Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
How well does a normal term newborn see?
Term newborns can often fixate on a target. The ability to track an object, however, does not generally develop until approximately 2 months after birth. Visual acuity, measured with visual evoked potentials, has been estimated around 20/400 at birth. Color vision and contrast sensitivity have only rudimentary function in the newborn. Best-corrected visual acuity gradually improves during early childhood as the brain and retina mature.
Does visual development differ in the preterm infant?
The central retina is still actively developing throughout the 20th and 30th weeks of gestation. Myelination of the optic nerves and radiations continues during this time as well. Infants have fused eyelids until 25 weeks’ gestational age (GA), and the lids can remain fused in some cases until 30 weeks’ GA. The effects of these changes on the development of visual function are still being studied. It is unknown whether earlier exposure to visual stimuli has a positive or negative effect on eventual visual development. However, premature infants may demonstrate delayed visual milestones in early infancy.
What type of visual function can be observed in the NICU?
The pupillary light reflex should be observable after 31 weeks’ gestational age. A blink reflex to light can often be observed a few days after birth. 1 2 3
1 Mills MD. The eye in childhood. Am Fam Physician 1999;60(3):907–16.
3 Repka MX. Ophthalmological problems of the premature infant. Mental Retardation and Developmental Disabilities Research Reviews 2002;8:249–57.
What are the basic components of a typical NICU eye examination?
The lids should be examined for any abnormalities, including malformation, swelling, or discharge. Pupils should be examined for signs of irregular shape. The cornea, lens, and retina should be assessed with the red reflex test.
How does the red reflex test work? Who should get it?
The red reflex test was well described in a policy statement by the American Academy of Pediatrics in 2008, part of which is included here. The test uses the transmission of light from an ophthalmoscope through all the normally transparent parts of a subject’s eye, including the cornea, aqueous humor, lens, and vitreous. The light reflects off the retina and optic nerve, is transmitted back through the optical media and through the aperture of the ophthalmoscope, and is imaged in the eye of the examiner. Any factor that impedes or blocks this optical pathway will result in an abnormality of the red reflex.
The test is performed by holding an ophthalmoscope close to the examiner’s eye with power set at “0” and projecting the light simultaneously onto both eyes of the infant from a distance of approximately 18 inches away in a darkened room. Abnormalities include a diminished reflex, white reflex, or asymmetric reflexes.
Before discharge from the neonatal nursery, all children should have an examination of the red reflex of the eyes performed by a pediatrician or neonatologist. The test is important for the early detection of vision disorders and systemic diseases with eye manifestations. All infants with an abnormal or absent reflex should be referred immediately to an ophthalmologist.
Do the eyes need to be dilated to perform the red reflex test?
In general, no. An adequate examination can usually be performed through the undilated pupil. There has been some question as to whether pupil-dilated red reflex examinations improve identification of conditions such as retinoblastoma and congenital cataract, but this has not been definitively established.
What is leukocoria?
Leukocoria means “white pupil.” Differential diagnosis includes retinoblastoma, retinal detachment, cataract, retinopathy of prematurity (ROP), coloboma, primary persistent hyperplastic vitreous, congenital infection, and vitreous hemorrhage. Prompt ophthalmologic consultation is important in cases of suspected leukocoria.
What are some causes of corneal clouding in a newborn?
Sclerocornea, Peters anomaly, forceps trauma, congenital glaucoma, congenital hereditary endothelial dystrophy, mucopolysaccaridoses, and corneal dermoids can result in a white or clouded appearance to the cornea and cause an abnormal red reflex. Prompt ophthalmologic consultation is important in cases of corneal clouding.
Does a newborn make tears?
In a term baby tears are produced with crying beginning between month 1 and month 3 of life. Excessive tearing in the early stages of life most often represents congenital nasolacrimal duct obstruction, which is common and spontaneously resolves in approximately 90% of cases within the first year. However, excessive tearing associated with other abnormalities, such as blepharospasm and photophobia (in congenital glaucoma), or periocular erythema and edema (in dacryocystitis), warrants urgent evaluation.
What is strabismus?
