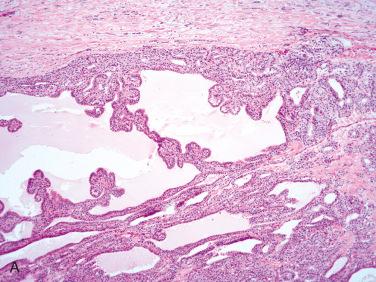
Follicular adenoma bizarre nuclei ( Fig. 28-20 )
Presence of scattered bizarre hyperchromatic and pleomorphic nuclei in an otherwise usual follicular adenoma
Nuclear changes referred to as endocrine atypia
Cells are immunoreactive for thyroglobulin, TTF-1 (nuclear), and PAX8 (nuclear)
Negligible to low proliferation rate by Ki67 staining
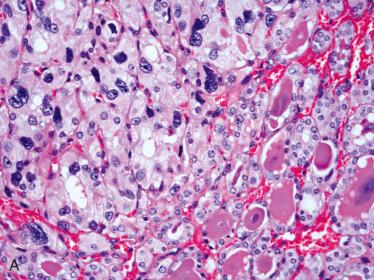
Follicular adenoma with mesenchymal cell components ( Fig. 28-21 )
Associated mesenchymal components may include:
Lipomatous stroma (thyrolipoma, adenolipoma)
Cartilage (chondroid metaplasia)
Bone (chondroid and osseous metaplasia)
Smooth muscle
Fibrous tissue
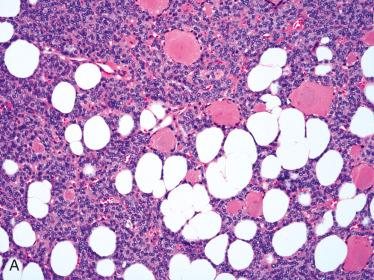
Post-FNAB-infarcted follicular adenoma:
Infarction may be partial or complete.
Infarction may compromise histology, making recognition of nature of lesion difficult.
Infarction appears as coagulative necrosis with associated hemorrhage and a variable amount of inflammatory cells
With time, granulation tissue and macrophages may be present.
Peripheral rim of residual, viable tumor may be present, which may show marked reactive nuclear atypia.
In infarcted foci, architectural pattern of lesion is retained despite cellular necrosis.
PAS staining for colloid may be helpful in recognizing lesion as a follicular epithelial neoplasm.
Antigenicity of tumor often is retained, including cytokeratin and thyroglobulin reactivity.
Tumors with cytoplasmic oxyphilia due to high content of oxygen-sensitive mitochondria are more easily traumatized, potentially resulting in infarction and additional retrogressive changes.
Hyperfunctioning adenoma (toxic or “hot” adenoma)
Rare occurrence
Associated with symptoms of hyperthyroidism due to autonomous production of thyroxine
“Hot” appearance on radioactive iodine scanning
Histologically shows hyperplastic foci with papillary growth with follicles lined by tall cuboidal cells
Histology reminiscent of that seen in Graves disease
Definition: Malignant follicular epithelial cell, not belonging to papillary carcinoma, characterized by invasive growth (i.e., capsular and/or vascular invasion) and/or metastatic disease.
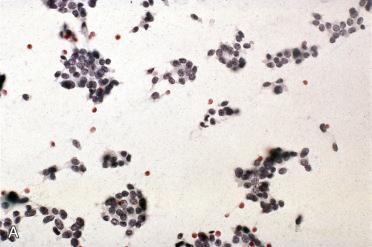
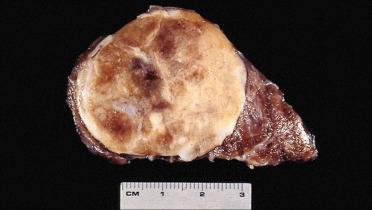
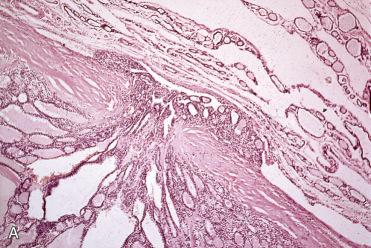
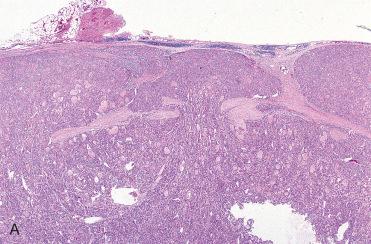
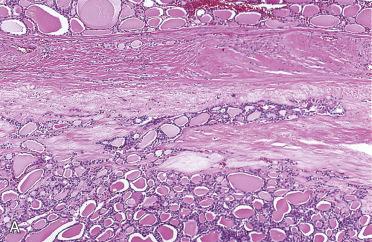
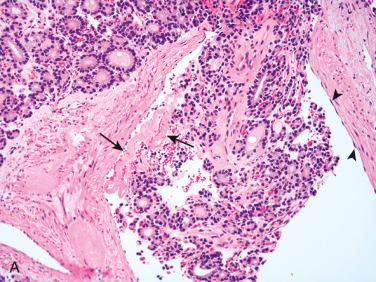
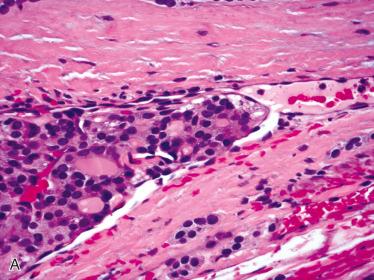
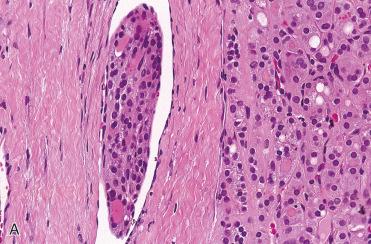
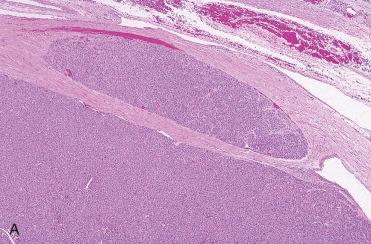
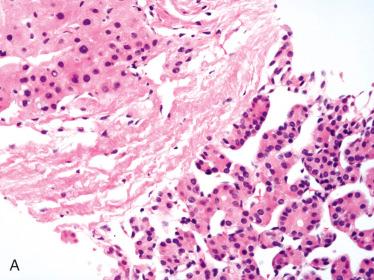
Represents approximately 10% to 20% of all malignant thyroid tumors
More common in women than in men; occurs over a wide age range but is most common in the fifth to sixth decades of life:
Approximately one decade older than patients with papillary thyroid carcinoma
Rare but does occur in children and adolescents
Clinical presentation usually solitary, painless neck mass:
Pain may occur later in disease course.
Initial presentation may be as a pulmonary metastasis or pathologic fracture secondary to osseous metastasis.
Patients are usually euthyroid:
Uncommonly, patients may present with clinical manifestations of hyperthyroidism.
Radiology
On thyroid scan ( 123 I), most often solitary, “cold,” or hypofunctioning nodules
Etiology:
Incidence greater in iodine-deficient regions of the world
Partly for this reason occurs in glands that have been enlarged for long periods
Addition of supplemental iodine to diet associated with decrease in incidence of follicular carcinoma
Development linked to:
Exposure to ionizing radiation:
Risk not as high as compared to that associated with papillary thyroid carcinoma
Pre-existing thyroid disease including:
Follicular adenoma:
Direct precursor lesion to follicular carcinoma
Goiter (adenomatoid nodules):
Predisposition owing to prolonged TSH stimulation, which in turn increases rate of cell proliferation that may result in increase chance of mutations in dividing cells
Familial syndromes such as phosphatase and tensin homolog (PTEN) hamartoma syndrome, which includes Cowden disease and other rare syndromes:
Cowden disease characterized by multiple mucocutaneous hamartomas and predisposition to malignant neoplasms, mostly breast and thyroid:
10% to 20% develop follicular carcinoma
Excellent screening tool in evaluation of a thyroid mass but utility limited in presence of differentiated (nonanaplastic) follicular epithelial cell lesion differentiating follicular adenoma from follicular carcinoma
Differentiation primarily predicated on presence or absence of invasive growth (capsular or vascular) and not cytomorphology, a feature not seen in needle aspiration
Often FNAB diagnosis is “follicular neoplasm or suspicious for a follicular neoplasm (Bethesda IV),” which informs treating physician that a neoplasm is present that requires surgical removal (e.g., lobectomy)
Cellular with minimal to absent colloid
Cells often arranged in microfollicular pattern but trabecular pattern can also be seen.
