Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]()
Small bowel malignancies are relatively rare and account for only approximately 3% of all cancers involving the gastrointestinal (GI) tract and less than 0.5% of all cancers in the United States. In 2022, an estimated 11,790 new cases of small bowel cancers will be diagnosed, and this disease accounts for nearly 1960 deaths annually. The four major histologic subtypes, which make up over 90% of small bowel cancers, include carcinoid, adenocarcinoma, lymphoma, and gastrointestinal stromal tumors (GISTs). Over the past several decades, the incidence of small bowel tumors, and in particular carcinoid tumors, has increased significantly in the U.S. and worldwide, in part because of improved diagnostic techniques.
For small bowel tumors, the mean age at diagnosis is 65 years. Sarcomas and lymphomas present at a slightly younger age, in the range of ages 60 to 62 years, whereas adenocarcinomas and carcinoid tumors present at a mean age of 68 years. These cancers are slightly more common in men compared with women and in Blacks compared with Whites.
Adenocarcinomas typically arise from adenomas, and the adenoma-carcinoma process is driven by specific genetic alterations. Large-scale genomic analysis has identified distinct genomic differences between small bowel adenocarcinomas and colorectal cancers. HER2 point mutations, microsatellite instability, and high tumor mutational load are more common in small bowel adenocarcinomas, and potentially targetable mutations in PIK3CA and MEK1 also are seen. Small bowel adenocarcinomas comprise approximately 35 to 40% of all malignant small bowel tumors, and the duodenum is the most common site of presentation in the small bowel. The majority of these adenocarcinomas are moderately or well differentiated.
Small bowel carcinoids ( Chapter 213 ) are derived from enterochromaffin cells in the crypts of Lieberkühn, and they account for up to 40 to 45% of cancers that arise in the small bowel. Carcinoids are typically well-differentiated cancers and, in contrast to adenocarcinomas, they tend to occur in the distal ileum. Up to 30% of cases are multifocal. Other less common small bowel neuroendocrine tumors include gastrinomas and somatostatinomas ( Chapter 213 ). Small cell cancers occur rarely in the small bowel.
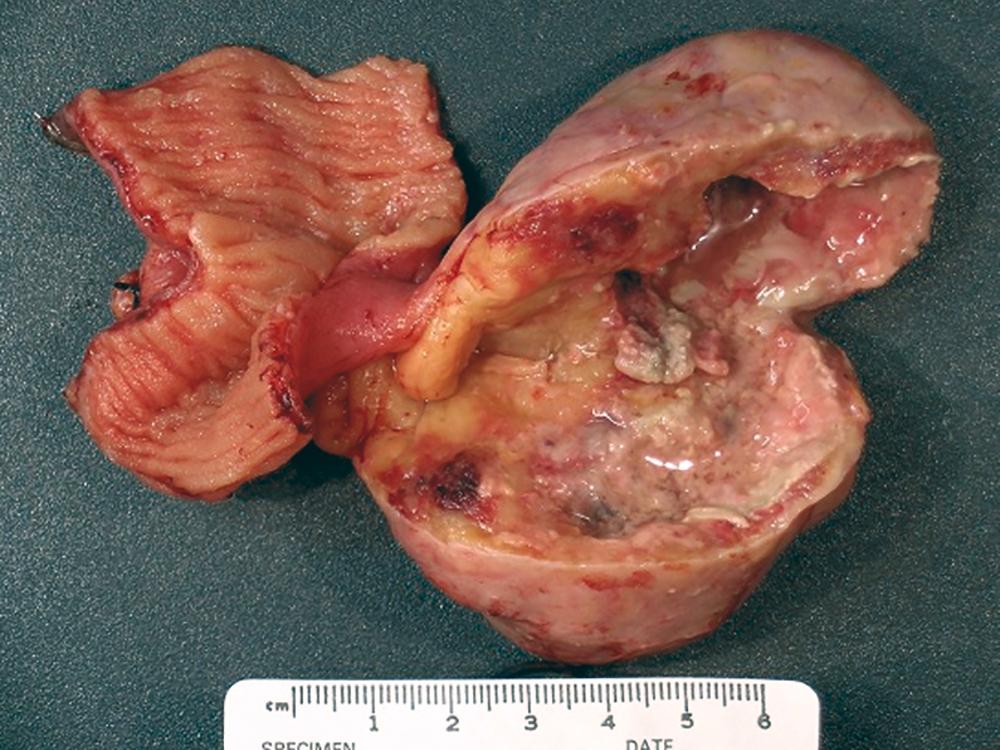
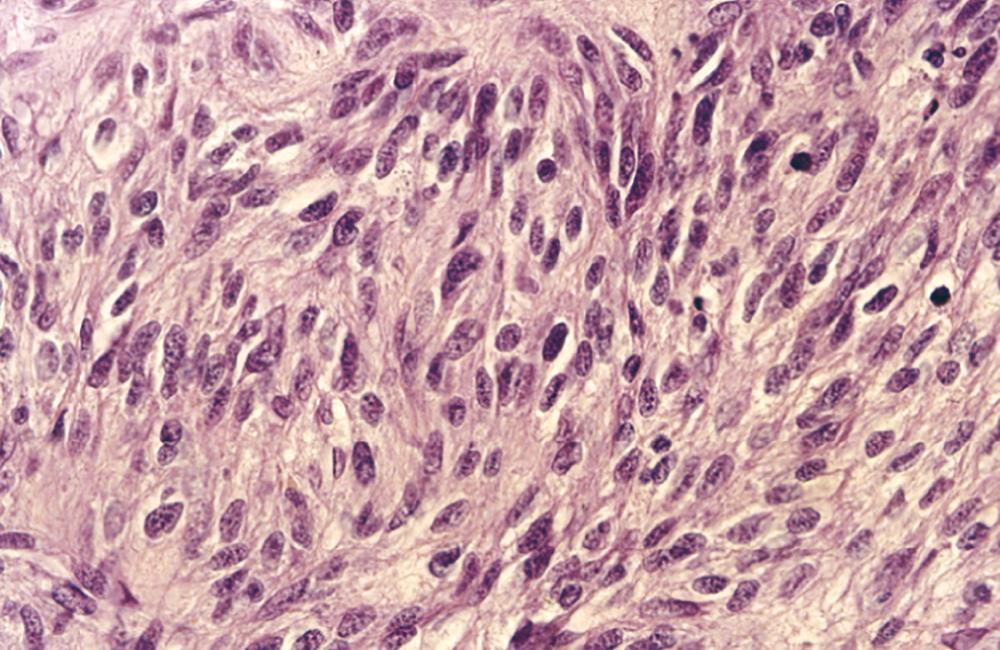
