Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Similar to tumors of other upper aerodigestive tract sites, the most common tumors of the oral cavity are of epithelial origin:
Most common benign tumor is a (squamous) papilloma
Most common malignant tumor is squamous cell carcinoma or variant thereof
Although epithelial neoplasms are the most common tumor type, other epithelial tumors including those of minor salivary gland origin, as well as nonepithelial tumors, occur in the oral cavity.
Benign
Epithelial
Squamous papilloma
Minor salivary gland tumors
Ectomesenchymal chondromyxoid tumor of the anterior tongue
Others
Mesenchymal/Neuroectodermal
Peripheral nerve sheath tumors (e.g., schwannoma, neurofibroma, granular cell tumor, mucosa neuroma, others)
Fibrous tumors (e.g., fibromatosis, myofibroma/myofibromatosis)
Vascular neoplasms (e.g., angiofibroma, hemangioma, lymphangioma)
Lipoma(s)
Leiomyoma(s)
Rhabdomyoma(s)
Fibrous histiocytic tumors (e.g., fibrous histiocytoma)
Osseous (e.g., ossifying fibroma, giant cell tumor, osteoma, osteoblastoma, others)
Cartilaginous (e.g., chondroma, chondroblastoma, others)
Others
Odontogenic
Ameloblastoma
Squamous odontogenic tumor
Odontogenic keratocyst (keratocystic odontogenic tumor)
Adenomatoid odontogenic tumor
Calcifying epithelial odontogenic tumor
Calcifying cystic odontogenic tumor
Odontogenic myxoma/fibromyxoma
Malignant
Potentially Malignant Disorders
Leukoplakia
Proliferative verrucoid leukoplakia
Erythroplakia
Actinic cheilitis
Keratinizing and nonkeratinizing dysplasias
Epithelial
Squamous cell carcinoma including conventional-type and variants (e.g., verrucous carcinoma, papillary [exophytic] squamous cell carcinoma, spindle cell squamous carcinoma, basaloid squamous cell carcinoma, adenosquamous carcinoma, carcinoma cuniculatum, others)
Minor salivary gland tumors
Cribriform adenocarcinoma of minor salivary glands
Others
Nonepithelial
Mucosal malignant melanoma
Neuroendocrine carcinomas
Malignant hematolymphoid (e.g., non-Hodgkin lymphomas, Hodgkin lymphoma, plasma cell
neoplasms)
Sarcomas
Rhabdomyosarcoma
Leiomyosarcoma
Liposarcoma
Malignant peripheral nerve sheath tumors
Fibrosarcoma
Undifferentiated pleomorphic sarcoma
Alveolar soft part sarcoma
Angiosarcoma
Kaposi sarcoma
Matrix-forming malignant neoplasms (e.g., osteosarcoma, chondrosarcoma, others)
Malignant odontogenic tumors
Secondary neoplasms
Definition: Benign, exophytic epithelial neoplastic growth composed of branching fronds of squamous epithelium with fibrovascular cores.
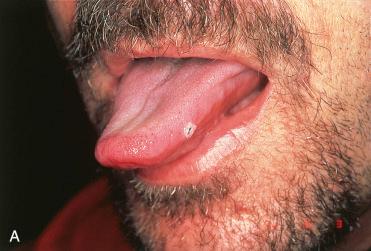
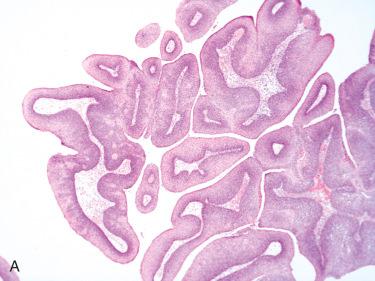
Synonym: Squamous papilloma
Most common benign neoplasm of the oral cavity
No gender predilection; most commonly seen in the third to fifth decades of life:
May occur in pediatric ages
Any site can be affected but most frequently involves tongue, palate, buccal mucosa, tonsil, and uvula
Symptoms relate to a painless mass.
Majority are solitary but may be multiple:
Multiple papillomas may occur in association with focal dermal hypoplasia syndrome or in focal epithelial hyperplasia (Heck disease)
Focal dermal hypoplasia:
Autosomal dominant disorder with incomplete penetrance
Predominantly occurs in women
Features include:
Multiple papillomas
Dermal hypoplasia with fatty penetrance
Syndactyly
Colobomas of the iris and choroids
Strabismus
Ductal hypoplasia
Focal epithelial hyperplasia or Heck disease includes:
Multiple oral papillomas
Caused by HPV types 13 and 32
Papillomas may occur anywhere in the oral cavity but most common on the labial and buccal mucosa and the tongue
Florid oral papillomatosis:
Clinical term for diffuse papillomatous change but not a defined clinicopathologic entity
May be associated with:
Cowden syndrome:
Autosomal dominant disease characterized by facial trichilemmomas associated with GIT, CNS, musculoskeletal, and thyroid abnormalities
Down syndrome, nevus unis lateris syndrome (ichthyosis hystrix), acanthosis nigricans, tuberous sclerosis, and focal dermal hypoplasia syndrome (Goltz-Gorlin syndrome)
Immunosuppressed patients, in particular HIV infection, may be associated with florid oral papillomas/papillomatosis (immunodeficiency and papillomas/papillomatosis):
Entire oral mucosa may be papillomatous.
Lesions tend to be larger than nonimmunosuppressed-related lesions and may be multiple; lesions may coalesce to form extensive mucosal patches.
Multiple HPV subtypes, including unusual ones, may be identified.
Cause:
Human papillomavirus (HPV) proposed as causative:
Many HPV subtypes have been detected, but most common are HPV types 6 and 11.
HPV DNA identified in greater than 80% of cases
No definitive association between HPV type and the type of papilloma
Exophytic, pink to tan-white lesion with a warty or cauliflower-like appearance; variation in size from a few millimeters up to 3.0 cm in greatest dimension
Multiple finger-like projections with prominent fibrovascular cores covered by hyperplastic squamous epithelium:
Typically there is an absence of keratosis.
Squamous cell component generally is free of any dysplastic change.
Variable amount of hyperkeratosis, parakeratosis, and orthokeratosis may be seen.
On rare occasions, an “inverted” growth may be seen.
Viral-associated cytopathic changes (i.e., koilocytes) may be seen in uppermost epithelial cell layers.
Verruca vulgaris
Syringocystadenoma papilliferum
Verrucous carcinoma
Exophytic squamous cell carcinoma
Inflammatory papillary hyperplasia:
Benign, reactive oral epithelial hyperplasia often associated with an intraoral inflammatory process (e.g., stomatitis)
Relatively common oral lesion
More common in men than in women; typically occur in the third through fifth decades of life
May occur in any intraoral site but is most often found on the palate
Painless, appearing as multiple warty or papillary, red-appearing lesions
Development linked to:
Patients with stomatitis, the result of ill-fitting dentures or dental prostheses, especially in those people who retain their prosthesis while sleeping and who exhibit poor oral hygiene
Candida
May also occur in dentulous patients without known history of dental prosthesis use
Histologically:
Similar to pseudoepitheliomatous hyperplasia with hyperkeratosis, parakeratosis, and an absence of epithelial dysplasia
Edematous change and a mixed chronic inflammatory cell reaction can be seen in the submucosa.
Secondary reactive or degenerative changes of seromucous glands, including squamous metaplasia, fibrosis, atrophy, mucin pool formation, and mixed inflammation may be present in areas overlying minor salivary glands; despite these findings the lobular configuration of the seromucous glands is retained.
Fungi (i.e., Candida albicans ) may be present.
Resolution may occur by initial treatment approach that includes:
Replacement of prosthesis with a better fitting one and education in the proper oral hygiene indicated
Topical antifungal (e.g., miconazole) used to treat presence of fungi
Failure for lesions to regress/resolve after conservative (nonsurgical) approach may require surgical excision
Not considered to be a premalignant lesion
Complete surgical excision usually is curative.
Recurrences occur infrequently and relate to inadequate excision.
Malignant transformation does not occur.
Benign salivary gland tumors of the oral cavity are fairly common.
Pleomorphic adenoma is the dominant histologic type seen; less often, monomorphic adenomas such as myoepithelioma and oncocytoma occur.
For a more complete discussion see Section 6, Salivary Glands.