Small three-dimensional clusters with syncytial configuration can be seen.
Isolated cells are often found.
In general, cells are monomorphic and enlarged as compared to non-neoplastic follicular epithelial cells characterized by uniform, round to oval nuclei with evenly distributed, finely granular (coarse) chromatin, small to inconspicuous nucleoli, and pale to clear cytoplasm with indistinct cell margins.
Nuclei may vary in appearance:
Anisokaryosis and anisochromatosis can be seen.
Similar to their benign counterparts follicular carcinomas are encapsulated tumors:
Typically (but not always), capsule tends to be thicker than capsule in follicular adenoma:
May vary in thickness from 0.1 to 0.3 cm to thicker
For widely invasive follicular carcinomas a distinct, readily identifiable capsule may be absent.
In general, usually shows single architectural pattern but may show admixture of growth patterns, including:
Follicular and/or microfollicular:
Uniform-appearing colloid-filled follicles
Solid
Trabecular
Insular:
Presence of increased mitotic activity and necrosis (as well as so-called cleaved nuclei) required for diagnosis of poorly differentiated thyroid carcinoma (see later in chapter)
Cellularity and cytologic appearance vary from tumor to tumor and even within the same tumor:
Tendency to demonstrate greater cellularity as compared to follicular adenoma
Lesional cells generally uniform with defined cell borders
Nuclei are:
Regularly shaped (round to oval), often aligned along basal aspect of cell
Small to medium in size, hyperchromatic with coarse nuclear chromatin
Absent to inconspicuous nucleoli
Variable amount of cytoplasm
Cytoplasm may be amphophilic, eosinophilic, oncocytic, and/or clear
In presence of oncocytic cytoplasmic changes, nuclei may be enlarged with prominent nucleoli but retain uniformity in shape and coarse nuclear chromatin.
Nuclear pleomorphism may be present:
Endocrine atypia characterized by markedly enlarged and hyperchromatic nuclei may be focally identified.
Colloid filled follicles are generally readily apparent but in some instances may be difficult to identify:
Periodic acid Schiff (PAS) stains of assistance in delineating presence of colloid
Mitotic figures can be seen but are usually uncommon:
Increased mitotic activity may be present in more widely invasive follicular carcinoma.
Necrosis typically absent
Intratumoral vascularity in form of delicate capillaries may be present but often inconspicuous by routine light microscopy.
Retrogressive changes often seen in adenomatoid nodules not commonly present but may be seen in particular follicular carcinomas with predominance of cells with oncocytic cytoplasmic changes
Immunohistochemistry:
In general, immunohistochemical staining unnecessary in diagnosis and differential diagnosis of follicular epithelial-derived tumor, including follicular carcinoma
Thyroglobulin most useful stain:
Reactivity in cytoplasm and luminal colloid
TTF-1 (nuclear), PAX8 (nuclear) positive
Cytokeratins, including AE1/AE3, CAM 5.2, CK7:
CK20 negative
Calcitonin, synaptophysin, chromogranin negative
CD56 may be positive.
Cytogenetics and molecular genetics:
RAS mutations
30% to 50%
PAX8/PPARγ rearrangement:
Occurs in approximately 20% to 50% of follicular carcinomas
Strong evidence of malignancy
Reported in approximately 8% of follicular adenomas
Diagnosis of follicular carcinoma predicated on invasive growth, including:
Capsular invasion
Vascular invasion
Invasion into adjacent thyroid parenchyma and/or outside the thyroid gland (extrathyroidal extension)
Tumors showing vascular invasion frequently also show capsule invasion.
Although presence of invasive growth conceptually appears to be straightforward, there are discrepancies among pathologists, including experts in thyroid pathology, as to which microscopic findings constitute capsular invasion and/or angioinvasion.
Owing to formation of fibrous tissue along advancing edge of tumor, rarely identify direct contact between lesional cells and adjacent normal thyroid parenchyma
Certainly, diagnosis of follicular carcinoma includes presence of metastatic tumor but in general uncommon for encapsulated thyroid follicular neoplasm to metastasize in absence of invasive growth
Extent of capsular invasion source of contention:
Some authorities believe that any degree of extension of tumor into capsule qualifies as capsular invasion:
Too low a threshold
Not considered diagnostic as invasion
Partial invasion of capsule:
Owing to reported absence of recurrence or metastasis many authorities (although not all) do not accept partial capsular invasion as diagnostic for carcinoma.
Most authorities require penetration of entire thickness of capsule to be regarded as unequivocal evidence of capsular invasion.
Elastic stains may be helpful in determining whether capsular invasion has occurred.
Problematic features relative to diagnostic interpretation include:
Irregular contour(s) of the tumor:
May be caused by presence of thick-walled vascular spaces in capsule
Tangential sectioning or folding of capsule at edge of section
Changes secondary to prior fine-needle aspiration biopsy, including capsular pseudoinvasion:
May take the form of a needle tract in which there is a linear-appearing “bud” into fibrous capsule
Low magnification appearance suggests capsular invasion, but at higher magnification follicular epithelium does not violate capsule.
Needle tract composed of dense fibrosis as well as chronic inflammatory cells infiltrate, including macrophages and giant cells with or without hemorrhage (recent and remote, latter in form of hemosiderin deposition); cholesterol granuloma formation may be present
Epithelial displacement or entrapment:
May take form of small clusters of follicular cells within capsule
Separate nodule lying immediately outside the capsule of main tumor mass:
In this setting, serial sections to determine whether there is a connection present or not is indicated
Presence of continuity between main mass and nodule outside the capsule would be indicative of invasion and diagnostic for a carcinoma.
Absence of any connection does not exclude a diagnosis of carcinoma.
May be indicative of multiple adenomatoid nodules
Important to try to determine if separate nodule(s) is or is not histologically similar to main lesion:
Histologic similarity with or without direct continuity may be indicative of invasion.
Histologic dissimilarity may be indicative of separate adenomatoid nodule(s).
In a follicular neoplasm with worrisome features (i.e., thickly encapsulated, high cellularity with increased mitotic figures and necrosis) but initial sections lack definitive evidence for a diagnosis of carcinoma, most critical issue is adequate and appropriate sectioning of tumor to evaluate the tumor-capsule-thyroid parenchymal interface for evidence of invasive growth.
No set criteria for number of sections required for adequate histologic evaluation:
Guideline to number of sections considered adequate to exclude presence of invasion is:
For tumor measuring <6 cm = submit the entire tumor
For tumor measuring 6 cm = submit at least 10 blocks
For tumor measuring >6 cm = submit one additional block per centimeter of tumor
Represents more reliable feature of malignancy than capsular invasion
As nodal metastasis is rare in follicular carcinoma, invaded vascular space are not lymphatics
Vascular space must lie within capsule or beyond the capsule:
Involvement of vascular space within substance of tumor does not qualify as vascular invasion.
Criteria for diagnosing vascular invasion:
Presence of tumor within endothelial-lined space:
Tumor adherent to wall lined by identifiable endothelial cells with or without associated thrombus formation
Tumor cells protruding into vascular space with endothelial layer identified over bulging tumor nests (endothelialization):
Includes vessels within capsule and/or outside capsule
Tumor cells within vessel lumen not adherent to wall and not endothelialized still qualifies as vascular invasion if there is thrombus formation
Minimal requirements for clinically meaningful vascular invasion currently point of controversy:
Historically, presence of endothelialized tumor alone has been minimal criterion to identify vascular space invasion, a finding supported in literature.