About 15% of small bowel cancers are malignant connective tissue tumors. GISTs, which are derived from the interstitial cells of Cajal, account for approximately 85% of the connective tissue tumors in the small bowel ( Fig. 179-1 ). GISTs most commonly present in the duodenum, and they often resemble soft tissue sarcomas morphologically ( Fig. 179-2 ). However, in contrast to classic sarcomas, GISTs express the c-Kit protein (CD117). Soft tissue sarcomas, such as leiomyosarcomas, present more commonly in the stomach and rarely in the small bowel ( Chapter 187 ).
Primary GI lymphomas represent the most common location for extranodal lymphomas, and the second most common site in the GI tract is the small intestine. The non-Hodgkin histopathologic subtype is most commonly observed, and they account for nearly 10% of all small bowel cancers. The ileum is the most common region for these extranodal GI lymphomas, presumably because this region of the small intestine is rich in submucosal lymphoid follicles. These cancers may be low- or high-grade in nature. Although they are most commonly of B-cell origin ( Chapter 171 ), they can also arise from precursor T lymphocytes.
The small intestine is also a site for metastatic spread of advanced solid cancers. Metastatic spread can occur via direct invasion, hematogenous spread, or extension of peritoneal metastases. For example, the small bowel is the most common GI site for metastatic malignant melanoma ( Chapter 188 ). Ovarian ( Chapter 184 ), breast ( Chapter 183 ), lung ( Chapter 177 ), and other GI cancers ( Chapter 178 ) can also spread to the small bowel.
Benign tumors originating in the small bowel include adenomas, lipomas, and leiomyomas. Desmoids, hamartomas, and hemangiomas are other benign tumors but are relatively rare. These benign tumors usually present in the distal small bowel.
Age is an important risk factor for small bowel cancers as it is for colorectal cancer. These cancers occur in the older age population, with a mean age of 66 years. Chronic mucosal inflammatory conditions are linked to the development of small bowel adenocarcinomas and lymphoma. Inflammatory bowel disease ( Chapter 127 ), in particular Crohn disease, is associated with an increased risk of adenocarcinoma within the involved region of the small intestine, and the risk of small bowel adenocarcinoma increases with extent and duration of small bowel involvement. Patients with chronic immunodeficiency states and autoimmune disorders, such as celiac disease ( Chapter 126 ), are at increased risk for developing small bowel adenocarcinomas and small bowel lymphoma.
