Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The fetal-neonatal immune system becomes activated at birth to play a role in host defense and eliminate environmental pathogens but also to tolerate self-antigens, nutrients, and commensals.
The innate immune system is comprised of neutrophils, the monocyte-macrophage lineage, natural killer cells, and the noncytotoxic innate lymphoid cells.
Neutrophils are the most numerus subgroup of leukocytes, but unlike in the adult, these cells are developmentally deficient in many host defense functions such as migration, phagocytosis, and microbial killing.
Increasing heterogeneity is being recognized in the macrophage system.
Cord blood contains a higher number and proportion of plasmacytoid dendritic cells than adult peripheral blood, but there are important functional differences.
The adaptive immune system also displays innate-like properties to compensate for limitations in antigen-specific acquired immune responses. T cells show important limitations in the repertoire of the antigenic specificity but show rapid maturation after birth. The immunoglobulin repertoire is relatively restricted in the fetus and neonate.
The B-1 lymphocytes show broad poly-specificities and may have a role in innate, not adaptive, immunity.
Fetal and neonatal natural killer (NK) cells have a significantly lower cytolytic activity than in adults. These cells should not be confused with the natural killer T cells, a heterogeneous group of T cells that express an alpha beta T-cell receptor besides some of the NK cell markers.
Noncytotoxic innate lymphoid cells are morphologically similar to other lymphocytes, but unlike adaptive T and B cells, they do not show antigen specificity. These cells function more as innate immune cells.
In the developing immune system, birth is an important functional watershed. The fetus is continuously exposed to maternal antigens in utero and its immune responses must remain suppressed for survival. In the neonatal period, the immune system is exposed to a diverse set of environmental antigens and needs a dichotomous set of responses to “contain” certain microorganisms on various cutaneous and mucosal surfaces, and at the same time, develop tolerance to other commensals and dietary macromolecules. Some components of the immune system are reasonably mature at birth, but other arms are yet to gain full functional reactivity. The deficient responses could be viewed as a developmentally regulated state of immunodeficiency. This chapter highlights the quantitative and qualitative differences in major leukocyte subsets ( Fig. 56.1 ) during the neonatal period.
During development, most leukocyte lineages can be traced back diverging from hematopoietic stem cells during the embryonic and fetal period ( Fig. 56.2 ). Downstream, during the neonatal period and beyond, the leukocyte subsets show increasing heterogeneity with multiple, specialized subsets ( Fig. 56.3 ). We have recognized many of these subsets of T cells for some time, including the T-helper cells, regulatory T cells, cytotoxic T cells, and γδ T cells. The two B-cell lineages, B-1 and B-2, which may be involved in innate and adaptive immune responses, respectively, have also been known. We, and others, have investigated the role(s) of various macrophage subclasses. The antigen-specific heterogeneity of lymphocytes has also been studied. Emerging information on the functional proficiency of innate lymphoid cells (ILCs) during the neonatal period, when adaptive immunity is still immature, is exciting. There is evidence that some of these subsets, such as the group 3 ILCs (ILC3), can be modulated by the microbiome and inflammation. Most recently, some heterogeneity has been noted in neutrophils in patients with malignancies; there may be two distinct neutrophil lineages, N-1 and N-2, that play different roles in immune regulation. This polarization could very well extend to the normal immune system. These details are discussed later in the chapter.