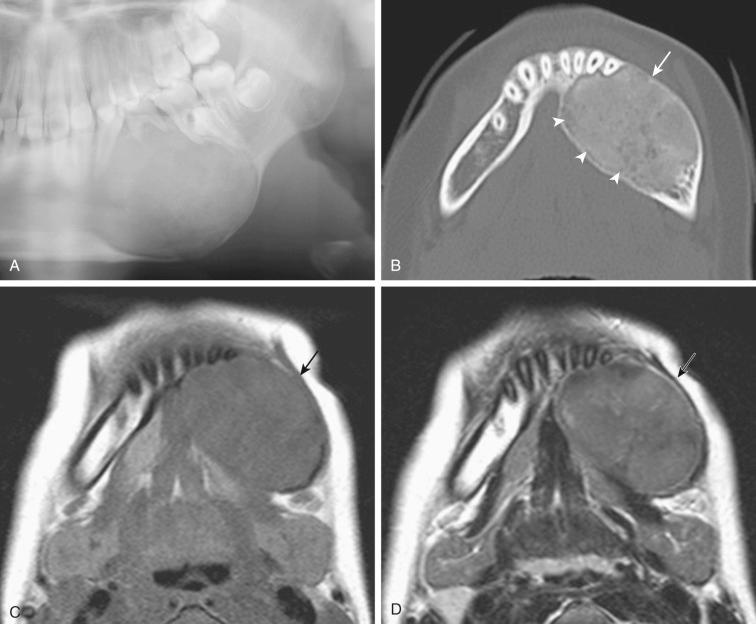
Definition: Benign tumor of the anterior dorsal tongue of presumed origin from an undifferentiated ectomesenchymal cell but owing to morphologic and immunohistochemical resemblance to soft tissue myoepitheliomas it is more likely a myoepithelial neoplasm of soft tissues.
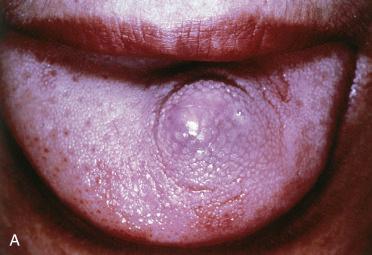
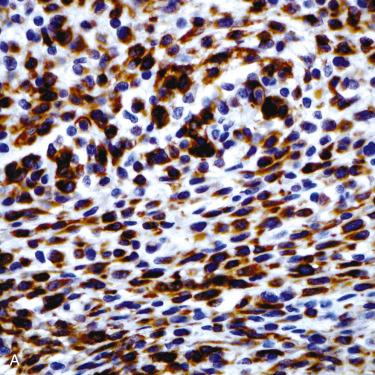
Synonyms: Oral myoepithelioma of soft tissue origin; reticulated myxoid tumor of the tongue; myoepithelioma of the tongue
Uncommon tumor
No gender predilection; occurs over wide age range including first to eighth decades of life; median age of 32 years
Asymptomatic, slow-growing, painless, submucosal nodular lesion of the anterior dorsal tongue
Submucosal nodular growth appearing tan-yellow and measuring from 0.5 to 3 cm in greatest dimension; on cut section has a gelatinous appearance/consistency
Submucosal unencapsulated but well-delineated or circumscribed nodule(s) separated by a fibrous stroma
Cells are round to polygonal to fusiform with uniform-appearing small hyperchromatic nuclei and ample basophilic-appearing cytoplasm.
Cells may be arranged in cords, strands, and so-called net-like sheets set in a chondromyxoid stroma; hyalinized foci may be present.
Generally, nuclear pleomorphism, multinucleation, and mitotic figures are not present but in occasional cases hyperchromatic and pleomorphic nuclei as well as mitotic figures may be identified.
Atypical mitoses and necrosis are not identified.
Swirling formations suggestive of neural differentiation may be present.
Absence of glands and/or myoepithelial cells (spindle shaped, plasmacytoid)
Lesional cells may extend into and/or entrap soft tissue structures, including:
Skeletal muscle
Nerves
Histochemistry:
Tumor cells:
Mucicarmine, periodic acid Schiff with and without diastase negative
Extracellular matrix:
Alcian blue (pH 0.4 and 2.5) positive
Mucicarmine faintly positive
Immunohistochemistry:
Glial fibrillary acidic protein (100%), cytokeratins (>90%), S100 protein (60%), smooth muscle actin (>50%); vimentin positive
Epithelial membrane antigen, desmin negative
No reports include staining for p63 or calponin.
Electron microscopy (limited to a single case):
Presence of partial basal lamina
Absence of desmosomes or thin filaments
Cytogenetics and molecular genetics:
No reported cases with EWSR1 gene rearrangements as identified in association with soft tissue myoepitheliomas
Monomorphic adenoma of salivary glands:
Myoepithelioma:
Myoepithelioma of the anterior dorsal tongue is rare.
Immunohistochemical findings would be compatible with myoepithelial cells, but presence of chondromyxoid stroma and absence of plasmacytoid and/or spindle-shaped myoepithelial cells by light microscopy weigh against this diagnosis.
Pleomorphic adenoma:
Rarity of salivary gland tumors localized to the anterior dorsal tongue and absence of identifiable glandular differentiation by light microscopy weigh against this diagnosis.
Extraskeletal myxoid chondrosarcoma:
Location is rare for chondrosarcomas of any type
Presence of cytokeratin and glial fibrillary acidic protein are not features seen in chondrosarcomas.
Conservative but complete excision is curative
Rarely (<10%) recur; recurrence likely a function of inadequate excision
Benign peripheral nerve sheath tumors include neurilemoma and neurofibroma (see Section 4, Neck).
Other benign tumors of nerve sheath or presumed peripheral nerve sheath origin include:
Granular cell tumor
Mucosal neuroma
Palisaded encapsulated neuroma (solitary circumscribed neuroma)
Dermal nerve sheath myxoma (neurothekeoma) (see Section 4, Neck)
Perineurioma (see Section 4, Neck)
See Section 5, Larynx, for discussion of mucosal granular cell tumor.
Definition: Benign tumor of neural (Schwann cell) origin.
Two forms of granular cell tumor can occur:
Mucosal granular cell tumor
Congenital granular cell epulis
See Section 6, Larynx, for a more complete discussion, including illustrations.
Synonyms: Congenital epulis; gingival granular cell tumor of newborn; congenital granular cell myoblastoma.
Use of the term epulis refers to any mass on the gingiva.
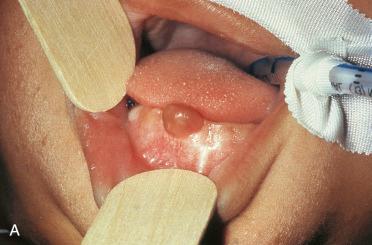
More common in females than males; occurs exclusively in newborns (at or immediately after birth)
Identified on gum pads in oral cavity on the crest of the alveolar ridge in the incisor region:
Localizes to labial aspect of the dental ridge with a predilection of the upper jaw
Affects the maxilla more often than the mandible but can occur in both locations simultaneously
Approximately 10% are multiple.
Radiology:
May be diagnosed prenatally by ultrasound:
Ultrasound examination shows marked blood flow in the tumor.
Histogenesis:
Owing to absence of S100 protein reactivity, this tumor is felt to originate from a non-neural cell of origin but the histogenesis remains uncertain.
Some authorities believe it to be a nonneoplastic (hamartomatous) lesion.
Smooth, pink multilobulated mass ranging in size from millimeters up to 5 cm
Similar to granular cell tumor with the following exceptions:
Greater degree of vascularity
Absence of associated pseudoepitheliomatous hyperplasia
Less conspicuous nerve bundles
Incorporation of odontogenic epithelium may be seen
Absence of S100 protein and laminin immunoreactivity
Immunoreactivity for:
Vimentin, CD68 (KP1), alpha-1-antitrypsin
Smooth muscle differentiation may be present.
Usually require complete surgical excision
May regress spontaneously
Definition: Benign tumor of nerve sheath origin involving mucosal surfaces of the oral cavity, eyelids, and intestines occurring associated with multiple endocrine neoplasia (MEN) syndrome type 2B.
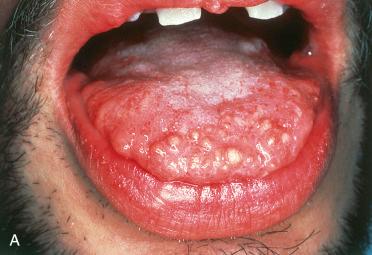
Synonyms: Multiple endocrine neoplasia 2B-associated mucosal neuroma; oral mucosal neuroma
MEN 2B (see Section 10 for a more complete discussion):
Occurs sporadically or inherited in autosomal dominant manner
Caused by germline mutations of RET proto-oncogene
Characterized by:
Medullary thyroid carcinoma
Pheochromocytoma
Parathyroid proliferative disease (adenoma, hyperplasia)
Mucosal neuromas, intestinal ganglioneuromatosis, and musculoskeletal abnormalities
Slightly more common in females than males; noted at birth or during first few years of life
Common sites of involvement include lips, tongue, and eyelids:
Oral cavity:
Vermilion border of the lips, anterior third of the ventral or dorsal tongue, buccal mucosa
Ocular:
Eyelids
Clinical appearance includes the presence of multiple small, sessile, mucosal-covered nodules.
Polypoid, dome-shaped, or diffuse submucosal proliferation of numerous irregular tortuous, highly branched and loosely arrayed nerves of varying size with prominent perineurium and presence of focal myxoid change:
Perineurium of affected nerve is thickened.
Mucoid or myxoid endoneurial matrix may separate nerve fibers.
Reactive perineural fibrosis not a feature.