Strabismus refers to misalignment of the eyes. Intermittent strabismus is often observed in the newborn and tends to resolve. Strabismus that persists beyond the first few months of life should be referred to an ophthalmologist for further evaluation. 4 5
4 Ramasubramanian A, Johnston S. Neonatal eye disorders requiring ophthalmology consultation. NeoReviews 2011;12(4):c216–c222.
5 Buckley EJ, Ellis GS, Glaser S, for the American Academy of Pediatrics section on ophthalmology. Red reflex examination in neonates infants, and children. Pediatrics 2008;122:1401–1404.
2 Hoyt CS, Good W, Petersen R. Disorders of the eye. In: Taeusch HW, Ballard RA, Avery ME, editors. Schafer and Avery’s diseases of the newborn. 6th ed. Philadelphia: Saunders; 1991.
What does “ROP” stand for?
ROP stands for retinopathy of prematurity. ROP is a vascular disease affecting the developing retina that is a leading cause of childhood blindness in the United States and throughout the world.
How does this disease happen?
Retinal vascular development begins during the second trimester of pregnancy, and full maturation typically occurs during or after the third trimester of pregnancy. In premature babies much of this development is taking place ex utero. The abnormal retinal development seen in ROP is in response to the artificial environment experienced by the neonate after birth.
In the first phase of pathogenesis, hyperoxia leads to cessation of the normal vascular development of the peripheral retina. In the second phase increased metabolic demand causes relative hypoxia to the peripheral retina, which leads to increased production of pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF) in the eye. This in turn stimulates abnormal proliferative vascular development. The proliferation can cause traction on the retina and bleeding inside the eye, which leads to vision loss ( Fig.15-1 ).
Which infants are at risk for ROP?
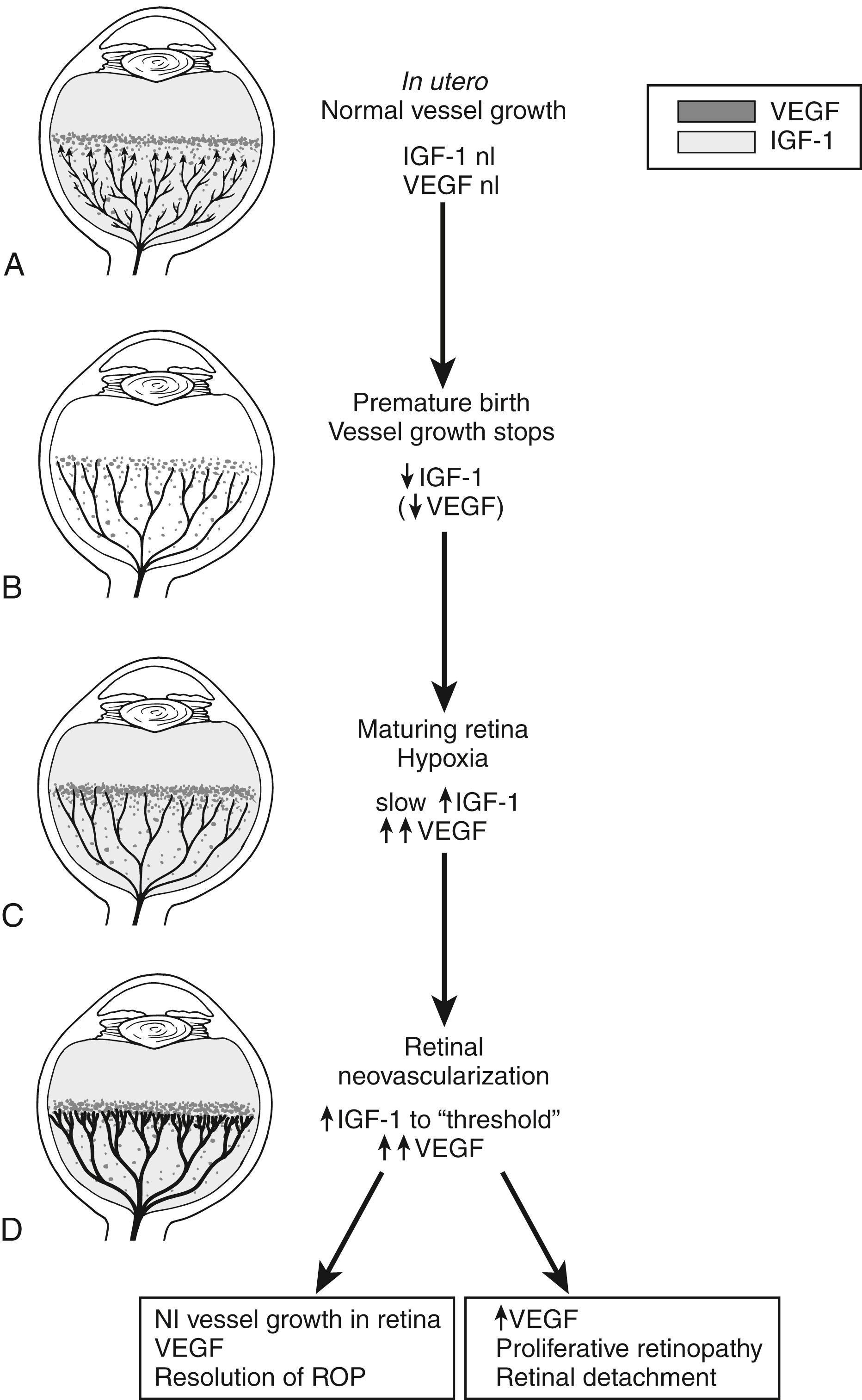
The “first epidemic” of ROP occurred in the 1950s and involved premature babies exposed to high levels of oxygen after birth. In most developed countries the danger of high levels of oxygen to the neonatal eye is a well-known risk factor. Survival rates of extremely-low-birth-weight (ELBW) infants have increased as neonatal and oxygen management has improved in developed countries, and these ELBW infants are at high risk for ROP (“second epidemic”). In developing countries, where neonatal intensive care is still developing and infants are often exposed to high levels of supplemental oxygen, larger and more mature babies are once again getting ROP. This has been termed a potential “third epidemic” of ROP.