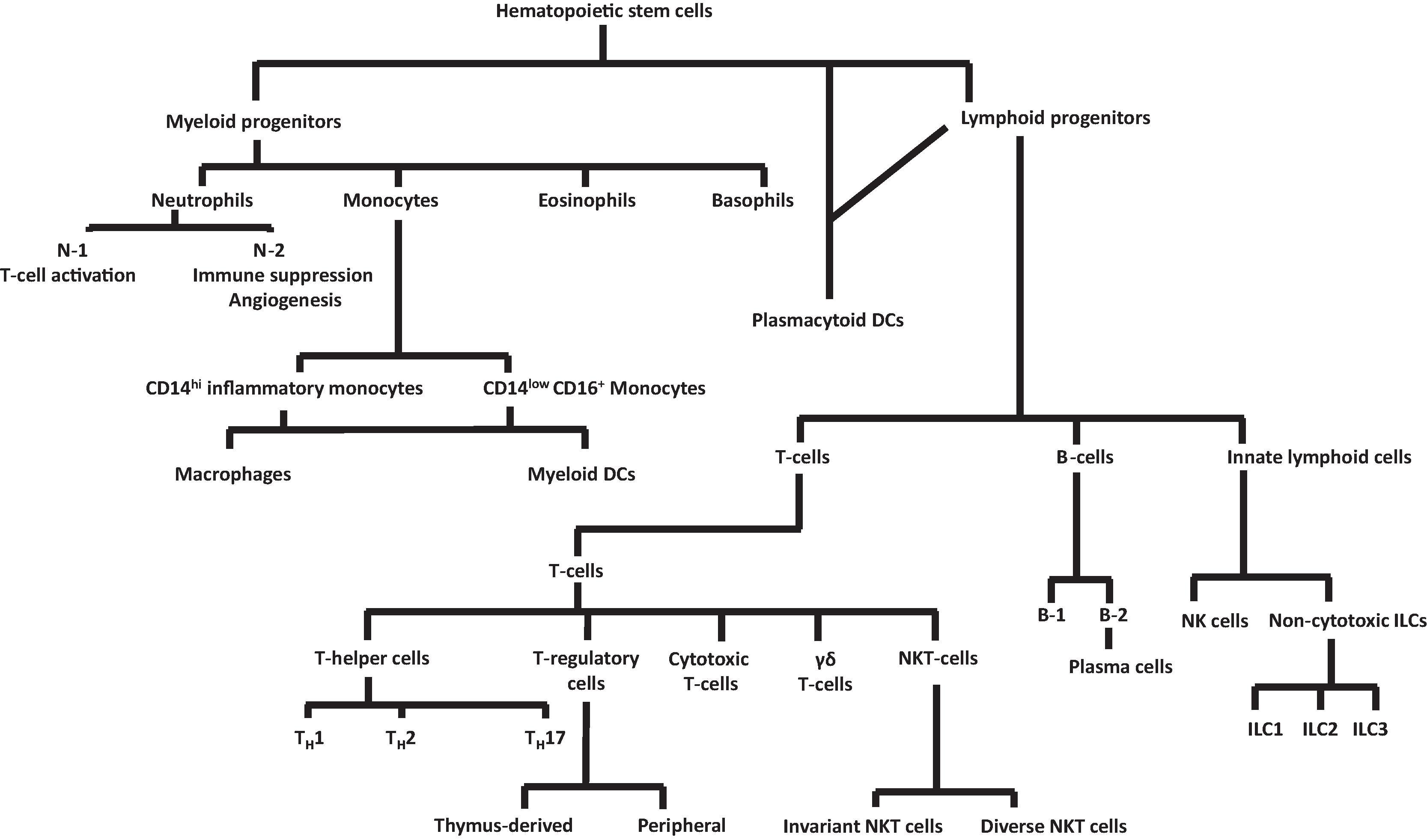
This section outlines the development and functional maturation of various cellular lineages in the innate immune system (as mentioned above; see Fig. 56.3 ) ( Box 56.1 ).