More recently stricter criteria proposed for “significant” vascular invasion requiring both of the following findings:
Tumor cells invade through vessel wall and endothelium.
Thrombus adherent to intravascular tumor:
Application of rigid criteria felt to predict distant metastasis in thyroid carcinoma especially well-differentiated thyroid carcinoma
Risk of metastasis when strict criteria not fulfilled is not entirely absent:
Strict criteria requiring tumor cells invading through vessel wall and endothelium with thrombus adherent to intravascular tumor may be too strict.
Presence of tumor cells protruding into vascular space with endothelial layer identified over bulging tumor nests but without obvious thrombus represents “organization” of a tumor thrombus and is still considered significant.
Problematic features relative to diagnostic interpretation of VI include:
Presence of tumor within fibrous capsule conforming to contour of a blood vessel (rounded edges) suggests but not definitively diagnostic for vascular invasion:
Special stains (e.g., CD31) of limited to no utility as tumor may obscure identification of endothelial cells
Tumor cells “floating” within space not adherent to vessel, without endothelialization, and without thrombus formation do not represent vascular invasion:
Represent artifactual placement within vessel lumen
Tumor cells in capsule associated with artifactual retraction in tissue suggesting vascular space but lacking identifiable endothelial cells is not diagnostic for vascular invasion.
Tumor cells surrounding vessels or slightly pushing against wall without protrusion into lumen is not diagnostic for vascular invasion.
Presence of papillary endothelial hyperplasia (Masson tumor-like reaction):
May suggest presence of vascular invasion
May be associated with dilated vascular spaces and intravascular organized thrombus
Reactive phenomenon secondary to prior fine-needle aspiration biopsy
Temporal sequence from FNAB to surgical removal important factor in interpretation
Histochemistry:
Elastic tissue stains or trichrome may be helpful but because a continuous smooth muscle layer may not be present they usually are of only limited assistance.
Immunohistochemistry:
Stains for endothelial markers including Factor VIII-related antigen, CD31, CD34, Fli1 (nuclear), ERG (nuclear) may assist confirming presence or absence of tumor cells within endothelial cell-lined space.
Traditional classification of follicular carcinoma includes two categories:
Minimally invasive follicular carcinoma
Widely invasive follicular carcinoma
Based on extent of invasion and biologic behavior an expanded classification can be used, including:
Follicular carcinoma with capsular invasion only
Follicular carcinoma with limited vascular invasion, including less than four vascular spaces
Follicular carcinoma with extensive vascular invasion, including four or more vascular spaces (also referred to as moderately invasive follicular carcinoma)
Widely invasive follicular carcinoma
Definition: Encapsulated follicular epithelial neoplasm histologically showing limited evidence of invasion and lacking features of papillary thyroid carcinoma.
Synonyms: Encapsulated type of follicular carcinoma; low-grade follicular carcinoma
Essentially similar to that of follicular adenoma
Within this category, tumors may be further subdivided on basis of whether there is:
Capsular invasion only
Limited vascular invasion (less than four vascular spaces)
Extensive vascular invasion (four or more vascular spaces)
Primarily but not exclusively follicular adenoma
Adenomatoid nodule(s)
Papillary thyroid carcinoma
Medullary thyroid carcinoma
Treatment options include conservative versus more aggressive radical approaches:
Conservative therapy includes limited resection (lobectomy or subtotal thyroidectomy) without radioactive iodine therapy:
Can be used in presence of low-risk patients, including:
Females ≤50 years; men ≤40 years
Tumors less than 5 cm in greatest dimension
No distant metastases
Radical therapeutic intervention includes total thyroidectomy followed by administration of radioactive iodine:
Used in presence of high-risk patients, including:
Females >50 years; men >40 years
Tumors greater than 5 cm in greatest dimension
Presence of distant metastases
Some authorities would argue that any evidence of VI even less than 4 vascular spaces potentially confers aggressive behavior requiring more aggressive (radical) management.
Prognosis considered excellent but varies depending on invasive component:
Follicular carcinoma with capsular invasion only:
Essentially no risk of distant metastasis
Very low to no risk of mortality
Follicular carcinoma with limited vascular invasion (less than four vascular spaces):
Low risk of distant metastasis (approximately 5%):
If occurs may do so years following diagnosis
Risk of death less than 5%
Follicular carcinoma with extensive vascular invasion (four or more vascular spaces):
Distant metastasis may occur.
Higher risk of mortality
Definition: Follicular epithelial neoplasm with widespread invasion (grossly and/or histologically) of thyroid parenchyma, vascular invasion, and/or extrathyroidal invasion but lacking features of papillary thyroid carcinoma:
Often tumor capsule not present
Capsule may be identified with obvious complete transgression by tumor.
Much less common than minimally invasive counterpart
Tend to occur in patients slightly older than those with minimally invasive follicular carcinoma
Fleshy, solid mass usually measuring greater than 4 cm and may be as large as 10 cm
Extensive invasive growth can be seen.
Yellow to red-pink, tan-brown or orange
Central fibrosis may be seen but irregular fibrosis and cyst formation are uncommon.
Less diagnostic dilemma with less subjectivity as compared to minimally invasive follicular carcinoma
Clear-cut invasion beyond capsular delimitation of tumor with extension into adjacent thyroid parenchyma:
Owing to extensive invasion a capsule may not be readily identifiable.
Vascular invasion readily identified often into medium and large caliber-sized vascular spaces
Tend to:
Show solid or trabecular growth patterns
Be hypercellular with nuclear hyperchromasia
Papillary thyroid carcinoma
Poorly differentiated thyroid carcinoma
Medullary thyroid carcinoma
Aggressive management indicated including total thyroidectomy and postoperative radioactive iodine therapy
Prognosis varies but generally considered poor:
Commonly recur and metastasize:
Metastatic rates from 30% to 70%
Hematogenously metastasize to bones, lungs, and brain
Cutaneous metastasis also occurs.
Metastatic disease may be identified at initial presentation.
Metastatic tumor treated with radioactive iodine therapy, which may offer long-term palliation but not a cure.
Metastatic foci are histologically similar to primary tumor and may appear bland, lacking cytologic atypia.
Mortality rate of approximately 50%
Adverse prognostic factors include:
Presence of extrathyroidal extension into adjacent soft tissues
Presence of distant metastasis
Lymph node metastasis in follicular carcinoma:
Exceedingly rare
Many cases reported with nodal metastasis likely were variants of papillary thyroid carcinoma
In general nodal metastasis in association with thyroid carcinoma confers a diagnosis of papillary thyroid carcinoma:
Exception is in association with follicular carcinoma, oncocytic type, which may metastasize to lymph nodes in 5% to 10% of cases
Definition: Follicular epithelial cell-derived neoplasm exclusively or predominantly (at least 75%) composed of cells with prominent granular eosinophilic cytoplasm (rich in mitochondria) with evidence of invasion but without diagnostic features for papillary thyroid carcinoma:
Similar to nononcocytic follicular carcinomas; classification includes low-grade (minimally invasive) and widely invasive depending on extent of invasion
Simply because a tumor has oncocytic cells does not correlate to an aggressive neoplasm; however, higher proportion of solitary encapsulated oncocytic follicular tumors are invasive and therefore malignant as compared to solitary encapsulated nononcocytic follicular tumors.