A number of dietary factors, including red meat, salt-cured and smoked food, refined sugar, and alcohol, are associated with an increased risk of small bowel adenocarcinomas. Although tobacco exposure and obesity may increase the risk of small bowel cancers, the data on the potential role of these two factors are conflicting.
Several familial polyposis syndromes are associated with small bowel neoplasms. Familial adenomatous polyposis syndrome is associated with a 100% risk of developing colorectal cancer, and patients with this familial colorectal cancer syndrome have a 4 to 12% lifetime risk of developing cancers in the duodenum or periampullary region. The risk of cancer is related to the number of polyps and to their size, histologic type, and degree of dysplasia. Hereditary nonpolyposis colon cancer is associated with an 80% lifetime risk of colorectal cancer and a 1 to 4% lifetime risk of small bowel cancer, which represents a 100-fold increased risk when compared with the general population. Hereditary nonpolyposis colorectal cancer–associated small bowel cancers tend to present 10 to 20 years earlier than the usual age of onset for small bowel cancer. Peutz-Jeghers syndrome is associated with a 57% lifetime risk of GI cancers, with colorectal cancer the most common tumor followed by small bowel adenocarcinoma.
Small bowel malignant tumors are usually associated with symptoms, whereas benign tumors are asymptomatic in up to 50% of patients. The most common symptom is abdominal pain. Other symptoms include weight loss, nausea and vomiting, reduced appetite, fatigue, GI bleeding, and symptoms related to GI obstruction. Duodenal primary tumors, and especially tumors in the periampullary region, can be associated with obstructive jaundice.
Small bowel carcinoid tumors are usually asymptomatic. With advanced disease and especially in the presence of hepatic metastasis, they secrete bioactive amines that can lead to symptoms typically associated with the carcinoid syndrome: flushing, wheezing, diarrhea, and right-sided heart failure (related to valvular fibrosis) ( Chapter 213 ). Systemic symptoms are more frequent with tumors that originate in the jejunum or ileum.
Patients with lymphoma may present with evidence of lymphadenopathy, hepatomegaly, and intestinal obstruction (e.g., abdominal distention and hyperperistalsis). GI lymphomas also may present with constitutional symptoms, such as fever, night sweats, and weight loss. Benign tumors usually are incidental findings, although larger tumors may eventually cause obstruction or bleeding.
The diagnosis of small bowel cancer is often delayed due to its rare and nonspecific presentation. The mean duration of symptoms prior to making a definitive diagnosis of small bowel cancer ranges from 8 to 10 months. In patients with small bowel tumors, the physical examination is typically unremarkable, although some patients develop a palpable mass, distended abdomen, or ascites. Patients with a primary duodenal tumor may present with scleral icterus and jaundice resulting from biliary obstruction.
The common laboratory findings may include iron deficiency anemia ( Chapter 145 ) or elevations in hepatic enzymes (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase). The serum bilirubin level can be elevated in patients who have liver metastases or biliary obstruction. In small bowel adenocarcinoma, serum levels of the carcinoembryonic antigen (CEA) may be elevated, especially in advanced cases, but this marker is neither sensitive nor specific for routine diagnosis because several tumor types may present with elevated levels. In patients with neuroendocrine tumors, elevated levels of serotonin, chromogranin A, tumor-specific bioactive amines (e.g., gastrin), or urinary 5-hydroxyindoleacetic acid (5-HIAA) are commonly observed.
Imaging is required to diagnose and stage small bowel tumors, but no imaging modality is optimal. Standard imaging techniques include upper GI series with small bowel follow-through, enteroclysis, transabdominal ultrasound, computed tomography (CT) scan, or magnetic resonance imaging (MRI). Enteroclysis is a double-contrast imaging study that is superior to an upper GI series with small bowel follow-through, and it has greater sensitivity to detect malignant small bowel tumors. A major limitation of enteroclysis is the inability to detect flat lesions. CT scanning of the abdomen is the most common imaging modality used in daily clinical practice, and this imaging modality is good for diagnosing primary tumors as well as metastatic disease. MRI appears to be superior to CT, especially as it relates to the detection of liver metastases as well as characterizing soft tissue sarcomas.