Neutrophil function includes emigration from the bloodstream, phagocytosis, and microbial killing
Neutrophils from both term and preterm neonates adhere poorly to endothelium; neonatal neutrophils have less selectin and β 2 -integrin (Mac-1/CD11b) expression; transendothelial migration, which is dependent on cell deformability, is comparable to adults
Neonatal neutrophils show impaired chemotaxis; term neutrophils show normal chemotaxis at 2 weeks; late preterm infants achieve normal function at term PCA; neutrophils from very low birth weight (VLBW) neonates begin to mature at 2–3 weeks after birth and progress very slowly
Neutrophils from preterm neonates phagocytose slowly and ingest fewer bacteria; corrects by term PCA
Preterm neutrophils display poor respiratory burst and impaired killing of Staphylococcus aureus or Escherichia coli ; improves by 2 months postnatal; term neonates are normal in this regard
Preterm monocytes show slightly impaired chemotaxis; however, activated monocytes show normal trafficking and adhesion molecule expression
Preterm monocytes can kill pathogens ( Staphylococcus aureus , S. epidermidis , E. coli , and Candida albicans ) comparable to adult monocytes
During sepsis, neonatal monocytes express TLRs and cytokines at levels similar to adults; monocytes from VLBW neonates may be slightly more impaired than in term infants
Skewed pattern of cytokine expression with low levels of Th1-polarizing cytokines such as interleukin (IL)12p70 and interferon-α, and more of the antiinflammatory cytokine IL-10
Cord blood contains fewer DCs (0.5%) than adult blood (1%)
Neonatal myeloid DCs are immature (lower CD83, CD86 expression) and produce less IL-12 and IFN-γ but more IL-10 than adult DCs
Neonatal DCs also perform poorly as accessory cells for T-cell mitogenic responses
Most T cells carry a T-cell receptor (TCR) composed of α and β chains, but in about 5% of all T cells, the TCR is composed of γ and δ chains
Each TCR protein chain contains an immunoglobulin-like extracellular region with a variable (V) domain at its N -terminal and a constant (C) domain at the C -terminal end; TCR diversity results from recombination of variable (V), diversity (D), and joining (J) gene segments in individual T cells
Term infants have more T cells in peripheral blood than adults; neonates have CD4/CD8 ratios of 5:1, which decline to adult values (2:1) by 4 years of age
Preterm infants have lower CD4 + and CD8 + counts than term infants
80% T cells in cord blood have a naïve CD45RA phenotype, compared with <50% of circulating T cells in adults; memory CD45RO T cells reach adult levels in adolescence
Naïve CD4+ cells differentiate into three effector T-helper (Th) subsets: Th1, Th2, and Th17
Th1 cells defend against intracellular pathogens and virus-infected cells and produce IL-2, IFN-γ, TNF, IL-13, and GM-CSF
Neonates have low Th1 function, produce less IL-12 and IFN-γ, and express less CD154 (CD40 ligand) than adults