Whether follicular carcinoma, oncocytic type is a histologic variant of conventional follicular carcinoma or a distinct type of follicular carcinoma remains debatable:
Similar pathologic features, aside for presence of oncocytes, support inclusion as variant of conventional follicular carcinoma
Differences in biologic behavior and natural history support distinct tumor type
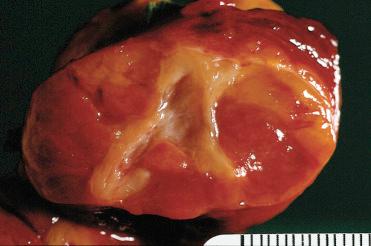
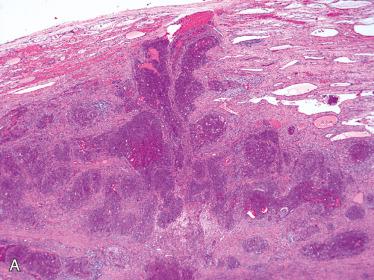
Synonyms: Hürthle cell carcinoma, oncocytic follicular carcinoma, oxyphilic cell carcinoma
More common in women than in men; tends to occur in patients approximately 10 years older than those with follicular adenoma, oncocytic type
As a group follicular carcinomas with oncocytic cells tend to:
Occur in older patients than adenomas (approximately 1 decade older)
Be larger tumors (approximately 2.0 cm larger)
Such features often associated with higher frequency of malignancy as compared to nononcocytic follicular neoplasms.
Clinical and radiographic features are similar to other follicular carcinomas:
Painless thyroid nodule
Most are solitary; rarely may be multifocal or bilateral
Cytology essentially similar to follicular adenoma with oncocytic cells
Smears or aspirates are dominated by enlarged oval to polygonal cells, often in sheets, that have abundant, granular-appearing cytoplasm.
Due to the oncocytic cytoplasm, there is nuclear enlargement:
Nuclei are round to oval and eccentrically located
Binucleated cells can be seen.
In contrast to its benign counterpart, in follicular carcinoma with oncocytic cells there may be greater nuclear enlargement and increased nuclear pleomorphism, but these findings are not diagnostic for a malignancy.
Prominent round, eosinophilic nucleoli are seen.
Colloid is minimal to absent.
Macroscopic appearance is similar to their benign counterparts, although carcinomas tend to be larger than adenomas.
Findings similar to follicular adenoma, oncocytic type except that invasion is present:
Criteria identical to those described in conventional follicular carcinoma including capsular and/or vascular invasion (see previous)
Classification includes minimally and widely invasive categories.
Histologic features that may be different from follicular adenomas with oncocytic cells include:
Thicker capsule
Greater degree of solid or trabecular growth
Increased nuclear-to-cytoplasmic ratio
Presence of increased mitotic activity
Presence of necrosis (individual cell or confluent areas)
Colloid:
May be readily apparent or limited in extent
May calcify including concentric laminations, suggesting presence of psammoma bodies and a possible diagnosis of papillary thyroid carcinoma:
Typically located within lumens, not usual location in papillary thyroid carcinomas
Cytomorphologic (i.e., nuclear) features not those of papillary thyroid carcinoma
Oncocytic cells owing to oxygen-sensitive nature of mitochondria more readily undergo retrogressive changes either spontaneously or following traumatic event such as post-fine-needle aspiration biopsy; such alterations may include:
Infarction
Necrosis
Hemorrhage (recent and remote in form of hemosiderin-laden macrophages)
Papillary architecture
Cyst formation
Fibrosis
Calcifications
Histochemistry:
Stains for mitochondria may be positive and include:
Phosphotungstic acid hematoxylin (PTAH): red staining
Novelli stain: dark purple staining
Immunohistochemistry:
Cytokeratin, thyroglobulin, and TTF-1 positive:
Thyroglobulin reactivity less intense than in nononcocytic follicular cells
CK14 reported as selective marker for thyroid oncocytic cells
Chromogranin, synaptophysin, and calcitonin negative
Electron microscopy:
Oncocytic cells are packed with mitochondria.
Mitochondrial abnormalities can be seen including quantitative and qualitative (size, shape, content) changes.
Cytogenetics and molecular genetics:
RAS mutation in 12% to 25%
PAX8/PPARγ rearrangement in 27%:
Most with follicular architecture
RET/PTC rearrangement in 38%:
All with solid pattern of growth
Identification of RET/PTC using highly sensitive methods possibly reflecting very low rearrangement level disputes this finding
Other studies failed to identify RET/PTC in oncocytic follicular tumors.
GRIM-19 mutation
TERT C228T promoter mutation detected (in widely and minimally invasive tumors)
Identification of aneuploidy in an oncocytic follicular neoplasm does not differentiate an oncocytic follicular adenoma from an oncocytic follicular carcinoma because both tumor types may show aneuploid cell populations.
PIK3CA-Akt-mTOR and Wnt/β-catenin pathways reported to differentiate follicular carcinoma, oncocytic type from follicular adenoma, oncocytic type
Follicular adenoma, oncocytic type:
Term Hürthle cell neoplasm of uncertain malignant potential ascribed to those tumors showing worrisome but inconclusive features of malignancy, including:
Smaller cells with high nuclear-to-cytoplasmic ratio
Increased mitotic activity
Because these tumors have uniformly followed a benign clinical course this terminology is not advocated, and designation of follicular adenoma, oncocytic type is preferred.
Papillary thyroid carcinoma, oncocytic type
Medullary thyroid carcinoma, oncocytic type
Oncocytic tumor of parathyroid gland origin
Treatment often includes total thyroidectomy and postoperative radioactive iodine therapy
Generally less avidity for uptake of radioactive iodine as compared to nononcocytic follicular carcinomas
5-year mortality rates of 20% to 40%
Metastases occur to:
Lungs and bone
Less commonly to regional lymph nodes:
Reported 5% to 10% of cases
Tends to occur in association with locally advanced disease or distant metastases
In presence of nodal metastasis strict criteria must be applied as to exclude diagnosis of papillary thyroid carcinoma, oncocytic type
When spreads to neck occurs usually does so as soft tissue implants rather than to lymph nodes:
Likely resulting from spread within venous channels
Overall mortality rate of 30% to 70%:
Worse prognosis as compared to nononcocytic follicular carcinomas may correlate to:
Tend to occur in an older population, which carries a greater risk of aggressive behavior
Higher percentage show presence of extrathyroidal extension
Higher percentage of tumors recur more often and metastasize more frequently.
Less avidity to take up radioactive iodine
Unfavorable prognosis factors may include:
Occurrence in men
Older age
Larger tumor size (≥4 cm in greatest dimension)
Aneuploidy tumors behave more aggressively than diploid tumors.
Recent literature reports improvement in survival for follicular carcinoma, oncocytic type such that survival rates now similar to those of conventional follicular carcinoma
Improvement in survival reported for both genders, in patients ≥45 years of age, in local and regional disease, and for tumors >4 cm
These variants of follicular carcinoma share similar diagnostic criteria, therapeutic interventions, and prognostic indicators as conventional type of follicular carcinoma.
Follicular carcinoma with clear cells:
Defined as follicular epithelial cell-derived neoplasm predominantly composed (>75%) of cells with clear cytoplasm, evidence of invasion, and absence of nuclear features diagnostic for papillary thyroid carcinoma
Presence of cells with clear cytoplasm result of accumulation of glycogen, lipid, and thyroglobulin or ballooning of mitochondria
Intracytoplasmic diastase-sensitive, PAS-positive material present indicative of glycogen
Clear cells are thyroglobulin and TTF-1 (nuclear) positive.
Must be differentiated from metastatic renal cell carcinoma ( Table 28-8 )
Follicular carcinoma with signet ring cells:
Defined as follicular epithelial cell-derived neoplasm characterized by cells with cytoplasmic vacuoles displacing the nuclei to one side and creating a signet ring appearance, evidence of invasion, and absence of nuclear features diagnostic for papillary thyroid carcinoma
Signet ring cells may be focal or diffuse
Intracytoplasmic diastase-resistant, PAS-positive but negative mucicarmine and alcian blue staining
Cytoplasmic vacuoles are immunoreactive for:
Thyroglobulin
TTF-1, CK7
Lesional cells immunoreactive for:
Thyroglobulin, TTF-1 (nuclear), CK7
Ultrastructural analysis:
Cytoplasmic vacuoles shown to be intracellular lumina or dilated vesicles lined by microvilli
Follicular carcinoma, mucinous variant:
Follicular epithelial cell-derived neoplasm with evidence of invasion characterized by presence of abundant extracellular basophilic mucinous material with evidence of invasion
Growth patterns of the follicular epithelial proliferation may include microcystic, multicystic, and reticular.