Absence of ganglion cells (present in intestinal ganglioneuromatosis, noted in cases of mucosal neuromas of lingual and ciliary nerves and lesions in the roof of the iris and uveal meshwork)
Immunohistochemistry:
S100 protein positive (Schwann cells)
Neurofilament protein positive (axons)
Traumatic (amputation) neuroma:
Exuberant, non-neoplastic proliferation of nerves occurring in response to injury or surgery
Presents as firm nodule occasionally tender or painful
Circumscribed nodule(s) located in continuity with proximal end of injured or transected nerve
Unencapsulated lesion consisting of haphazard proliferation of nerve fascicle including axons Schwann cells and fibroblasts:
Less well-myelinated than parent nerve
Enveloped in collagen
May be embedded in mucoid matrix
Immunoreactivity present for neurofilament protein (axons), S100 protein (Schwann cells), and EMA (perineurial cells)
Plexiform neurofibroma
Palisaded encapsulated neuroma (solitary circumscribed neuroma) ( Fig. 6-7 ):
Distinct form of true neuroma consisting of Schwann cells as well as axons within a perineurial-derived capsule
No association with NF-1 or MEN 2B
Usually a cutaneous lesion
May occur in the oral cavity (palate, lips)
Small asymptomatic solitary circumscribed nodule
No gender predilection; occurs in adults
Histology:
Submucosal circumscribed nodular or multinodular, solid proliferation of Schwann cells lacking stromal alterations, including hyalinization or myxoid change often associated with schwannomas or neurofibromas
Most are small, measuring approximately 3 mm, and localized to the reticular dermis or submucosa.
In spite of its description most do not show “palisading” and are not encapsulated but often are incompletely encapsulated.
Composed of bland-appearing spindle cells set in a variably fibrous stroma and focally separated by artifactual clefts or cracking artifact:
Clefts or cracks likely related to fixation
Surrounds or results in slit-like spaces between cell bundles
Schwann cells are diffusely and strongly S100 protein positive.
Presence of axons traverse the lesion in close association with Schwann cells:
Not evident on hematoxylin and eosin staining
Best seen with silver stains
May be highlighted by neurofilament protein (NFP) immunostaining
Simple surgical excision is curative.
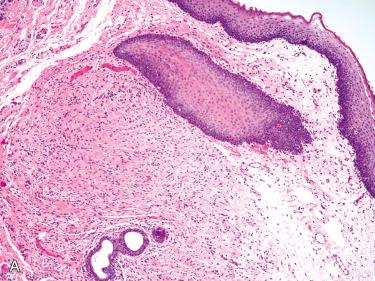
Surgical excision may be performed for cosmetic purposes.
Diagnosis should prompt work-up for possibility of MEN 2B:
Early diagnosis of MEN 2B, especially evaluation for the possibility of medullary thyroid carcinoma, allows for initiation of earlier treatment.
Definition: Locally aggressive, nonmetastasizing (myo)fibroblastic neoplasm characterized by locally infiltrative growth.
Synonyms: Desmoid-type fibromatosis; desmoid tumor; aggressive fibromatosis; extraabdominal desmoid, extraabdominal fibromatosis; tumefactive fibroinflammatory tumor; inflammatory pseudotumor
See Section 5, Neck, for a more complete discussion.
Fibromatosis of the head and neck region occurs primarily in the soft tissues of the neck, including the supraclavicular region and the anterolateral neck.
Excluding the neck, the common sites of occurrence are the sinonasal tract, nasopharynx, tongue, and oral cavity.
Approximately 10% to 15% of fibromatoses occur in the head and neck; in children, more than one third of cases occur in the head and neck.
Definition: Benign neoplasm composed of myoid cells arranged around thin-walled blood vessels.
Two types:
Solitary (myofibroma)
Multicentric (myofibromatosis)
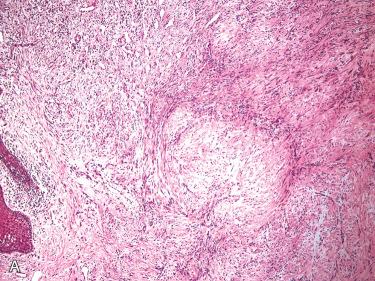
Synonym: Infantile myofibromatosis
Myofibromas:
Most common type occurring three times more often than multicentric form
More common in males than females; occurs over a wide age range, including infants and adults but represents the type most commonly seen in adults
Tend to occur in the head and neck region:
Most common in bone (mandible and skull) followed by the oral cavity, especially the buccal mucosa and tongue; less common intraoral sites of involvement include the gingival, palate, lip, retromolar trigone
May rarely involve other head and neck sites, including sinonasal tract and neck
Presents as a painless mass usually measuring less than 3 cm
Myofibromatosis may occur with or without visceral involvement:
Without visceral involvement:
More common in males than females; majority (up to 90%) occurs within the first 2 years of life with many presenting at birth
Sites of involvement include skin, subcutis, soft tissue, and bone:
Multiple lesions may be limited to a single general anatomic site (e.g., only head and neck) or may be generalized in distribution
Absence of visceral involvement
Presents as a painless mass usually measuring less than 3 cm
Possible but not definitive for familial inheritance
With visceral involvement:
Up to 40% have visceral involvement
More common in males than females; majority (up to 90%) occur within the first 2 years of life with many presenting at birth
Sites of involvement include skin, subcutis, soft tissue, and bone as well as visceral involvement, the latter including:
Lungs, heart, gastrointestinal tract, liver, kidney, pancreas, and rarely central nervous system
Present with symptoms related to the organs involved
Possible but not definitive for familial inheritance
Radiology:
Osseous lesions appear as circumscribed radiolucent lesions often with sclerotic margin
Central mineralization may be present.
Familial occurrence has been reported:
Inherited in autosomal dominant manner
NOTE: Pathologic findings remain similar, whether solitary or multifocal.
Well-circumscribed, rubbery to firm, gray-white to tan-brown mass ranging in size from 0.5 to 7 cm with a median size of 2.5 cm
Degenerative changes may be present, including cyst formation, hemorrhage, and necrosis.
Nodular or multinodular growth with characteristic biphasic or “zoning” appearance due to regional variation of cell types, including:
Peripheral (light staining) zone composed of plump-appearing, spindle-shaped cells with elongated, cigar-shaped vesicular nuclei, small nucleoli, and pale pink cytoplasm; cells are arranged in short fascicles or whorls
Central (dark staining) zone composed of primitive-appearing round cells with round to polygonal to spindle-shaped, vesicular to hyperchromatic nuclei, and scant eosinophilic to clear cytoplasm arranged around thin-walled, irregularly branching blood vessels showing features similar to the vascularity seen in hemangiopericytomas
No significant pleomorphism or increase in mitotic activity
Extensive coagulative necrosis may be identified.
Hemorrhage, cystic degeneration, calcification, and stromal hyalinization may be focally present.
Intravascular growth is frequently present:
Subendothelial location
No prognostic significance
Peripherally located chronic inflammatory cells, including lymphocytes and plasma cells, may be present.
Immunohistochemistry:
Vimentin positive
Actins (smooth muscle and muscle specific) focally and weakly positive
S100 protein, desmin, and epithelial markers are negative.
Proliferation rate indices of up to 10% seen by Ki67 (MIB1) staining
Electron microscopy:
Findings compatible with myofibroblasts, including prominent rough endoplasmic reticulum, intracytoplasmic filament bundles with dense bodies, and focal basal lamina
Cytogenetics and molecular genetics:
Absence of ETV6-NTRK3 gene fusion that is identified in infantile fibrosarcoma
Fibromatosis
Nodular fasciitis
Smooth muscle neoplasms (leiomyoma, leiomyosarcoma)
Sarcomas:
Due to increased cellularity, rich vascularity, and presence of necrosis, myofibromas/myofibromatosis may be mistaken for a sarcoma.
Spontaneous regression may occur in solitary or multifocal (without visceral involvement) lesions.
Simple excision of solitary lesions is curative.
Surgical resection of multiple lesions may be indicated in cases in which there is functional impairment or involvement of vital organs.
Recurrence rates after surgery usually are less than 10% of cases.
Prognosis less favorable in newborns and infants with multiple visceral lesions:
Increased morbidity and mortality:
Mortality rates >70% reported
Death may be due to cardiopulmonary or gastrointestinal complications.
Low-dose chemotherapy (methotrexate and vinblastine) may be effective in treating patients with multicentric visceral involvement.
See Section 1 on the Sinonasal Tract for a more complete discussion.
May include a variety of hemangioma subtypes, including capillary, cavernous, mixed capillary-cavernous
Majority occurs in adults
Most common sites of occurrence include lips, buccal mucosa, and tongue.
These tumors may be mucosal based or within muscle (intramuscular) or bone (intraosseous).
Benign lipogenic tumors of the oral cavity are uncommon.
See Sections 4, Neck, and 5, Larynx and Trachea, for discussion.
Benign myogenic tumors of the oral cavity are uncommon and include tumors of smooth muscle differentiation (leiomyomas) and skeletal muscle differentiation (rhabdomyomas).
For leiomyoma see Section 1, Sinonasal Tract.