Risk factors for ROP include degree of prematurity; low birth weight; slow weight gain after birth; and general health factors, such as anemia; intraventricular hemorrhage; and acidosis. There may be a genetic predisposition to ROP, and advanced maternal age may also be an independent risk factor.
The frequency of ROP in the United States has been found to be approximately 65% in infants with birth weight below 1251 g. In most cases the disease is mild and spontaneously regresses. A small percentage of these infants will progress to disease requiring treatment, usually between 36 and 40 weeks postmenstrual age.
Who should be screened for ROP, and what does the screening process entail?
Infants with a birth weight of ≤1500 g or gestational age of 30 weeks or less (as defined by the attending neonatologist), and selected infants with a birth weight between 1500 and 2000 g or gestational age of >30 weeks with an unstable clinical course, should have retinal screening examinations. This examination is to take place either at 31 weeks’ GA or 4 weeks after birth, whichever is later.
The examination involves dilating the child’s pupils with eyedrops (e.g. Cyclomydril, which is a combination of 1% phenylephrine, a sympathomimetic, and 0.2% cyclopentolate, an anticholinergic). These drops are usually administered in each eye by the nursing staff. During the eye examination the ophthalmologist will typically look at the anterior segment (cornea, iris, and lens) of the eye with a penlight, and then examine the retina using indirect ophthalmoscopy (a headlamp with a handheld lens). The examination may include using a lid speculum to keep the eyelids open during the examination and pressing gently on the sclera using a small rod to view the peripheral retina. If retinopathy is noted, the severity (stage), extent (clock hours), location relative to the central retina (zone), and degree of vascular tortuosity (plus) are recorded. Examinations continue every 1 to 2 weeks until the peripheral retina is fully vascularized or more frequently if warranted by clinical findings ( Table 15-1 ).