Presence of abundant extracellular basophilic mucinous material, which may also be identified within follicular lumens
Mucinous material stains for mucicarmine and alcian blue and is diastase-resistant, PAS-positive.
In presence of such histologic findings it is imperative to exclude a metastatic mucinous carcinoma from another site, including the gastrointestinal tract, breast, and others.
Hyalinizing trabecular carcinoma:
Considered to be malignant counterpart of hyalinizing trabecular adenoma
Some cases reported may in fact represent cribriform-morular variant of papillary thyroid carcinoma that may be associated with familial-adenomatous polyposis (FAP).
Measure from 2.5 to 4 cm
Histology is identical to that hyalinizing trabecular adenoma except there is capsular and/or vascular invasion.
These minimally invasive tumors are biologically low grade.
Conservative surgical removal is indicated with close follow-up.
Definition: Malignant epithelial cell-derived neoplasm with evidence of follicular cell differentiation defined or diagnosed on basis of characteristic nuclear features:
Papillary architecture is not a requisite feature for diagnosis.
Invasive growth is not a requisite feature for diagnosis.
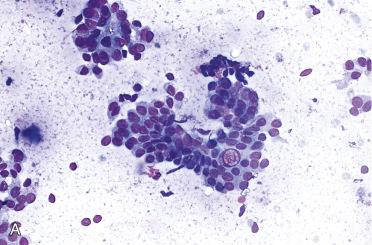
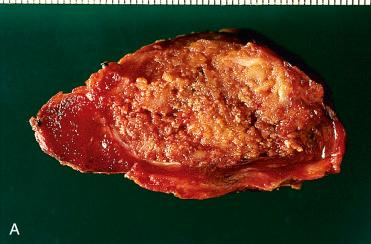
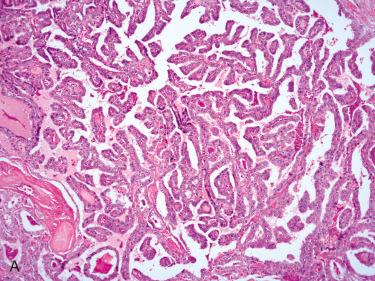
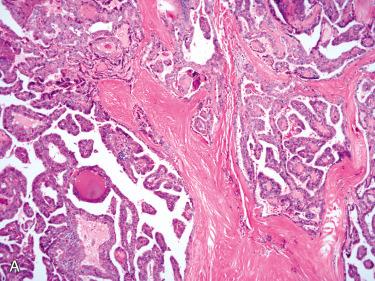
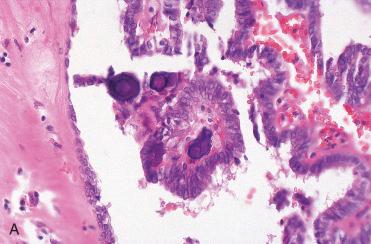
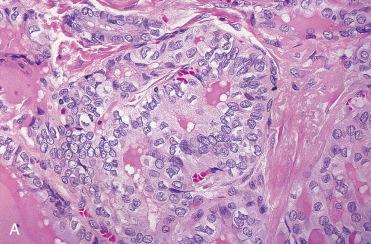

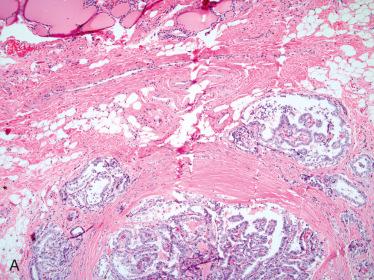
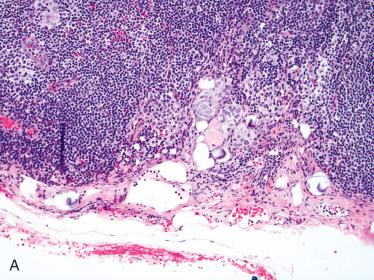
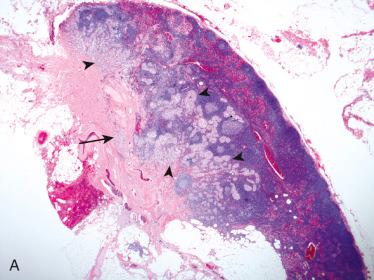
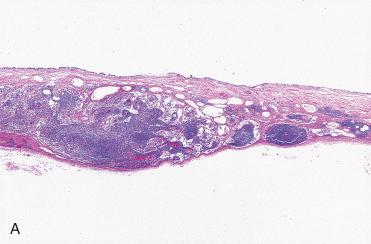
Most common malignant thyroid neoplasm
In countries with iodine-sufficient or iodine-excess diets (i.e., nonendemic) makes up to 80% of all thyroid malignant tumors
Tends to occur more frequently in women than in men; occurs in all age groups including pediatric and adolescent but is most common in third through fifth decades of life:
Most common thyroid malignant tumor in prepubertal age group
Presentation:
Usually asymptomatic (painless) thyroid or neck mass:
May or may not be palpable
With or without enlargement of regional (cervical) lymph nodes
May initially present as lateral neck mass representing nodal metastasis from primary thyroid cancer:
Reported in 27% of patients at presentation
Primary cancer most often in ipsilateral thyroid lobe
Primary cancer may be very small and difficult to detect by clinical and/or radiologic imaging.
Metastasis may be predominantly/exclusively cystic and clinically considered to be branchial cleft cyst especially in absence of thyroid mass.
Hoarseness and dysphagia may occur:
Seen in approximately 20% of patients
Indicative of recurrent laryngeal nerve involvement with vocal cord paralysis or tracheal compression
Any part of the thyroid gland can be affected.
Radiology
Thyroid scan ( 123 I), most often “cold” or hypofunctioning nodules
Etiology:
Radiation exposure:
Radiation exposure to neck region is known etiologic factor associated with the development of thyroid cancer in general and PTC in specific
Types of radiation exposures include:
External beam radiation therapy for treatment of malignant neoplasms and benign conditions:
Use of radiation in treatment of benign conditions such as acne relatively common in the earlier part of the twentieth century but essentially abandoned starting the mid- to latter part of twentieth century
γ radiation from nuclear reactor accident or nuclear weapon explosion
Development of carcinoma is dose dependent (linear correlation) and may arise in a relatively short time period if exposure is large (e.g., following Chernobyl exposure or atomic bomb) or decades later if radiation exposure is less intense (e.g., treatment of acne).
Thyroid cancer risk following radiation exposure:
Highest following radiation at a young age:
Highest in children exposed during infancy
Decreases with increasing age at treatment
Increases with follow-up duration
Radiation-induced thyroid cancers:
Most commonly are PTCs
Show solid or mixed solid and follicular growth patterns
Primarily have chromosomal rearrangements (e.g., RET/PTC ) rather than point mutations (e.g., BRAF, RAS )
Excess dietary iodine:
In areas of endemic goiter, addition of iodine to diet associated with an increase in incidence of PTC and decrease in incidence of follicular carcinoma
Genetic predisposition:
Approximately 5% of PTCs are familial:
As component of known hereditary cancer syndrome, including:
Familial adenomatous polyposis (FAP): autosomal dominant disease caused by germline mutation of APC gene located on 5q21; approximately 1% to 2% of patients develop thyroid carcinoma, primarily PTC and, in particular, cribriform-morular variant (see later in this chapter)
Other hereditary cancer syndromes that may develop PTC include: Carney complex, Werner syndrome.