For rhabdomyoma see Section 4, Neck.
Benign fibrohistiocytic tumors of the oral cavity are uncommon and include fibrous histiocytoma; see Section 1, Sinonasal Tract.
Gnathic (jaw bones) benign fibro-osseous lesions include ossifying fibromas (and variants thereof) and fibrous dysplasia.
Fibrous dysplasia is not a neoplastic lesion but is included here as part of the broader category of benign fibro-osseous lesions.
Gnathic fibro-osseous lesions (ossifying fibroma and fibrous dysplasia) may be histologically indistinguishable; therefore a definitive diagnosis may not be achievable on morphology alone, and the diagnosis rests on the clinical-radiologic-histopathologic correlation.
In the head and neck, benign fibro-osseous lesions occur most often in relation to gnathic (maxillary and mandibular) bones.
Given its localization to the sinonasal tract, psammomatoid ossifying fibroma is discussed in Section 1, Sinonasal Tract.
| OF | POF | FD | |
|---|---|---|---|
| Gender/age | F > M; 2nd-4th decades | F = M; younger age groups (1st-2nd decades), but may occur in older individuals | MFD: F = M; 2nd-3rd decades PFD: F > M; 1st decade |
| Location | Most common in the mandible (posterior or molar region) | Ethmoid sinus; supraorbital frontal region | No specific site of involvement |
| Focality | Single site | Single site or involvement of multiple (contiguous) sites/sinuses | MFD (75%-80%) PFD (20%-25%) MAS (1%-3%) |
| Radiology | Well-circumscribed or sharply demarcated lesion with smooth contours | Lytic or mixed lytic/radiopaque osseous and/or soft tissue mass varying from well demarcated to invasive with bone erosion | Poorly defined expansile osseous lesion with a thin intact cortex; predominantly fibrous lesions are radiolucent; predominantly osseous lesions are radiodense; lesions with an equal admixture of fibrous and osseous components have a ground glass appearance |
| Histology | Randomly distributed mature (lamellar) bone spicules rimmed by osteoblasts admixed with a fibrous stroma; central portions may be woven bone with lamellar bone at the periphery | Bony spicules and distinctive mineralized or calcified “psammomatoid” bodies or ossicles admixed with a fibrous stroma; psammomatoid bodies vary from a few in number to a dense population of innumerable spherical bodies; osteoclasts are present within the ossicles, and osteoblasts can be seen along their peripheral aspects; the bony trabeculae vary in appearance and include odd shapes with a curvilinear pattern; trabeculae are composed of lamellar bone with associated osteoclasts and osteoblastic rimming. | Fibrous tissue component is nondescript and of variable cellularity; osseous component includes irregularly shaped trabeculae of osteoid and immature (woven) bone that is poorly oriented with misshapen bony trabeculae with odd geometric patterns including C - or S -shaped configurations; the trabeculae typically lack osteoblastic rimming |
| Syndromes | No known association | No known association | MAS (1% to 3%) |
| Treatment | Surgical resection | Surgical resection | Disease may stabilize at puberty and, in children, therapy should be delayed if possible until after puberty; surgical resection indicated in cases with compromise of function, progression of deformity, associated pathologic fracture(s), or the development of a malignancy |
| Prognosis | Excellent | Good after complete excision; recurrence(s) often occurs due to incomplete excision; may behave in an aggressive manner with local destruction and potential invasion into vital structures | Good prognosis; recurrence rates are low and death due to extension into vital structures rarely occurs |
| Malignant transformation | Not known to occur | Not known to occur | Malignant transformation (osteosarcoma) occurs in less than 1%; dismal prognosis |
Definition: Well-demarcated, slow-growing benign fibro-osseous neoplasm composed of fibrocellular tissue admixed with varying amounts of mineralized material (i.e., bone, cementum) of varying appearances.
Synonyms: Cemento-ossifying fibroma; cementifying fibroma; central intraosseous ossifying fibroma; fibrous osteoma; osteofibroma
More common in women than men; occurs over a wide age range but is most frequently seen in the second to fourth decades of life
Most common site of occurrence is the mandible, especially molar or posterior area followed by the premolar area, incisor area, and cuspid area
May also occur in association with the maxilla, but maxillary involvement occurs much less often as compared with the mandibular region
Generally asymptomatic unassociated with pain or swelling and diagnosed incidentally after radiographic examination; symptomatic tumors manifest by displacement of teeth or as an expansile mass that may include facial asymmetry.
Typically presents as solitary mass but infrequently may be multifocal:
Multifocal tumors can occur in the mandible, in the maxilla, or in both regions.
Polyostotic involvement of extragnathic bones as seen in fibrous dysplasia is not a finding associated with ossifying fibromas.
Radiology:
Well-circumscribed lesion with smooth contours having a variation in appearance based on the maturity of the tumor, including:
Completely radiolucent:
Immature lesion
Completely radiopaque:
Mature lesion
Mixed radiolucent and radiopaque:
Increased mineralization with age results in radiopaque foci admixed with radiolucent areas
Presumptive origin is from the periodontal ligament, which:
Is a layer of fibrous connective tissue surrounding and attaching the roots of teeth to alveolar bone
Is capable of forming cementum, bone, and fibrous tissue
Supports close relationship to cementifying fibroma and cemento-ossifying fibroma; in fact, all of these lesions are considered variants of ossifying fibroma
Well-demarcated, tan/gray to white, gritty and firm lesions varying in size from 0.5 to as large as 10 cm
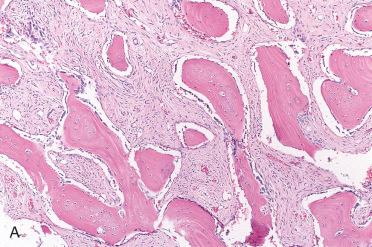
Well-delineated, demarcated, or encapsulated proliferation composed of randomly distributed mature (lamellar) bone spicules rimmed by osteoblasts admixed with a fibrous stroma
Although the osseous component is generally described as mature, the central portions may be woven bone with lamellar bone at the periphery.
Complete bone maturation is seldom seen.
Fibrous stroma may be densely cellular; mitotic figures are rare to absent.
Secondary changes, including hemorrhage, inflammation, and giant cells, may be seen.
Lesions with associated cementum are referred to as cementifying fibroma and those lesions with cementum and bone are referred to as cemento-ossifying fibroma:
Cementum is mineralized material covering the surface of roots of teeth.
There is no clinical relevance in distinguishing cementum from bone.
Immunohistochemistry:
Reactivity for osteocalcin:
Weak in ossifying fibroma
Strong throughout calcified regions in fibrous dysplasia
Cytogenetics and molecular genetics:
Guanine nucleotide-binding protein/α-subunit (GNAS) mutational analysis by PCR reported to be:
Absent in ossifying fibromas, cemento-ossifying fibromas, and cemento-ossifying dysplasias:
Also reported to be absent in odontogenic myxomas
Present in fibrous dysplasia
Primarily with fibrous dysplasia (see below and Table 6-1 )
Differentiation of ossifying fibroma from fibrous dysplasia is important because the therapeutic rationale differs for these lesions.
Surgical excision is the preferred treatment:
Well-circumscribed nature of this lesion allows for relatively easy removal.
Prognosis is excellent after complete excision.
Recurrences are rare.
Represents an oral mucosal or soft tissue (nonintraosseous) ossifying fibroma:
Similar to intraosseous (central) ossifying fibroma; also presumably arises from periodontal ligament
Believed to represent a reactive process rather than a neoplastic proliferation
More common in women than in men; most common in the second decade of life
Unique to gingival mucosa:
Majority occurs anterior to molar region, equally affecting mandible and maxilla.
Presents as firm, sessile to pedunculated mass measuring up to 1 cm in greatest dimension:
Overlying epithelium may be intact and smooth in appearance or ulcerated.
Histology includes:
Unencapsulated cellular lesion composed of connective tissue, plump-appearing fibroblasts with large, round to oval vesicular nuclei and mineralized material, the latter including interlacing trabeculae of bone or osteoid, calcification, or spherules of cementum
Multinucleated giant cells associated with bone and calcified material may be present.
Chronic inflammatory cell infiltrate may be present along the periphery of the lesion.
Complete surgical resection is the preferred treatment, often requiring excision of the lesion as well as the periodontal ligament and periosteum:
Tooth extraction is not usually required.
Up to 20% of cases may recur.
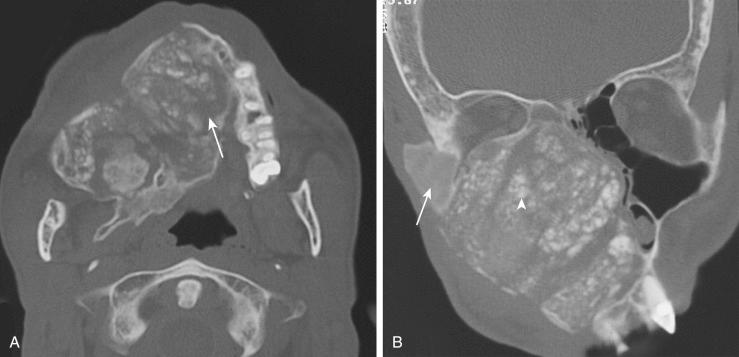
Definition: Idiopathic nonneoplastic bone disease in which normal medullary bone is replaced by structurally weak fibro-osseous tissue.