Th2 cells participate in allergic reactions; they produce IL-4, IL-5, IL-9, IL-10, IL-12, and IL-13
Th17 cells protect against infections and activate neutrophils and macrophages; they produce IL-17A, IL17-F, IL-21, IL-22, and IL-26; cord blood T cells show limited capacity to produce IL-17
Derived from naïve CD4 + cells; suppress immune responses by expressing IL-10, TGF-β, cytotoxic molecules, and modulators of cAMP
Two populations: thymus-derived (tT-regs) and peripherally derived T-regs (pT-regs)
In the fetus/preterm infant, T-regs are tolerogenic and promote self-tolerance
During midgestation, CD4 + CD25 + Fox P3 + T-regs constitute 20% of all CD4 + cells in lymphoid tissues; represent <5% of CD4 + cells in cord blood from term infants and in the adult blood
Preterm and term T-regs are less functional than adults; they limit contact between DCs and effector T cells, causing decreased DC immunogenicity and impaired T-cell activation
CD8 + T cells can differentiate into cytotoxic T lymphocytes; they protect against intracellular pathogens—cause cytotoxicity by releasing pore-forming mediators (perforin/granzyme) or by activating fas-mediated apoptosis
Neonatal CTLs are less efficient than in adults; circulating αFP and prostaglandins may inhibit CTL activity in neonates
γδ T Cells
γδ T cells respond to nonpeptide microbial metabolites, show cytotoxicity, and produce interferon-γ and TNF. γδ IELs protect epithelial tissues from injury
Constitute 10% of circulating T cells during midgestation, but the number declines to about 3% at term in skin and mucosa
In the intestine, 30% of IELs, 10% of mucosal lymphoid tissue lymphocytes, and 5% of LPLs have the γδ TCR
NKT cells express TCR-αβ chains and NK cell markers; classified into two main subsets, that is, type I or invariant NKT cells and type II or diverse NKT cells
Invariant NKT cells have limited TCR diversity and recognize lipid antigens such as α-galactosylceramides in the context of the major histocompatibility complex (MHC)-like molecule CD1d; this works similar to PRRs, but for microbial lipids; I-NKT cells secrete anti- and proinflammatory cytokines and activate adaptive immune responses
Diverse NKT cells express a variety of TCRs and may recognize lipid antigens with cross-reactivity between mammalian and microbial phospholipids, possibly representing the adaptive immune arm for lipid antigens
Preterm and term neonates have more circulating B cells than adults; B-cell counts peak at 3 months and then decline to adult levels by 6 years of age
More than 90% of B cells in the fetus/neonate express CD5 and are called B-1 cells; the proportion drops to 75%–80% during infancy and to the 25% adult levels by late adolescence; they express activation markers (CD25) and a CD11b + sIgM high sIgD low phenotype
Localize in the spleen and peritoneum; broad polyspecific specificities; the restricted immunoglobulin repertoire suggests a role in innate, rather than in adaptive, immunity