Familial PTC development in individuals in families with thyroid cancer but no known hereditary cancer syndrome:
PTC occurs within families
Reported inheritance as autosomal dominant disorder with incomplete penetrance
Tend to be multifocal and arise in association with benign lesions (nodules)
Pre-existing thyroid disease including:
Increase risk of PTC in association with:
Adenomatoid nodules and adenoma:
Mechanisms not fully understood
Chronic lymphocytic (Hashimoto) thyroiditis:
More controversial association with mixed evidence weighing for but also against risk for PTC in setting of chronic lymphocytic (Hashimoto) thyroiditis
Graves disease:
No clear-cut evidence to support Graves disease as risk factor for developing PTC
In contrast to follicular adenoma and follicular carcinoma, cytologic features of PTC are diagnostic by FNAB, making needle aspiration an excellent diagnostic tool.
Findings include:
Cellular aspirates and smears
Colloid is scant and may be absent.
Cells may be arranged in papillary formations, monolayers, follicles, small or large cell clusters (syncytium-like formations), or individually dispersed.
Papillary formations may be sharply outlined with complex branching and central vascular core.
Key diagnostic components are nuclear features, which include:
Enlargement with irregularities in size and shape
Powdery or dusty chromatin pattern:
Nuclear clearing “orphan Annie” seen in histologic preparations not found in cytologic preparations
Intranuclear (pseudo)inclusions
Nuclear grooves
Nuclear crowding or overlapping
Cytoplasm usually abundant and includes pale, vacuolated, or foamy appearance
Cytoplasmic features are not of much assistance in diagnosis.
Psammoma bodies can be seen and are helpful in the diagnosis.
Multinucleated cells can be seen and sometimes are abundant.
Most are solid with poor circumscription:
May be circumscribed with or without identifiable capsule:
Capsule typically absent
Capsule may be seen in certain variants such as follicular variant.
May or may not show infiltration into adjacent thyroid parenchyma
Most confined to thyroid without grossly identifiable extrathyroidal extension, but some may show gross evidence of extension into perithyroidal soft tissues
Variations in size but most measure from 1 to 3 cm in greatest dimension
Cut section varies and may include:
Tan-white, solid tumors with rubbery to firm consistency
Partly or wholly cystic tumors filled with clear to yellow/brown fluid
Papillary appearance may be apparent by macroscopic examination.
May have gritty consistency owing to presence of psammoma bodies:
Extensive foci of calcification or ossification may be present.
Fibrosis is common finding in and around tumor appearing as white area with firm consistency.
Multifocal disease is common.
Can be divided by size and extent of invasion into:
Microcarcinoma (occult, minute, or microscopic): <1.0 cm
Intrathyroidal: encapsulated; invasive; diffuse; cystic
Extrathyroidal (massive)
Includes architectural and cytomorphologic features
Growth patterns:
Papillary, follicular, solid, trabecular, organoid; multiple growth patterns in a single lesion can occur
Elongated or twisted follicles with little colloid
Other findings:
Psammoma bodies (concentric laminations)
Intratumoral irregular fibrosis
Inspissated colloid (darker-appearing colloid as compared to the surrounding thyroid)
Nuclear alterations:
Nuclear enlargement
Nuclear irregularities in size and shape of nuclear contour
Dispersed (very fine) to optically clear-appearing (“Orphan Annie”) nuclear chromatin
Margination of chromatin along the nuclear membrane
Loss of nuclear basal polarity with haphazardly arrayed nuclei within the cell
Nuclear crowding and overlapping
Nuclear grooves
Nuclear (pseudo)inclusions
* From Lloyd RV, Erickson LA, Casey MB, et al: Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma, Am J Surg Pathol 28:1336-1340, 2004.
[Brackets contain percentage of cases showing these features.]
Cytoplasmic invaginations (pseudoinclusions) into nucleus [25%]
Abundant nuclear grooves [100%]
Ground glass nuclei [98%]
Psammoma bodies [16%]
Enlarged overlapping nuclei [99%]
Irregularly shaped nuclei [100%]
Dark staining colloid [86%]
Irregular contours of follicles [64%]
Scalloping of colloid [59%]
Elongated follicles [80%]
Multinucleated macrophages in lumen of follicles [14%]
Classic example includes presence of papillary growth:
Papillae tend to be narrow with thin fibrovascular cores and show complexity in growth with arborization.
Papillary growth not required for diagnosis and some variants devoid of papillary architecture entirely composed of a tumor with a follicular growth (see Follicular Variant later in this chapter)
Parenthetically, papillary growth can be seen in other thyroid lesions, including:
Diffuse hyperplasia (Graves disease; dyshormonogenetic goiter)
Adenomatoid nodules: as retrogressive change occurring spontaneously or following a traumatic event such as prior fine-needle aspiration biopsy
Follicular adenoma: as retrogressive change occurring spontaneously or following a traumatic event such as prior fine-needle aspiration biopsy
Other growth patterns include:
Solid, trabecular, microfollicular, macrofollicular, insular, and cystic:
Any of these patterns may be sole pattern seen or multiple admixed patterns can be seen in any one tumor
Predominantly solid tumors are those in which solid elements make up nearly all of the neoplasm.
Follicles often are elongated or twisted in appearance:
Extremely variable (but not pathognomonic) feature especially in papillary cancers without a papillary architecture and may be useful in diagnosis at time of frozen section
Tend not to be features seen in follicular adenoma/carcinoma or medullary thyroid carcinoma
Nuclear features are paramount in diagnosis:
In presence of diagnostic nuclear features, a diagnosis of PTC can be rendered in absence of invasion (i.e., invasion is not a requisite finding for diagnosis)
Nuclear alterations remain constant finding irrespective (with some exceptions) of type of PTC under consideration.
Diagnosis is predicated on constellation of nuclear alterations and should not be decided on basis of a single alteration as many individual nuclear alterations may be present in lesions that are not PTC.
NOTE: To properly evaluate nuclear features in thyroid lesions well-fixed and thin sections (4 microns) are recommended.
Nuclear enlargement:
As a general rule, nuclei in papillary carcinoma are always larger (two to three times) than those of normal thyroid follicles, adenomatoid nodules, and follicular tumors (adenomas, carcinomas).
Presence of oncocytic cytoplasm may induce nuclear enlargement, suggesting a diagnosis of PTC.
Irregularities in nuclear contour: Irregularities in size and shape of nuclei are a hallmark finding:
Nuclear membrane contour is irregular and may take on many appearances including semi-lunar, crenated, or convoluted.
Nuclear chromatin, including:
Nuclear clearing or dispersion with margination of chromatin along nuclear membrane creating optically clear-appearing nuclei (so-called Orphan Annie eyes nuclei):
Represent an artifact of fixation
Not identified in frozen sections or fine-needle aspiration
Often includes very fine (evenly dispersed) to ground glass appearance
Nuclear grooves:
Discrete longitudinal fold appearing as linear line usually along long axis of nucleus
Result from irregularity of nuclear contours
By electron microscopy represents deep invagination of nuclear membrane
Often used as an essential and diagnostic feature of PTC but not specific or unique to PTC and can be seen in non-neoplastic and other thyroid neoplasms (benign and malignant)
Nuclear orientation:
Loss of basal polarity of nuclei with random dispersion in all portions of cell resulting in crowded and overlapping of nuclei
Nuclear pseudoinclusions:
Formed by cytoplasmic invaginations into nucleus
Appear as large, round eosinophilic inclusions with sharp borders
If identified, represent reliable but not pathognomonic feature in diagnosis
Least common feature:
Identified in a minority of cases
Absence does not exclude diagnosis.
Presence does not ensure diagnosis.
Distortional changes in processing may result in intranuclear “bubbles” that simulate appearance of “true” intranuclear pseudoinclusions.
To prevent this artifact, proper fixation and thin sections are recommended.
Nucleoli:
When present, nucleoli are located along nuclear membrane but do not play a significant component in diagnosis.