Three variants are identified: monostotic, polyostotic, polyostotic with endocrinopathy (McCune-Albright syndrome).
Only a single osseous site is involved.
Represents greater than 75% to 80% of all cases
Frequently occurs in older children and young adults
Most commonly affects ribs, femur, and tibia
Involves head and neck bones in up to 25% of cases:
In head and neck most common sites of involvement include maxilla (zygomatic process) > mandible (region of premolar and molar teeth) > frontal bone > ethmoid and sphenoid bones > temporal bone
Involvement of two or more bones
Represents approximately 20% to 25% of all cases
May be limited to a few bones in one anatomic region or may be diffuse, affecting virtually every bone in the skeleton
In greater than half the cases osseous involvement includes the long bones of the extremities, pelvic bones, ribs, metacarpals, metatarsals, and the humerus:
Craniofacial or jaw regions are included in up to 50% of patients.
Triad includes:
Polyostotic fibrous dysplasia
Endocrine dysfunction:
Hyperthyroidism and/or sexual precocity, the latter predominantly identified in females
Cutaneous hyperpigmentation
Least common variant representing approximately 1% to 3% of all cases
Monostotic type:
No gender predilection or slightly more common in women
Polyostotic type:
More common in women than men
Regardless of type, majority of patients affected are under 30 years of age, although older individuals may be affected:
In monostotic type most common in second to third decades of life
In polyostotic type and McCune-Albright syndrome tend to occur in the first decade of life
Craniofacial symptoms include:
Painless, asymmetric, nonmobile swelling associated with functional disturbances
Displacement or malocclusion of teeth, failure of tooth eruption in children
Headaches, proptosis, nasal obstruction, especially for sinonasal tract lesions
Hearing loss (conductive)
Laboratory findings:
Serum calcium and phosphorous levels are normal; alkaline phosphatase may be elevated.
May be associated with:
Oncogenic osteomalacia
Cholesteatoma
Radiology:
Poorly defined expansile osseous lesion with a thin intact cortex
Predominantly fibrous lesions are radiolucent.
Predominantly osseous lesions are radiodense.
Equal admixture of fibrous and osseous components results in a ground glass appearance.
Usually no periosteal reaction is seen unless there is an associated fracture.
Cause remains unknown:
No familial or hereditary origin
Cherubism is congenital form of fibrous dysplasia:
Autosomal dominant disease with variable expressivity
Characterized by lateral swelling of the jaws:
Bilateral symmetric involvement is almost pathognomonic.
Usually involves the mandible with the ramus always involved.
Patients have characteristic upturned appearance of the eyes, resulting in a cherubic expression.
Rarely may be associated with soft tissue myxomas referred to as Mazabraud syndrome:
Defined as combination of one or more intramuscular myxomas and fibrous dysplasia of bone
Association is more common with polyostotic fibrous dysplasia and McCune-Albright syndrome
Myxomas tend to appear years (decades) after bone lesions
Soft tissue myxomas most common in the thigh (intramuscular)
Often multiple and tend to occur near to abnormal bones
These patients are at increased risk for malignant transformation:
Risk is greater than in those with fibrous dysplasia alone
Risk increases when patient has both McCune-Albright and Mazabraud syndromes
Tan-white to yellow, soft, rubbery, gritty, or firm tissue
Thin cortex
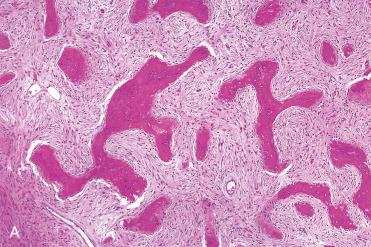
Fibrous tissue component is nondescript and of variable cellularity
Osseous component includes:
Irregularly shaped trabeculae of osteoid and immature (woven) bone arising metaplastically from fibrous stroma
Poorly oriented with misshapen bony trabeculae, increased cellularity, and irregular margins and forms odd geometric patterns including C - or S -shaped configurations, so-called Chinese characters
Trabeculae typically lack osteoblastic rimming.
Multinucleated giant cells, macrophages, increased vascularity, and calcification may be seen.
Under polarized light bone appears woven rather than lamellar; however, lamellar bone can be seen in fibrous dysplasia, and its presence does not exclude the diagnosis.
Immunohistochemistry:
Reactivity for osteocalcin:
Strong throughout calcified regions in fibrous dysplasia
Weak in ossifying fibroma
Cytogenetics and molecular genetics:
Guanine nucleotide-binding protein/α-subunit (GNAS) mutational analysis by PCR showed:
Presence in fibrous dysplasia
Absence in ossifying fibromas, cemento-ossifying fibromas, and cemento-ossifying dysplasias
Also reported to be absent in odontogenic myxomas
Ossifying fibroma (see Table 6-1 ):
Gnathic fibro-osseous lesions (fibrous dysplasia and ossifying fibromas) may be histologically indistinguishable; therefore the diagnosis and differentiation rest on the clinical–radiologic–histopathologic correlation.
Differentiation of ossifying fibromas from fibrous dysplasia is important because the therapeutic rationale differs for these lesions.
Conservative surgical excision is the preferred treatment and is indicated only in cases with compromise of function, progression of deformity, pain, associated pathologic fracture(s), or the development of a malignancy.
Disease may stabilize at puberty and, in children, therapy should be delayed if possible until after puberty.
Radiation treatment is not used because of the risk of inducing malignant change.
Recurrence rates are low and death due to extension into vital structures rarely occurs.
Malignant transformation:
Occurs in less than 1% of cases but is most feared complication
When it occurs is most often an osteosarcoma > chondrosarcoma > fibrosarcoma:
Also associated with angiosarcoma, Ewing sarcoma, and malignant mesenchymoma, including osteosarcomatous, chondrosarcomatous, and rhabdomyosarcomatous elements
Most common in craniofacial bones (maxilla and mandible) followed by femur and tibia
Peaks in third and fourth decades
May occur spontaneously or in patients treated by prior radiation
Identified more often in association with the monostotic type:
Risk increases when patient has both McCune-Albright and Mazabraud syndromes
Risk is greater than in those with fibrous dysplasia alone
Tends to occur years to decades after development of fibrous dysplasia
Treatment is similar to that of a primary malignant bone tumor.
Prognosis is poor with tendency to metastasize to lungs and short survival periods.
Definition: Benign but potentially aggressive primary tumor of bone composed of stromal mononuclear cells and osteoclast-like giant cells.
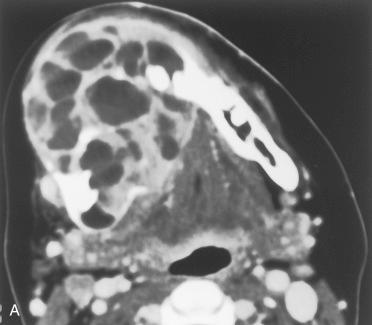
Synonym: Osteoclastoma
Most occur at ends (epiphyses) of long bones with distal femur the most common site followed by proximal tibia
Rare in the head and neck:
Less than 2% of all giant cell tumors occur in head and neck.
Propensity to affect sphenoid, temporal, and ethmoid bones
More common in women than in men; occur over a wide age range
Symptoms vary per site of involvement:
Sphenoid and ethmoid bones
Headaches, diplopia, visual disturbances, proptosis
Middle ear, temporal bone, petrous bone:
Conductive hearing loss
Vertigo and sensorineural hearing loss
Laboratory:
No abnormalities of serum calcium
Radiology:
Lytic lesions with or without involvement/destruction of adjacent bones
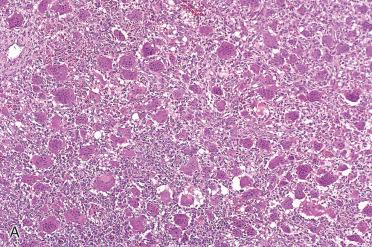
Characterized by the presence of abundant multinucleated giant cells and stromal mononuclear cells
Multinucleated giant cells:
Diffusely and evenly distributed, are large and have numerous nuclei (10 to 100)
Nuclei are round to oval with or without nucleoli and tend to cluster in the center of the giant cells.
Cause bony destruction
Are thought to be recruited from normal mononuclear stromal cells
Mononuclear cell stromal component:
Plump, ovoid, or spindle-shaped
Nuclei similar to those seen in the giant cells
No cytologic atypia
Variable associated thin-walled blood vessels:
Identification of intravascular tumor especially at the periphery of the tumor may be seen but has no clinical import.
Mitoses are seen in the stromal mononuclear cells and may be abundant but atypical mitoses are not present:
Presence of atypical mitoses identified as an indicator of malignancy
Additional findings may include the presence of foam cells, osteoid, and rarely chondroid:
Foam cells frequently present and in some case may be abundant
Reactive bone may be focal or abundant, usually in the form of seams of osteoid with prominent osteoblastic rimming.