Respond to T-cell-independent, carbohydrate antigens (unlike follicular B-2 cells that respond to protein antigens)
Preterm infants produce antibodies but may not respond to all antigens in a vaccine; may remain limited to immunoglobulin M (IgM) with delayed isotype switch and may produce antibodies of low affinity
Postnatal age is a better predictor of antibody response than gestation; both preterm and term infants respond weakly to diphtheria toxoid in the first week and better at 1–2 months of age; premature infants respond poorly to the hepatitis B vaccine in early infancy but are comparable to term infants in later infancy
Most immunoglobulins in cord blood are derived from maternal IgG (particularly IgG1 and IgG3)
Preterm infants have lower IgG levels; term infants have serum IgG levels (1000 mg%) similar to or higher than those in maternal serum
Immunoglobulin levels drop to 300–500 mg% at 3–5 months, when the infant starts producing more; this nadir is reached earlier and is lower in preterm infants
Noncytotoxic ILCs are derived from the common lymphoid progenitor but do not exhibit antigen specificity
ILC1s express Th1-associated cytokines such as IFN-γ and TNF to protect against intracellular bacteria and parasites
ILC2s express Th2 cytokines (including IL-4, IL-5, IL-9, and IL-13); detectable in cord blood and are implicated in intestinal inflammation in gastroschisis
ILC3s express Th17 cytokines IL-17A, IL-17F, IL-22, GM-CSF, and TNF, to promote antibacterial immunity and inflammation; abundant in gut mucosa and may be involved in enhancing IgA production and in shaping the local microbiome
Neutrophil defects, T-cell defects, severe combined immunodeficiency, and bone marrow failure syndromes can present in the neonatal period
T-cell defects may include DiGeorge syndrome, hyper-IgM syndrome, and ZAP-70 tyrosine kinase defects
SCID includes purine nucleoside phosphorylase deficiency (viral infections, severe varicella, GVHD), cartilage hair hypoplasia, IL2Rα defects, X-SCID (Jak3), and AR SCID (ADA, RAG1/2, IL7Ra)
B-cell defects present only after maternally derived antibody levels drop during infancy
Transient hypogammaglobulinemia of infancy may be considered after 6 months; low basal but normal stimulated Ig responses
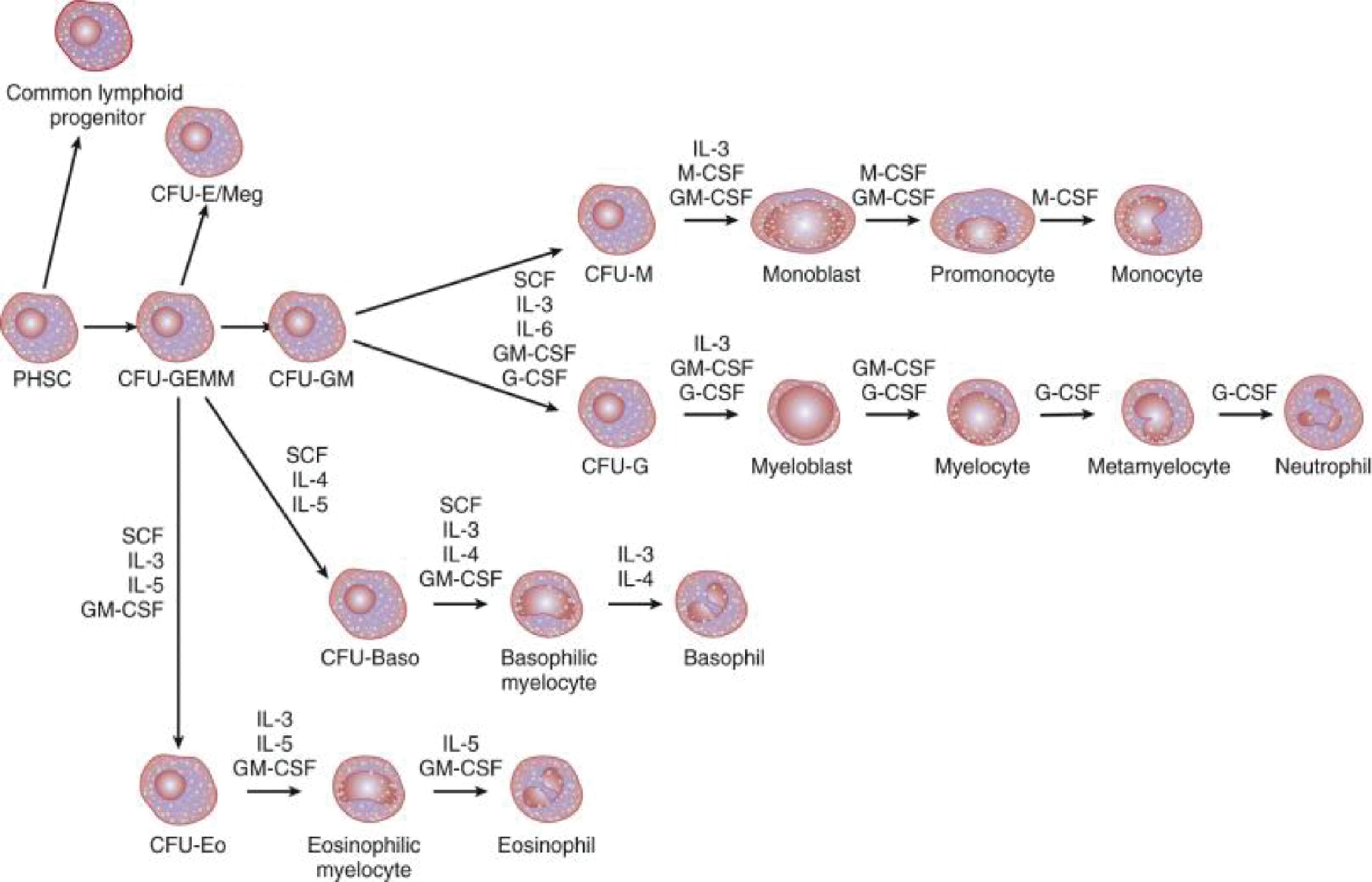
During development, hematopoiesis begins in the extraembryonic yolk sac in about the third week of embryogenesis. The two major hematopoietic progenitors committed to the neutrophil lineage are the colony-forming units-mix, which give rise to a mixture of various leukocyte populations, and the CFU-GEMM, which produce granulocytes, erythrocytes, megakaryocytes, and macrophages. Early hematopoiesis gets activated at about 7 to 8 weeks’ gestation with hematopoietic stem cells derived from the intraembryonic endothelial cells in the descending aorta. These hematopoietic stem cells subsequently seed the liver and the bone marrow later in gestation.