Cytoplasmic appearance:
No specific cytoplasmic changes that assist in diagnosis
Certain variants of PTC named according to cytoplasmic appearance, including:
Oncocytic PTC, clear cell PTC
Psammoma bodies
Round, calcified concretions with concentric lamination
Felt to represent necrotic tumor cell(s) that form nidus for deposition of calcium salts
Name derived from Greek and means “salt-like”
Identified in up to 50% of PTCs
Located in tip of papillary stalk but can be found in solid neoplastic component or in the stroma between neoplastic follicles
Isolated or “naked” psammoma bodies represent strong evidence for presence of PTC
True whether found in normal thyroid (intrathyroidal spread) from adjacent focus or from other lobe
In cervical lymph nodes (metastatic tumor)
Not specific for PTC but considered rare in benign thyroid diseases
Microcalcifications with an appearance similar to psammoma bodies may be found within follicle lumens but are not diagnostic and should be disregarded:
These microcalcifications usually lack concentric laminations seen in psammoma bodies.
Intratumoral fibrosis
Dense fibrosis in and around lesional cells with irregular pattern (variable thickness) common feature of PTC
Colloid:
Colloid in PTC tends to be thicker with more intense eosinophilia on H&E section than colloid of adjacent non-neoplastic thyroid follicles:
Weak criteria in diagnosis but in conjunction with other features may be helpful in overall histologic picture and diagnosis of PTC.
Additional features that can be seen in association with PTC include presence of:
Lymphocytic infiltration
Squamous metaplasia
Multinucleated giant cells and/or foamy histiocytes present in lumen of lesional follicles or between papillae
Can rarely be seen in benign thyroid lesions
Mitotic figures but are uncommon
Lymph-vascular invasion
Multifocality or multicentricity:
May represent intraglandular spread/metastasis
Based on ancillary testing many of these foci have been shown to demonstrate monoclonality and different RET/PTC profiles supporting concept that they are independent primary tumors.
Extrathyroidal extension (ETE):
When present most often manifests as minimal ETE
May occur in association with larger tumors or microcarcinomas (less than 1.0 cm in greatest dimension)
Lesional cells are immunoreactive for
Thyroglobulin (cytoplasmic and luminal colloid), TTF-1 (nuclear), PAX8 (nuclear):
Thyroglobulin staining is diminished to absent in foci of squamous metaplasia.
Cytokeratins including AE1/AE3, CAM 5.2, CK7
CK20 negative
Expression of Hector Battifora mesothelial cell 1 (HBME1) (strong membranous staining with apical accentuation), galectin 3, cytokeratin 19, and CITED-1 suggested as differentiating PTC (positive reactivity) from lesions other than PTC:
Such stains are not sensitive or specific.
Similar staining may be present in normal thyroid follicles, follicular cells in non-neoplastic lesions (e.g., chronic lymphocytic thyroiditis), and benign follicular neoplasms.
Loss of CD56 reactivity in PTC reported as potential useful marker as retention of CD56 staining is identified in benign thyroid lesions and non-PTC thyroid carcinomas
Calcitonin, chromogranin, and synaptophysin negative
NOTE: To date, there is no single immunohistochemical marker or panel of immunohistochemical markers that are specific (or diagnostic) for PTC.
RET/PTC rearrangement:
Reported in 10% to 30% of PTCs
RET/PTC-1 most common followed by RET/PTC-3
Frequency higher among:
Children and young patients
Radiation induced PTCs especially:
Following exposure to radiation fallout from Chernobyl accident
External radiation received during childhood
Reported prevalence in PTC highly variable owing to geographic variation and detection methods used
Not found in follicular neoplasms
BRAF mutations:
Reliable marker of PTC
V600 BRAF mutation
Constitutes majority of BRAF mutations
Found in approximately 45% of PTC
Not found in follicular tumors and medullary thyroid carcinoma
Virtually diagnostic for PTC
Found in classic PTCs, tall cell variant, Warthin tumor–like variant, and oncocytic variant
Found in 10% to 15% of follicular variant of PTC
Much less frequent in PTCs in children and young patients
Prognostic significance
RAS mutation
Found in 10% to 15% of PTCs essentially limited to follicular variant
NRAS codon 61 and HRAS codon 61 mutations most common
Not unique to PTC found in benign thyroid lesions and other malignant neoplasms
Lesions that may have a papillary architecture:
Adenomatoid nodules, Graves disease, dyshormonogenetic goiter, others
NOTE: Not all lesions with a papillary architecture are papillary carcinomas, and the absence of a papillary architecture does not exclude a diagnosis of thyroid papillary carcinoma.
Lymphocytic thyroiditis:
Lymphocytic infiltrate may be associated with oncocytic cytoplasmic change, which in turn may be associated with nuclear enlargement and vesicular nuclear chromatin raising concern for a diagnosis of PTC.
Despite nuclear enlargement and cleared chromatin, nuclei in lymphocytic thyroiditis remain round with regular contours and coarse nuclear chromatin lacking constellation of nuclear alterations seen in PTC.
Follicular adenoma, including hyalinizing trabecular adenoma
Follicular carcinoma
Medullary thyroid carcinoma
Standard treatment is surgery as well as radioiodine therapy and thyroid hormone therapy for nearly all patients:
Surgery
Extent of surgery may vary from lobectomy to subtotal thyroidectomy to total thyroidectomy.
Standard approach for tumors measuring ≥1.0 cm is total thyroidectomy, nodal sampling of palpable lymph nodes, and subsequent radioactive iodine ablation ( 131 I).
Total thyroidectomy traditionally advocated due to high frequency of tumor multifocality
For tumors measuring <1.0 cm more conservative approach can be taken to include lobectomy and subtotal thyroidectomy; however, recommendations for more aggressive surgical approach have been advocated in presence of smaller foci of PTC, including total thyroidectomy and radiodine ablation (see Micropapillary Carcinoma).
Conservative approach including lobectomy with or without isthmusectomy or subtotal thyroidectomy followed by suppression of thyroid-stimulating hormone secretion seems most reasonable in low-risk patient population (see below):
In patients falling into low-risk group, conservative therapeutic approach shown to be as effective with similar outcomes as more aggressive approaches to management
Complications of total thyroidectomy may include hypoparathyroidism and vocal cord paralysis.
Surgical treatment of cervical lymph nodes is subject of debate:
In absence of cervical lymph node enlargement, a (modified) neck dissection need not be performed.
In face of clinically involved lymph nodes, neck dissection is performed:
Central compartment neck dissection:
Central compartment nodal metastasis is common with reported incidence of up to 50% in patients with PTC
Therapeutic central neck dissection prevents:
Sequelae of compression and invasion of critical aerodigestive and neural structures
Decreases incidence of lymphatic recurrence
May improve survival
Benefits of therapeutic central neck dissection widely accepted
Role of prophylactic neck dissection in patients with clinically negative node status remains controversial.
Lateral neck dissection:
Indicated in presence of clinically positive lymph nodes
For patients with macroscopically positive lymph nodes, neck dissection is associated with statistically significant improvement in disease-specific survival as compared to thyroidectomy alone.
Modified radical neck dissection with preservation of sternocleidomastoid muscle is performed.
Utility in patients with clinically negative node status advocated for patients with significant risk factors for lateral metastasis, including:
Presence of extrathyroidal extension
Older age
Male gender
Larger primary tumor size
Large metastatic burden in central compartment
Goals of surgical excision in PTC include:
Complete excision of cancer, including involved cervical lymph nodes:
Important determinant of outcome
Residual metastatic lymph nodes represent most common site of disease persistence/recurrence
Permit accurate staging
Facilitate postoperative treatment with radioactive iodine
Permit accurate long-term surveillance, including:
Measurement of serum thyroglobulin:
Represents highest sensitivity (95% to 100%) of detection of persistent or recurrent disease
Radioiodine whole body scanning
Minimize risk of recurrent and metastatic spread
Rationale for radioactive iodine ( 131 I) ablation includes:
Destruction of all thyroid tissue to include occult foci of carcinoma
Facilitation of postablative thyroid scanning to exclude persistent disease
Administration of 131 I cannot be performed in presence of residual normal thyroid gland; hence desire to perform total thyroidectomy
Because normal thyroid would concentrate majority of radioactive iodine, goal of destroying occult foci of carcinoma may not be achieved.