Cartilage formation is uncommon, and presence of chondroid material should engender consideration of another lesion.
Secondary aneurysmal bone cysts are not uncommon:
May be present as microscopic cysts
Absence of collagenized or fibroblastic background unless previously biopsied or traumatized
Immunohistochemistry:
Multinucleated giant cells:
Express strong CD68 (KP1) staining (monocytic/histiocytic lineage) and vimentin staining
Smooth muscle actin negative
Exhibited an osteoclast phenotype expressing tartrate-resistant acid phosphatase and vitronectin receptor
Mononuclear stromal cells:
p63 reactivity (strong nuclear staining)
Exhibited an osteoblast phenotype, expressing:
Alkaline phosphatase, and the receptor activator for nuclear factor kappa B ligand (RANKL), a factor that is essential for osteoclast formation
Also expressed osteoprotegerin (OPG), an inhibitor of osteoclastogenesis, and TNF-related apoptosis-inducing ligand (TRAIL), a receptor that binds OPG:
These findings indicate that the mononuclear and giant cell components of giant cell tumor have similar phenotypic features and that the accumulation of osteoclasts in these giant cell–rich tumors occurs by a receptor activator of nuclear factor kappa-B ligand (RANKL)-dependent process
RANKL expression by osteoblast-like mononuclear stromal cells in these tumors stimulates osteoclast formation and resorption accounting for the osteolysis associated with these giant cell–rich tumors
Inhibitors of osteoclast formation and activity are likely to be effective in controlling the osteolysis associated with GCTB and possibly other giant cell–rich lesions.
Identification of angiogenic factors, including vascular endothelial growth factor (VEGF) and basic fibroblastic growth factor (bFGF) in mononuclear cells and giant cells, may play a role in the process of osteoclastogenesis, potentially contributing to additional growth in these lesions.
Cytogenetics and molecular genetics:
Telomeric associations (TAS) represent the most frequent chromosomal translocation.
Telomeric associations on 11p and dicentric chromosomes
Clonal abnormalities, such as del(17p), and losses of chromosomes 4, 13, and 18 and gains on chromosome 7
Comparative genomic hybridization: chromosomal imbalances with gains on chromosomes 1p31-q44, 6q12-q23, and 12q15-q22
Giant cell granuloma:
Share histologic similarities with giant cell tumor:
Divergent opinion whether giant cell tumor and giant cell granuloma represent spectrum of a single entity
Features in giant cell granuloma differing from giant cell tumor include:
Predilection to the gnathic bones, especially the mandible
Overall fewer numbers of giant cells with less even distribution of the giant cells
Frequent areas of hemorrhage and tendency for giant cells to cluster in areas of hemorrhage, as well as in proximity to vascular spaces
Greater amount of stromal collagenization
Brown tumor of hyperparathyroidism:
Absence of abnormalities of serum calcium in giant cell tumor assists in differentiating these lesions.
Chondroblastoma
Surgical excision (i.e., curettage) is the preferred treatment:
Treatment recommendation is based on the more common giant cell tumors of long bones.
In long bones recurrence rates vary from 20% to 50% after surgical curettage.
Radiation is not recommended because:
These tumors are not felt to be radiosensitive.
There is believed to be increased risk of malignant transformation after radiation treatment.
Medical therapies include diphosphonates and denosumab (RANKL inhibitors):
May be used in patients with untreatable disease, including refractory, recurrent, or metastatic giant cell tumor
Rarely (less than 10%) morphologically benign giant cell tumors may metastasize:
May be referred to as benign metastasizing giant cell tumor
No reliable predictors of which lesions may metastasize
Metastasis may be solitary or multiple.
Metastatic disease most often to lungs; less often to lymph nodes and other visceral sites
If solitary surgical resection is achievable (metastasectomy), prognosis is good.
Rarely, more diffuse metastatic disease occurs and may result in death.
Malignant transformation of giant cell tumors is uncommon, representing presence of histologically benign giant cell tumor in association with sarcomatous component:
Malignant giant cell tumors, primary and secondary:
Primary malignant giant cell tumor represents a de novo malignancy (i.e., at presentation).
Secondary malignant giant cell tumor represents malignant transformation of a previous tissue-verified benign giant cell tumor:
Most occur secondary to radiation treatment.
Could be considered postirradiation sarcoma
For primary and secondary malignant giant cell tumors, malignancy includes osteosarcoma, undifferentiated pleomorphic sarcoma, and fibrosarcoma.
Metastatic disease is most often to the lungs.
Poor prognosis with greater mortality associated with secondary as compared with primary malignant giant cell tumor
Rare malignant giant cell tumor of the sphenoid arising in setting of Paget disease.
Primary soft tissue neoplasm that is clinically and histologically similar to giant cell tumor of bone
Majority occurs in upper and lower extremities but approximately 7% may occur in soft tissues of the head and neck
Histology and immunoreactivity similar to that of giant cell tumor of bone
Complete surgical resection is the preferred treatment.
May locally recur in up to 12% of cases (often a function of inadequate excision)
Rarely metastasizes
Definition: Benign bone-forming neoplasm characterized by osteoblastic rimming of woven bony trabeculae (histologically similar to osteoid osteoma) but with potential for progressive/aggressive growth .
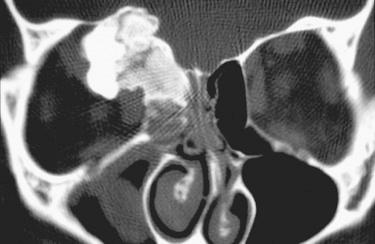
Synonym: Giant osteoid osteoma
Represent approximately 1% to 4% of all benign tumors of bone
Most common site of occurrence is in the vertebra:
Uncommon tumor of head and neck
In head and neck occurs most commonly in gnathic (jaw) bones:
More common in the mandible (body) than maxilla
Less common sites of occurrence include the cervical vertebrae, skull, sinonasal tract, and middle ear/temporal bone.
More common in men than in women; overwhelming majority of cases (greater than 90%) occur in patients under 30 years of age
Presentation is usually that of localized pain to involved site:
Unlike osteoid osteoma, pain is not nocturnal and aspirin does not typically assist in resolving the pain.
In addition to pain, other symptoms related to site of occurrence may include:
Jaws: swelling, loosening of teeth
Sinonasal tract: epistaxis, nasal obstruction
Middle ear/temporal bone: conductive hearing loss
Radiology:
Well-defined or sharply circumscribed to poorly defined radiolucent/radiopaque lesion with variable mineralization:
Depending on the degree of mineralization may appear:
Predominantly lytic
Predominantly sclerotic
Mixed lytic and sclerotic
Expansion of bone with cortical erosion and extension into adjacent soft tissue may be identified
Usually measure more than 2 cm but rarely larger than 10 cm
Absence of a nidus as seen in osteoid osteoma
In gnathic bones may be intimately associated with roots of teeth
Significant percentage of cases may radiographically show features similar to those identified in osteosarcoma.
Intact lesions are rarely seen by the pathologist, because curettage is the usual means of surgical treatment.
Intact lesions are:
Usually well circumscribed with a hemorrhagic appearance and granular to somewhat gritty texture, depending on the degree of calcification
Older lesions may be more heavily calcified, resembling cancellous bone.
In all skeletal locations range in size from 1.5 cm to 10 cm in greatest dimension
Often lack the sclerotic rim, which is so prominent in osteoid osteomas
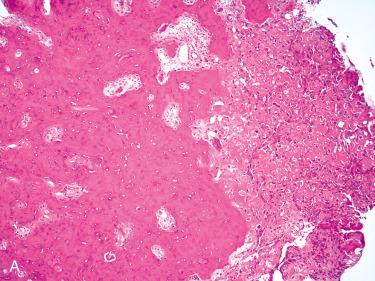
Well-circumscribed/sharply demarcated lesion composed of intricate complex (anastomosing) bony trabeculae in loose fibrovascular stroma:
Trabeculae may connect with cortical bone at periphery of the lesion, suggesting maturation
Bony trabeculae are lined by a single layer of plump osteoblasts, which may have small bland nuclei or may have enlarged nuclei with prominent nucleoli:
Scattered typical mitotic figures may be identified in osteoblastic cells.
May lack mineralization or may be rather heavily calcified
Lace-like osteoid may be present in small percentage of cases
Intertrabecular spaces contain a richly vascularized loose fibroblastic stroma.
Chondroid areas are uncommon but may be seen focally.
Rarely large atypical but degenerated-appearing hyperchromatic nuclei may be seen.