Carcinoids and primary neuroendocrine tumors are best imaged with nuclear medicine scans with indium-111 octreotide and/or meta-iodobenzylguanidine (MIBG). Over 90% of carcinoid tumors express somatostatin receptors, and the octreotide scan using the indium-111 tracer has high sensitivity (80 to 100%). A wide variety of small bowel cancers, including adenocarcinomas, lymphomas, and GISTs, may display uptake on positron emission tomograph (PET) scanning. However, PET is not useful for diagnosing carcinoid tumors because they are not typically FDG-avid.
Video capsule endoscopy ( Chapter 120 ) uses a wireless endoscopic device to visualize the entire small bowel. The primary indication for video capsule endoscopy is to evaluate GI bleeding of an unknown source. In about 2 to 3% of patients who undergo capsule endoscopy for GI bleeding, a tumor is found. Capsule endoscopy can also assess the small intestine in patients who have familial adenomatous polyposis and Peutz-Jeghers syndrome, but its clinical utility as a routine screening test in such patients is unknown.
Deep enteroscopy techniques ( Chapter 120 ) facilitate the intubation of the small bowel using long endoscopes. The small bowel may be examined antegrade (from the mouth) or retrograde (from the colon), thereby permitting direct examination of most of the small bowel. These methods are more invasive than capsule endoscopy but have the potential advantages for tissue biopsy, immediate polypectomy, and direct control of bleeding.
Staging for small bowel tumors varies by histology. Adenocarcinomas, GISTs, and neuroendocrine cancers are typically staged using specific classifications within the American Joint Committee on Cancer’s (AJCC) TNM system. In contrast, non-Hodgkin lymphomas ( Chapter 171 ) are staged in accordance with a modified Ann Arbor system originally used for the staging of Hodgkin disease ( Chapter 172 ).
Surgical removal is the treatment of choice for localized small bowel tumors, with the extent of the surgical resection dependent on the tumor’s histology and location. For adenocarcinomas in the first two portions of the duodenum, extensive pancreaticoduodenectomy is usually required, whereas small bowel carcinomas of the distal small intestine may require segmental or wide local resection of the tumor and the regional lymph nodes. En bloc resection is recommended for low-grade endocrine tumors, including regional lymph nodes. In contrast, GISTs do not require lymphadenectomy, unless the lymph nodes are grossly abnormal at the time of surgery. Surgery is not usually a primary treatment approach for lymphomas but can be considered for low-stage lymphomas. Surgery may also be required to treat complications such as bowel obstruction or perforation. Benign small bowel tumors, such as incidental lipomas, may simply be observed. Small adenomas can be removed by endoscopic polypectomy, but periampullary villous adenomas may require pancreaticoduodenectomy ( Video 179-1 ).
The role of adjuvant therapy depends on the specific type of small bowel cancer. Small bowel adenocarcinomas are generally treated similarly to colorectal cancer, with 6 months of fluoropyrimidine-based systemic chemotherapy under expert guidance for patients with lymph node involvement. Completely resected, moderately differentiated neuroendocrine cancers do not require adjuvant therapy.
For high-risk GISTs, postoperative imatinib reduces recurrence, and 3 years of treatment improves survival compared with 1 year of treatment, especially in patients with certain Kit exon 11 deletions as well as deletions that involve exon 11 codons 557 or 558. Emerging clinical data suggest that 5 years of adjuvant imatinib therapy may confer additional clinical benefit to patients with high-risk GISTs. For other soft tissue sarcomas, adjuvant therapy has no definitive role following surgical resection. Postoperative systemic chemotherapy is recommended for both low- and high-grade lymphomas ( Chapter 171 ).
Metastatic small bowel carcinomas are generally treated with systemic chemotherapy regimens known to be effective against colorectal cancer, but no treatment regimen is standard. , For patients with metastatic GISTs, imatinib therapy yields median survival rates of nearly 4 years, with an estimated 10-year survival rate of about 20%. When resistance to imatinib develops, sunitinib and/or regorafenib are effective second-line treatments under expert guidance. For metastatic carcinoids that are surgically unresectable, somatostatin analogues, such as octreotide and lanreotide, are the main treatment options ( Chapter 213 ), but agents such as 5-fluorouracil, streptozocin, and doxorubicin have been used with modest clinical activity (see Table 164-2).