When is ROP treated, and what are the treatment options?
| AGE AT BIRTH | TIMING OF FIRST ROP EXAMINATION |
|---|---|
| 24 weeks GA | 31 weeks GA |
| 25 weeks GA | 31 weeks GA |
| 26 weeks GA | 31 weeks GA |
| 27 weeks GA | 31 weeks GA |
| 28 weeks GA | 32 weeks GA |
| 29 weeks GA | 33 weeks GA |
| 30 weeks GA | 34 weeks GA |
| >30 weeks GA | 4 weeks after birth |
Large, multicenter studies have shown that zone I disease with stage 3 severity, zone I disease in any stage with plus disease, or zone II disease with stage 2 or 3 severity and plus disease warrant prompt treatment. These entities were collectively defined by the Early Treatment for ROP (ETROP) study as “type 1 ROP.” Without prompt treatment a significant percentage of these children will progress to severe vision loss. On average, type 1 ROP occurs at 37 weeks’ postmenstrual age.
Laser treatment to the peripheral retina is currently the standard of care for the treatment of type 1 ROP. The treatment attempts to halt the production of VEGF by ablating the metabolically active, yet hypoxic peripheral retina. Ophthalmologists are divided regarding whether the infant should be intubated and anesthetized for the laser procedure or whether it may be performed at the NICU bedside with topical anesthesia and intravenous sedation. This varies depending on surgeon preference, extent of treatment, and other systemic comorbidities.
New data suggest that an injection of anti-VEGF medications such as bevacizumab (Avastin) directly into the vitreous of the eye may be beneficial for treatment of ROP, and the standard of care is still evolving in this area.
If the ROP progresses to the retinal detachment stage, further surgery is often required. This may consist of a vitrectomy (incisional surgery to remove fibrous tissue and flatten retinal detachment) or a scleral buckle (insertion of an encircling band around the eye to flatten retinal detachment).
Does screening hurt the baby?
Studies have shown that transient, small elevations in both heart rate and blood pressure may occur. These may result from both the administration of the dilating eyedrops and the eye examination itself. There are typically no lasting effects after the examination, but infants should be monitored carefully during ROP examinations.
Is it possible to make a diagnosis with a special photograph? If so, how does this work?
In some centers bedside photographs of the retina with a specialized handheld camera (RetCam; Clarity Medical Systems, Pleasanton, Calif.) are obtained by trained staff in the NICU and sent electronically to an ophthalmologist for ROP screening and interpretation. This has been used in some areas, particularly where in-person ophthalmologic examination is difficult. The standard of care is still evolving in this area.
Why is outpatient follow-up so important for extremely premature babies?
Many babies are discharged or transferred from the NICU at just about the time when most type 1 (treatment requiring) ROP will develop: at 37 weeks’ GA. It is imperative that the neonatologist, ophthalmologist, and discharge planner communicate and coordinate appropriately regarding discharge and follow-up in children who are high risk. ROP can progress rapidly, and even a short delay in a necessary follow-up ophthalmic examination can be the difference between a lifetime of functional vision and a lifetime of blindness for a premature child. Loss to timely outpatient ophthalmologic follow-up at the time of NICU discharge or transfer has been a common source of both poor visual outcome and malpractice claims against care providers.
If a child does not require treatment for ROP, will the eye still develop normally?
Usually yes. Although severe complications are less frequent, follow-up with a pediatric ophthalmologist is recommended for infants with ROP who do not require treatment. This is because of the increased prevalence of myopia, strabismus, and astigmatic refractive errors in this population. 6 7 8 9 10 11 12 13
6 Chen J, Stahl A, Hellstrom A, et al. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr 2011;23(2):173–8.
7 Mills MD. Evaluating the cryotherapy for retinopathy of prematurity study (CRYO-ROP). Arch Ophthalmol 2007;125(9):1276–81.
8 Good WV. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233–50.
9 Mintz-Hittner HA, Kennedy KA, Chuang AZ for the BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603–15.
10 Darlow BA, Ells AL, Gilvert CE, et al. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed 2013;98(2):F170–4
11 Chiang MF, Melia M, Buffenn AN, et al. Detection of clinically significant retinopathy of prematurity using wide-angle digital retinal photography: a report by the American Academy of Ophthalmology.Ophthalmology 2012;119(6):1272–80.
12 Richter GM, Williams SL, Starren J, et al. Telemedicine for retinopathy of prematurity diagnosis: evaluation and challenges. Surv Ophthalmol 2009;54:671–85.
13 Laws DE, Morton C, Weindling M, et al. Systemic effects of screening for retinopathy of prematurity. Br J Ophthalmol 1996;80:425–8.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here