Designation ascribed to those osteoblastomas with atypical features suggestive of malignancy, including:
Increase in epithelioid-appearing osteoblasts
Increased mitotic figures
Sheetlike or trabecular areas of osteoid
Osteoclastic activity
Absence of radiographic distinctive findings
Metastatic disease and/or death not seen in the lesions
Validity of existence of aggressive osteoblastoma questioned
Another entity whose validity as a distinct lesion with reproducible features has been questioned:
Designation used for lesion showing borderline features between osteoblastoma and osteosarcoma, including:
Cellular pleomorphism of osteoblasts
Large number of giant cells
Speculated blue bone similar to that found in osteosarcoma
May recur but metastatic disease not reported
Osteoid osteoma:
Benign bone forming tumor with limited growth potential
Represents approximately 14% of all benign bone tumors
More common in men than in women; most common in the first to third decades of life
Majority occurs in femur and tibia
Rare in head and neck sites, where most common sites of occurrence include mandible and cervical vertebrae
Pain, especially occurring at night (nocturnal pain), is most common presenting complaint; pain typically responds (i.e., pain relief) to nonsteroidal anti-inflammatory drugs.
Radiology:
Circumscribed dense cortical radiolucency surrounding marked sclerosis (nidus)
Usually measures 1 cm or less
Essential identical histology to osteoblastoma:
Exception is presence in osteoid osteoma of a nidus representing interconnecting mass of osteoid and immature (woven) bony trabeculae of variable length and thickness rimmed by osteoblasts
Because of shared histologic features (except for the nidus), differentiation may not be achievable in curetted material, thereby requiring radiographs to assist in differentiating osteoid osteoma from osteoblastoma.
Complete surgical excision or ablation of the nidus (en bloc) is curative:
Depending on the site of occurrence may require multiple procedures
Medical management (nonsteroidal anti-inflammatory drugs) may be an option if surgical treatment is contraindicated.
Aneurysmal bone cyst
Fibrous dysplasia
Giant cell tumor
Odontogenic lesions/neoplasms:
Cementoblastoma, cemento-ossifying fibroma, cemento-osseous dysplasia
Osteosarcoma:
Features in osteoblastoma that assist in excluding osteosarcoma include:
Sharp circumscription with no permeation or entrapment of surrounding host bone
Bony trabeculae embedded in loose connective tissue; lining of trabeculae by a single layer of osteoblasts
Features in osteosarcoma that contrast to those of osteoblastoma include:
Greater nuclear pleomorphism and hyperchromasia
Greater number of mitoses with atypical mitotic figures
More compact stromal component
Penetration of neoplastic cells between existing bone/bony trabeculae
Presence of sheets of osteoblasts without osteoid production
Conservative but complete surgical resection by curettage or local excision is the preferred treatment and is curative in majority of cases.
Recurrent tumor is uncommon.
Features potentially associated with aggressive behavior include:
Location of lesion:
Tumors of the central neuroaxis associated with increased morbidity and mortality
Presence of secondary aneurysmal bone cyst component:
Associated with more destructive behavior
Local control of disease:
Ability to completely resect tumor associated with excellent long-term prognosis
Histology alone is not predictive of aggressive behavior.
Metastatic disease rarely, if ever, occurs.
Odontogenic tumors represent a broad group of relatively rare heterogeneous neoplasms.
The full spectrum of odontogenic neoplasms is beyond the scope of this text.
This section focuses on some of the more common odontogenic neoplasms with which the surgical pathologist may be confronted in daily practice.
Definition: Slow-growing, locally aggressive epithelial odontogenic jaw tumor recapitulating enamel organ development during tooth crown formation with a high propensity for recurrence:
Thought to arise from reduced enamel epithelium of the dental follicle, remnants of odontogenic epithelium, lining of odontogenic cysts, or basal cells of the overlying oral (alveolar) mucosa
May develop from an odontogenic cyst (e.g., dentigerous cyst, odontogenic keratocyst) or develop in association with another type of odontogenic neoplasm (e.g., adenomatoid odontogenic tumor, others) referred to as hybrid tumor.
Based on various clinical and pathologic features, ameloblastomas can be divided into four categories, including:
Solid/multicystic:
Are intraosseous
Unicystic:
Are intraosseous
Desmoplastic:
Are intraosseous
Peripheral:
Arise in extraosseous (mucosal) locations
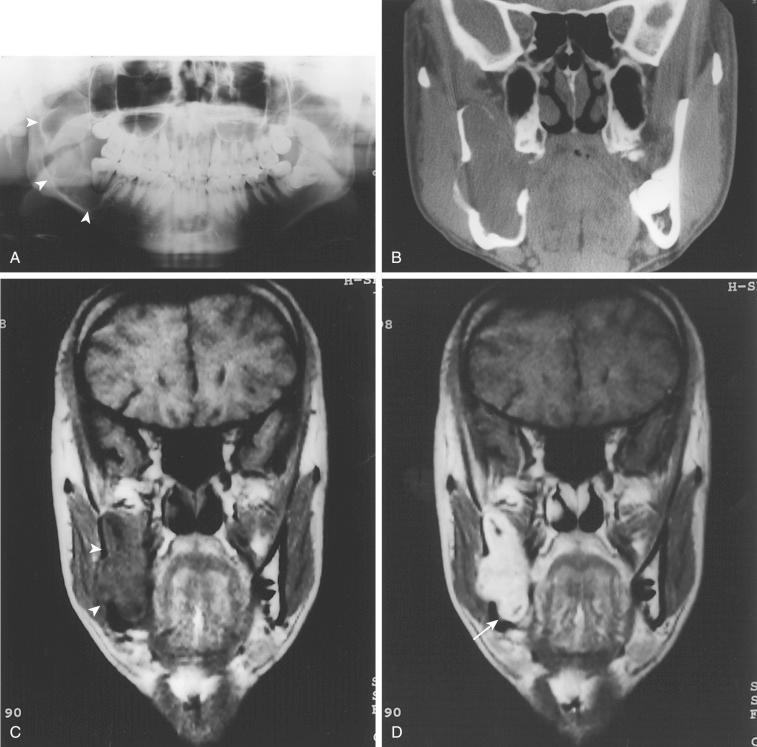
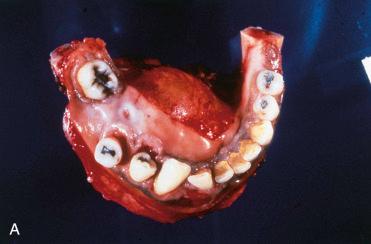
Second most common odontogenic tumor (odontoma is considered the most common odontogenic tumor)
Most common type of ameloblastoma, representing greater than 80% of all cases
No gender predilection; occurs over a wide age range but most commonly occurs in the fourth to sixth decades of life; rare under 20 years of age
Greater than 80% involve the mandible with predilection for the posterior mandibular region (molar-ramus area > premolar area > symphysis); often associated with unerupted third molar teeth:
Predilection to the molar-ramus area is thought to be the result of:
Aberrant tooth germs often found in this region
Posterior end of the dental lamina proliferates continuously.
Maxillary ameloblastomas occur primarily in the posterior (molar) region
May occur as a primary sinonasal tract neoplasm; see Section 1, Sinonasal Tract, for detailed discussion.
Most common clinical presentation is a painless swelling of the affected area; pain or paresthesia is rare
Radiology:
Unilocular or multilocular radiolucent lesion resembling cysts with a honeycomb appearance and scalloped borders
Extensive thinning of cortical bone can often be seen.
Desmoplastic type may present as mixed radiolucent/radio-opaque lesions
Predominantly solid but microcyst may be identified
Several histologic variants may be seen:
Histologic subtypes can be found independently or admixed within the same tumor.
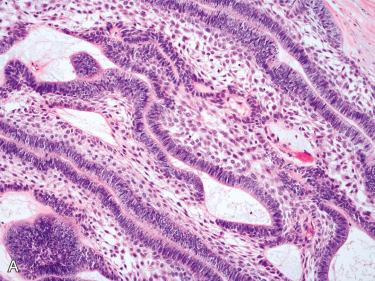
Follicular (solid and cystic) variant:
Most common histologic variant
Variably sized epithelial islands composed of central loosely arranged cellular areas identical to stellate reticulum of enamel organ with squamous cells, basal cells, or granular cells surrounded by of columnar epithelial cells with clear to vacuolated cytoplasm and hyperchromatic (basaloid) nuclei aligned at the periphery away from the basement membrane referred to as reverse polarity of the nuclei
Absence of significant nuclear pleomorphism and increased mitotic activity
Microcyst formation commonly present in this histologic subtype
Mucocytes may rarely be found.
Desmoplastic:
Considered a variant of follicular ameloblastoma characterized by presence of a markedly desmoplastic stroma
Plexiform variant:
Second most common histologic variant
Composed of long, anastomosing double columns and sheets of cuboidal or columnar epithelial cells with reverse polarity of the peripheral located nuclei with minimal to no evidence of central stellate reticulum
Acanthomatous variant:
Identical to follicular type but characterized by presence of extensive squamous metaplasia in central areas replacing typical stellate reticulum
Squamous metaplasia includes keratin pearl formation and individual cell keratinization:
Absence of significant nuclear pleomorphism or mitotic activity
Retention of peripheral columnar cells with reverse polarity of hyperchromatic nuclei
Keratoameloblastoma:
Term for ameloblastomas with extensive keratinization of central areas
Includes surface parakeratin
May show papillary growth (papilliferous keratoameloblastoma)
Granular cell variant:
Composed of varying numbers of cells with prominent granular, eosinophilic-appearing cytoplasm
Retention of peripheral columnar cells with reverse polarity of hyperchromatic nuclei
May show anastomosing trabeculae or cords
Basal cell variant:
Least common variant
Composed of small, ovoid, basaloid cells with scant cytoplasm in central areas replacing typical stellate reticulum
Retention of peripheral columnar cells with reverse polarity of hyperchromatic nuclei
May be histologic type seen in association with peripheral (extraosseous) ameloblastomas
Additional cell types that can be seen including mucous cells, clear cells, and cells with melanin pigment
Infiltrative growth may be seen with any histologic variant, including infiltration of bone.