In patients with any of the various familial colon cancer syndromes (including familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, and Peutz-Jeghers syndrome), regular surveillance of the small intestine is required. Patients with familial adenomatous polyposis also should undergo regular screening esophagogastroduodenoscopy as part of their initial diagnostic evaluation and again at intervals of 1 to 5 years, depending on the presence and degree of duodenal polyposis. Patients with hereditary nonpolyposis colorectal cancer are also at increased risk for small bowel carcinomas, which may, in fact, be the first manifestation of their disease. Hereditary nonpolyposis colorectal cancer–associated small bowel cancer typically presents at a younger age (median, 39 years), and the duodenum is the most common site of disease (about 50% of cases). Screening should be started at age 30 years. Patients with sporadic small intestine adenomas and patients with carcinoid tumors should undergo colonoscopy given their higher risk of colonic neoplasia. Currently, no specific guidelines are available for the routine surveillance of patients with resected small bowel carcinomas.
The overall prognosis for small bowel cancers depends on the specific pathologic subtype. For small bowel carcinomas, the 5-year survival is 50 to 85% for stage I to III patients but only 5% for stage IV. Tumor site is an important determinant of prognosis, with survival rates being worse for primary duodenal tumors compared with ileal or jejunal primary tumors. Other factors associated with poor prognosis include poor differentiation, lymph node involvement, distant metastases, and lymphovascular invasion. The tumor mutational burden and SMAD4 mutations may potentially be useful biomarkers for predicting the prognosis in small bowel cancers.
Carcinoids ( Chapter 213 ) are typically indolent, and 5-year survival rates range between 50 and 95% depending on the stage of disease. The prognosis of small intestine GISTs depends on the primary site (distal tumors have a worse prognosis), size, and mitotic activity, as well as the adequacy of surgical resection. However, 5-year survival rates approach 80% with imatinib therapy, even when complete surgical resection is not possible. For small intestinal lymphomas, the 5-year survival rate is over 60% but varies by histologic subtype.
In the United States, colorectal cancer is the third most common cancer, with an annual incidence of approximately 150,000. It is also the second-leading cause of cancer death, with approximately 53,000 deaths each year. Worldwide, an estimated 1.2 million new cases of colorectal cancer are diagnosed each year, and about 600,000 patients die from it. In the average individual, the lifetime risk of developing colorectal cancer is high, about 1 in 20.
About 75% of large bowel cancers arise in the proximal colon. Colorectal cancer is more common in the elderly, with a median age of approximately 73 years, but about 10% of cases are in patients younger than age 50 years. The overall incidence and mortality of colorectal cancer have declined significantly over the past 2 decades, especially for patients older than 50 years of age. However, this decline has not occurred equally in all populations and varies according to age. For example, the incidence of colorectal cancer in adults younger than age 50 years has increased by more than 20% since 2000, and this rise has been driven by tumors in the distal colon and rectum. The incidence in individuals between ages 20 and 49 years has also been increasing, with the largest increase, approaching 90%, in Hispanics ages 20 to 29 years. The overall incidence of left-sided or distal colon cancer has decreased, likely because of effective distal large bowel screening, but the incidence of right-sided or proximal colon cancer has been steadily increasing in the United States.
Colorectal cancer is slightly more common in men than in women, and men, on average, develop the disease 5 to 10 years younger than women. The mortality associated with colorectal cancer is also 25% higher in men. Of all the ethnic groups in the United States, Blacks have the highest rates of colorectal cancer, and they are diagnosed an average of 5 to 10 years earlier than White patients, with an especially increased frequency under age 50 years. The mortality associated with this disease is 20% higher in Blacks than in Whites.
Sporadic colorectal cancer accounts for 80 to 85% of cases, and the main risk factors include older age, male sex, African ancestry, family history, inflammatory bowel disease, obesity, diabetes mellitus and insulin resistance, tobacco use, and alcohol use. The consumption of red meat or processed foods and reduced intake of fiber, green leafy vegetables, and fruits also contributes to the risk of colorectal cancer. The presence of certain intestinal microbiota, including bacteria ( Streptococcus bovis , Fusobacterium , and various E. coli strains) and viruses (human papillomavirus), may be risk factors. Factors associated with a reduced risk of colorectal cancer include greater physical activity and exercise; higher dietary intake of fiber, fruit, and vegetables; regular use of aspirin and nonsteroidal anti-inflammatory agents; and supplementation with folic acid, calcium, and vitamin D.