Radioactive iodine ablation is generally not administered in low-risk groups (see below) because surgery is considered sufficient.
Relapse after initial therapy is highest in first decade and may be associated with increased mortality:
Relapses may be delayed for decades (20 to 30 years) after initial diagnosis.
Overall recurrence reported in 15% to 35% of patients occurring in:
Tumor bed
Cervical neck lymph nodes
At distant sites
Incomplete surgical resection associated with increased risk of recurrence
Metastatic spread:
Preferentially via lymphatic drainage manifesting as intrathyroidal and/or regional lymph node metastasis
Distant (visceral) metastatic disease unusual occurring in 5% to 7% of cases
Lung is most common visceral metastatic site.
Bone, liver, and brain metastasis may also occur.
Prognosis:
PTC tends to be biologically indolent with an excellent prognosis:
5-year survival of 96%
10-year survival 93%
20-year survival >90%
Prognosis influenced by clinical extent of disease (clinical stage):
Stage I: 99.8% 10-year survival
Stage IV: approximately 41% 10-year survival
Overall mortality rates for thyroid carcinoma is 0.2%:
Survival rates measured over 20 years vary per risk group:
Low risk: 99% 20-year survival
Intermediate risk: 88% 20-year survival
High risk: 43% 20-year survival
Prognosis in PTC can be categorized as to risk based on established criteria, including:
AMES: A ge, M etastasis, E xtent of primary cancer, and S ize ( Table 28-9 )
| Parameter | Low Risk | High Risk |
|---|---|---|
| Age (A) | Men ≤40 years of age; women ≤50 years of age | Men >40 years of age; women >50 years of age |
| Metastases (M) | Absent | Present |
| Extent (E) of primary cancer | Intrathyroidal without ETE Follicular carcinoma, minimally invasive |
Presence of ETE Follicular carcinoma, widely invasive |
| Size (S) | <5 cm | >5 cm |
DAMES: D NA, A ge, M etastasis, E xtent of primary cancer, and S ize
GAMES: G rade, A ge, M etastasis, E xtent of primary cancer, and S ize
AGES: A ge, G rade, E xtent of primary cancer, and S ize
MACIS: M etastases, A ge, C ompleteness of resection, I nvasion, S ize
Prognostic factors associated with PTC include:
Age and gender:
Mortality increases with age:
Patients <40 years generally not associated with death as compared to patients >40 years
Women fare better than men:
Low-risk group: men ≤40 years of age; women ≤50 years of age
High-risk group: men >40 years of age; women >50 years of age
Tumor size:
Risk of death increases with increasing tumor size.
Tumor recurrence and spread increases when the tumors are large (measuring >4 to 5 cm).
Best prognosis seen with tumors ≤1.0 to 1.5 cm in diameter
Staging
Extrathyroidal extension (ETE):
Represents presence of tumor beyond confines of thyroid gland into adjacent soft tissues
One of worst prognostic indicators in thyroid cancer
Extrathyroidal extension includes minimal extension and extensive extension.
Diagnostic findings for minimal extrathyroid extension include the presence of cancer extending into perithyroidal soft tissues, including infiltration of adipose tissue and skeletal muscle, as well as neurotropism.
Diagnostic findings for extensive extrathyroid extension would include the presence of carcinoma well beyond the thyroid gland proper with direct invasion (i.e., not metastasis) into one or more of the following structures: subcutaneous soft tissues; adjacent viscera, including the larynx, trachea, and/or esophagus; the recurrent laryngeal nerve, carotid artery, or mediastinal blood vessels.
Microscopic foci of PTC with extrathyroidal extension have outcomes that are better than those tumors with extensive invasion outside the gland.
Invasion into adjacent anatomic structures (e.g., trachea, esophagus, other) is an unfavorable prognostic finding associated with decreased survival.
Distant metastasis
Presence of distant metastasis associated with worse prognosis
Site of distant metastasis affects prognosis:
Osseous and visceral (other than pulmonary) metastasis represents an ominous prognostic finding.
Pulmonary metastasis is not associated with as dire a prognosis as with osseous (or other distant) metastatic disease but is associated with a moderate adverse outcome.
Nodal metastasis:
In general presence of nodal metastasis has limited impact on survival; however, the presence of extranodal extension (ENE) of tumor into soft tissues adversely affects survival with increased risk of distant metastasis and worse prognosis.
Histologically proven angioinvasion may be considered as a sign of an increased tendency toward hematogenous spread and consequent increase in the relative percentage of metastases affecting prognosis negatively.
Completeness of surgical excision:
Complete excision decreases risk of recurrence.
Incomplete excision increases risk of recurrence.
Tumor encapsulation:
Encapsulated tumors and/or tumors showing limited invasion without ETE are associated with a favorable prognosis.
Histology (type and differentiation):
Adverse prognosis has been related to cell type and/or growth pattern (e.g., columnar cell, tall cell, solid, hobnail, and diffuse sclerosing variants)
Molecular prognostic factors:
BRAF V600E mutation: conflicting information relative to prognostic import associated with presence of BRAF V600E mutation, including:
Association with more aggressive disease characteristics including:
Extrathyroidal extension, advanced stage at presentation, metastatic disease (locoregional, distant), increased patient-related mortality
More aggressive disease characteristics also associated with small size, low-stage tumors, and microcarcinomas
Alternatively, other publications found no significant or more limited association between presence of BRAF V600E with aggressive disease characteristics
Telomerase reverse transcriptase (TERT) promoter mutations reported to be:
An independent prognostic indicator of clinically aggressive tumors correlated with worse outcome and disease-specific mortality in differentiated thyroid cancers, in particular PTC
Highly prevalent in advanced thyroid cancers, particularly those harboring BRAF or RAS mutations
Other molecular findings that may be predictors of aggressiveness in PTC include STRN/ALK fusion and PIK3CA gene mutations.
Definition: Represents an incidentally identified focus of papillary carcinoma that measures 1.0 cm or less in greatest dimension ( Box 28-6 ).

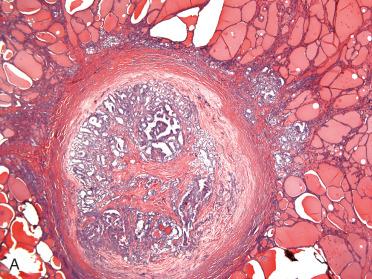
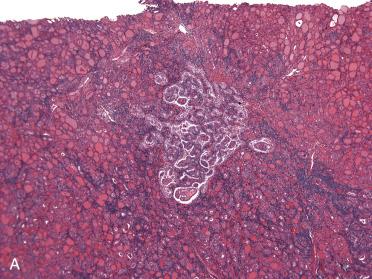
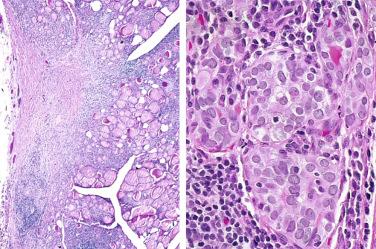
Papillary microcarcinoma
Encapsulated variant
Follicular variant
Macrofollicular variant
Oncocytic variant
Clear cell variant
Solid variant or radiation-induced pediatric variant
Cribriform-morular variant
Warthin-like variant
Diffuse follicular variant
Diffuse sclerosing variant
Tall cell variant
Columnar cell variant
Hobnail variant
Poorly differentiated thyroid carcinoma
Undifferentiated (anaplastic) thyroid carcinoma
Synonyms: Papillary microtumor; occult thyroid papillary carcinoma; occult sclerosing thyroid papillary carcinoma; microscopic papillary thyroid carcinoma; latent thyroid papillary carcinoma
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here