Special stains including histochemistry and immunohistochemistry are of limited utility in the diagnosis.
No specific cytogenetic or molecular genetic findings
Dentigerous cyst: see earlier in section
Odontogenic keratocyst: see later in section
Ameloblastic fibroma
Squamous odontogenic tumor:
Rare benign odontogenic epithelial neoplasm thought to arise from epithelial rests of Malassez of the periodontal membrane
Most common in third to fourth decades of life
Occur most often in the anterior maxilla or posterior mandible
Radiology: well-demarcated radiolucent lesion with sclerotic, osseous border
Histologic features include:
Irregular-shaped islands of bland-appearing mature squamous epithelium without nuclear pleomorphism, nuclear hyperchromasia, increased mitotic activity, or dyskeratosis
Flattened peripheral cells with smoothly contoured connective tissue interface
Absence of stellate reticulum and peripheral nuclear palisading with reverse polarity:
Assists in differentiating from acanthomatous variant of ameloblastoma
Cystic change may be present.
Abundant fibrous stroma
Epithelial islands may contain spherical eosinophilic hyaline material (reminiscent to Rushton bodies) that stain strongly with PAS
Absence of significant cytologic atypia allows for differentiation from intraosseous carcinoma or metastatic squamous cell carcinoma
Simple excision (enucleation or curettage) considered preferred treatment and is curative
Complete surgical resection is preferred treatment:
For small tumors that are well delineated, conservative but complete excision can be performed.
For larger tumors that have spread to adjacent tissues (e.g., bone, other) en bloc resection may be required to include at least 1 cm of normal tissue beyond radiographic margin.
Surgical curettage not an acceptable form of therapy
Considered radioresistant; chemotherapy has no proven efficacy.
Recurrence is not uncommon and may lead to extensive local destruction with facial disfigurement or may pose life-threatening complications as a result of extension into vital structures.
Metastases are rare and are generally related to long-standing tumors associated with multiple surgical procedures or radiation treatment.
Prognosis for ameloblastomas depends on tumor size, extent of disease, and location of the tumor:
Mandibular ameloblastomas tend to be confined tumors, due to the inherently thick cortical mandibular bone
Maxillary ameloblastomas are more likely to demonstrate extension beyond the bone, due to the absence of a thick cortical bone and the intimate association with the sinonasal cavity.
Malignant ameloblastoma:
Represents ameloblastomas with benign features yet metastasize
Diagnosis can be rendered only after identification of metastatic tumor:
Also referred to by designation metastasizing ameloblastoma
Rare occurrence
Lung followed by regional lymph nodes represent most common sites for metastases:
Other reported metastatic sites include bone, brain, kidney, intestine, and liver.
Interval between diagnosis of primary tumor and metastasis can be years
Ameloblastic carcinoma ( Figs. 6-20 and 6-21 ):
Represents rare malignant transformation of a benign ameloblastoma characterized by cytomorphologically malignant epithelial cells with marked nuclear pleomorphism, increased nuclear-to-cytoplasmic ratio, increased mitotic activity, necrosis, and lymph-vascular invasion:
Predilection for the mandible
Presentation may include pain, swelling, trismus, and odynophagia
Treated by surgical resection
May metastasize to regional lymph nodes and distantly to lungs, bone, and liver
Poor prognosis
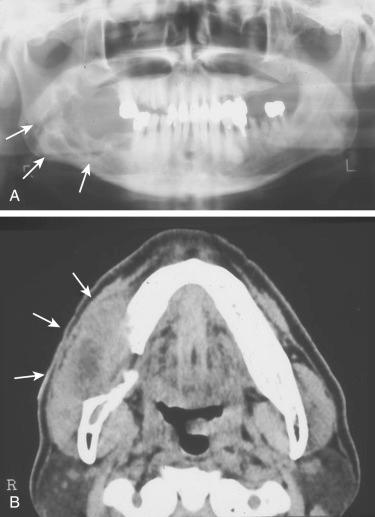
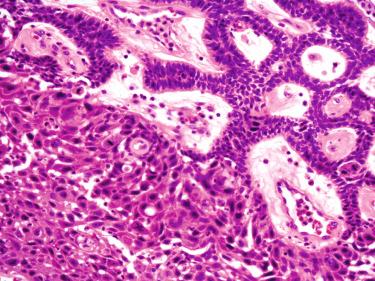
Ameloblastic fibrosarcoma (ameloblastic sarcoma) ( Fig. 6-22 ):
Rare malignancy in which there is mixed epithelial-mesenchymal odontogenic neoplasm composed of a sarcomatous mesenchymal component arising:
De novo
From pre-existing odontogenic mixed neoplasm such as an ameloblastic fibroodontoma or ameloblastic fibroma
Histology includes foci of ameloblastoma most often in a follicular pattern surrounded by sarcomatous proliferation with fascicular to herringbone pattern composed of malignant spindle-shaped cells with marked nuclear pleomorphism, increased mitotic activity including atypical mitoses and necrosis
Biomarker analysis showed alterations of p53 and c-KIT genes restricted to the sarcomatous component
Treatment follows that for other sarcomas, including radical extirpation and chemotherapy.
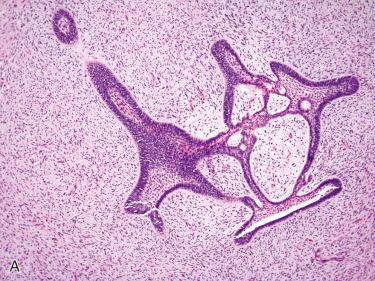
Definition: Variant of conventional ameloblastoma in which there is a single, often large unilocular cyst lined by ameloblastomatous epithelium.
May develop de novo
May develop in a preexisting odontogenic cyst
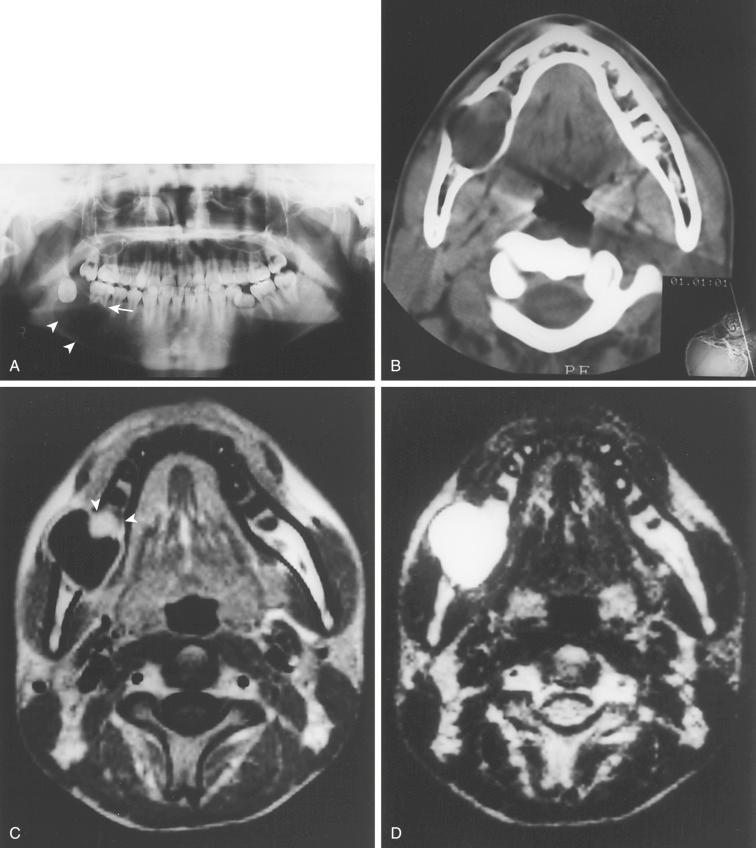
From 5% to 15% of all ameloblastomas are of the unicystic type
No gender predilection; tends to occur at a younger age than solid/multicystic ameloblastoma primarily in the second to third decades of life
Greater than 90% involve the mandible, usually the posterior portion of the mandible.
May be asymptomatic or present as a painless swelling of affected area
Often associated with an impacted tooth, making it indistinguishable from a dentigerous cyst
Radiology:
Well-delineated unilocular radiolucency
Occasionally may show demarcated, perilesional corticated rim
Radiolucency often associated with an unerupted tooth, making it indistinguishable from a dentigerous (follicular) cyst
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here