Age is the strongest risk factor for sporadic colorectal cancer, with risk increasing substantially starting at age 50 years. Nevertheless, 10 to 12% of colorectal cancers are diagnosed before age 50 years. Family history is important, even in the absence of defined familial colon cancer syndromes. If a first-degree relative has colorectal cancer, a person’s risk for developing colorectal cancer is two- to three-fold higher, with the risk rising to five- to six-fold when two first-degree relatives have colorectal cancer. Even if a first-degree relative has an adenoma, the risk of colorectal cancer is increased two-fold. Patients with a history of ulcerative colitis or Crohn disease ( Chapter 127 ) have a 60% higher risk of colorectal cancer compared with the general population, and the risk is related to the extent and duration of large bowel involvement.
Adenocarcinomas comprise more than 90% of colorectal cancers. Other relatively rare histologic subtypes include neuroendocrine cancers, epidermoid carcinomas, hamartomas, lymphomas, and sarcomas (including GISTs). Composite tumors are also observed, most commonly adenocarcinomas with neuroendocrine features.
Colorectal adenocarcinomas arise from the columnar glandular epithelium of the mucosa. Mucinous cancers make up the large majority of colorectal cancer, whereas only about 20% do not produce mucin. Mucinous cancers tend to develop in the right side of the colon. The medullary carcinoma subtype is characterized by large, eosinophilic cells and has a heavy infiltration of tumor-infiltrating lymphocytes. These tumors exhibit a high degree of microsatellite instability because they are deficient in one or more of the mismatch repair proteins. Medullary carcinomas arise more commonly on the right side of the colon, are seen in older women, and are less likely to spread to lymph nodes. Signet ring cancer is characterized by large numbers of single tumor cells with nuclear displacement by intracytoplasmic mucin.
Carcinogenesis for colorectal adenocarcinomas is caused by a multistep process when multiple genetic alterations occur over time ( Fig. 179-3 ) in one or more of three pathways: chromosomal instability, microsatellite instability, and CpG island methylator phenotype. The chromosomal instability pathway, which accounts for up to 70% of cases of sporadic colorectal cancer, is most commonly related to mutations in the APC (tumor suppressor gene) and in the KRAS gene (proto-oncogene that regulates transduction of mitogenic signals across cell membranes). In familial adenomatous polyposis, germline mutations in APC are the result of chromosomal instability that leads to aneuploidy (imbalance in chromosome number), genomic amplifications, and loss of heterozygosity (cells have only one gene allele because of the loss of individual chromosomes during mitosis). Several other gene mutations lead to colorectal cancer, including the colon cancer ( MCC ) gene (a tumor suppressor gene), p53 (a key regulator of the cell cycle), VEGF , MYC , MET , LYN , and PTEN . Many of the mutational events have downstream effects on the Wnt signaling pathway.
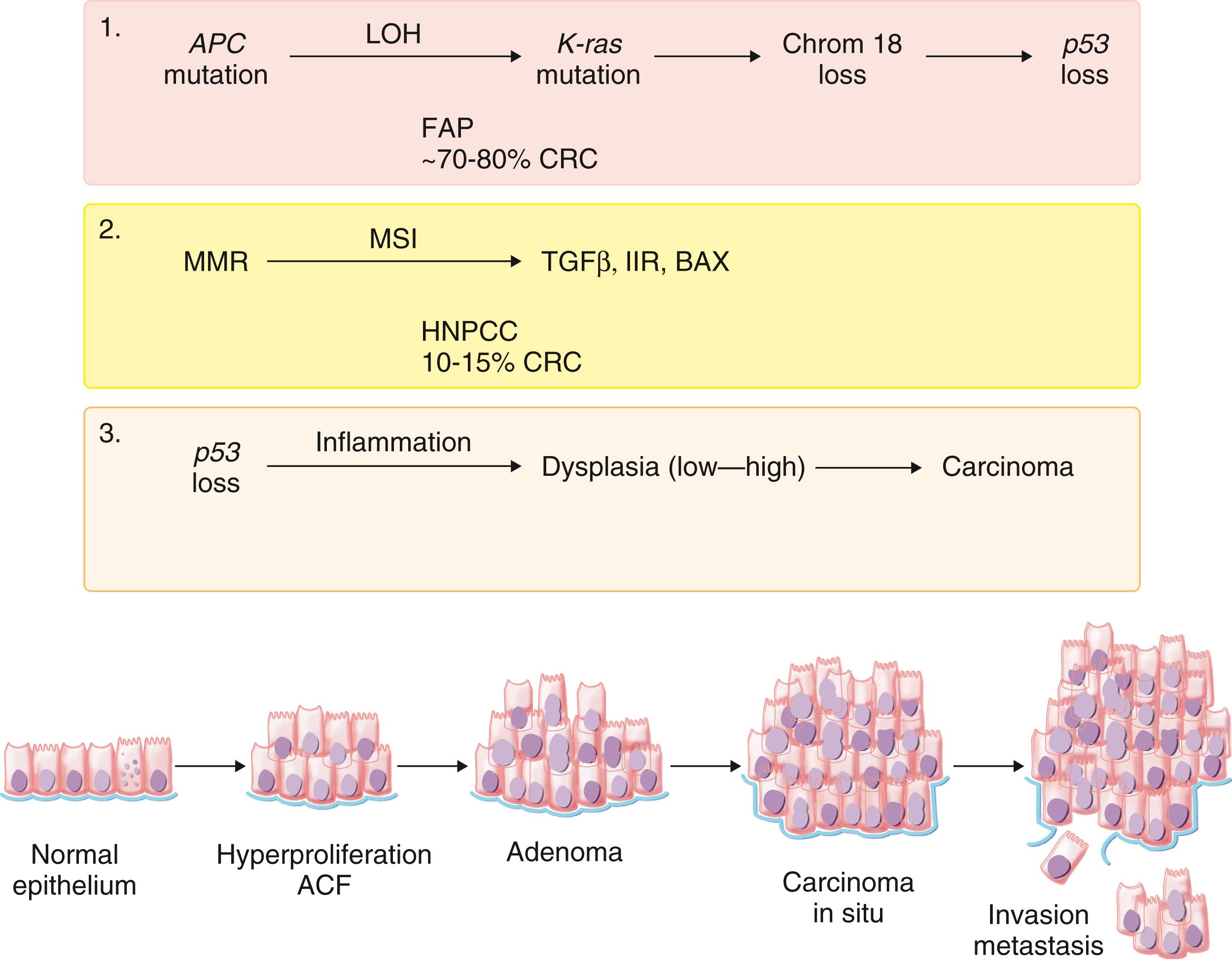
The microsatellite instability pathway is a result of defects in DNA mismatch repair. Microsatellites are short repeating nucleotide sequences that are prone to errors because of their repetitive nature. Microsatellite instability–high tumors are caused by defects in the function of mismatch repair genes, especially MLH1 , MSH2 , MSH6 , and PMS2 . Germline mutations in these genes cause HNPCC (Lynch syndrome), but silencing of MLH1 by hypermethylation is the most common molecular mechanism underlying mismatch repair defects in sporadic colorectal cancer. Microsatellite instability–high tumors are more common in women and in the right colon, and they are often poorly differentiated and associated with a lymphocytic infiltration.
In the CpG island methylator phenotype pathway, hypermethylation of DNA promoter regions silences tumor suppressor genes, thereby leading to carcinogenesis. In colorectal cancer, hypermethylated genes include APC , MCC , MLH1 , and MGMT . CpG island methylator phenotype-high tumors often have BRAF mutations, are poorly differentiated, have mucinous or signet ring morphology, and are microsatellite instability high. The precursor is likely to be a sessile serrated adenoma.
Neuroendocrine cancers include carcinoid tumors ( Chapter 213 ) and small cell carcinomas. Carcinoid tumors are the second most common colorectal neoplasms after adenocarcinomas, and are more common in non-Whites. In the distal bowel, carcinoids rarely are hormonally inactive.
Epidermoid carcinomas are usually of the squamous cell subtype. They tend to be more common in women and Hispanic patients. More than 90% of epidermoid carcinomas are located in the rectum, and they tend to be moderately or poorly differentiated.
Primary large intestinal lymphomas ( Chapter 171 ) make up about 10 to 20% of all GI lymphomas but less than 1% of all colorectal cancers. The cecum is the most common site, and they are more common in males and in the elderly. The large majority of these large bowel lymphomas are of B-cell origin.
Large bowel sarcomas are relatively rare, and a majority are leiomyosarcomas ( Chapter 187 ). These tumors commonly present in the rectum, where they are usually localized at the time of diagnosis, regardless of their grade. GISTs and Kaposi sarcomas ( Chapter 359 ) occur in the large bowel.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here