The neutrophil lineage can be viewed in a proliferating pool of early precursors, the neutrophil proliferating pool (NPP) that may have a capacity for 4 to 5 cell divisions, and a postmitotic neutrophil storage pool (NSP) that continues to differentiate ( Fig. 56.4A ). In adults, the NPP contains about 2 × 10 9 cells/kg body weight and the NSP contains about 6 × 10 9 cells/kg body weight. The NPP and NSP comprise nearly 90% of all neutrophils in the body; the other 10% of neutrophils ( Fig. 56.4B ) are either flowing in the circulation or are marginated (loosely attached to the microvascular endothelium). During late gestation and at birth, the blood contains 10- to 50-fold higher concentrations of CFU-GM than in adults, but the overall size of the pool of neutrophil progenitors is much smaller than in adults and gets easily exhausted during stress, such as during sepsis. , The NPP is only about one-tenth the size (per kilogram of body weight) seen in adults. ,
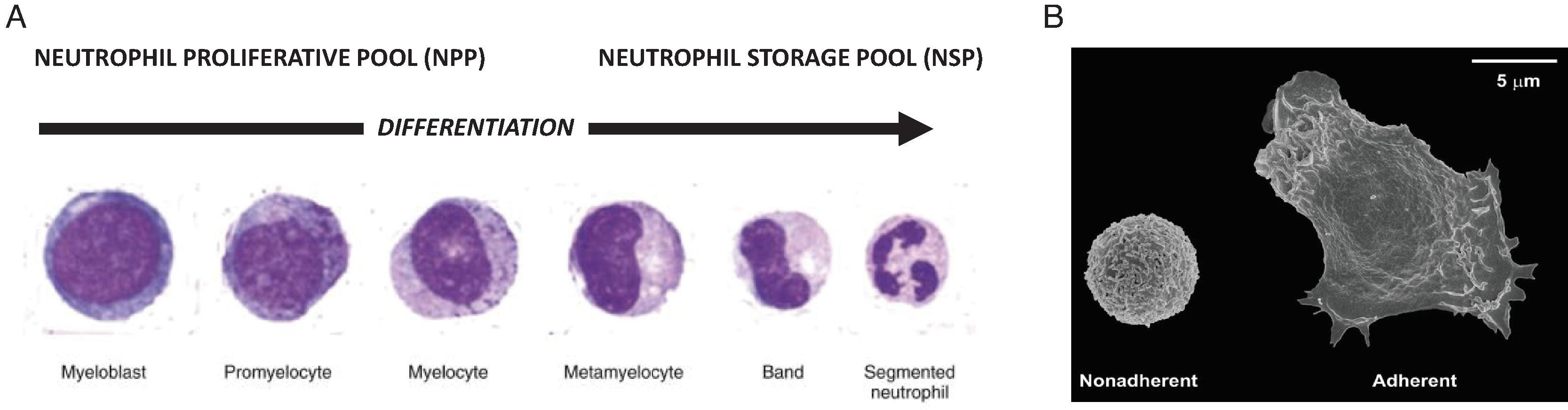
During acute inflammation, neutrophils are released first from the NSP, and once these limited stores are exhausted, progressively immature cells are mobilized (the “left” shift of sepsis). After circulating for 6 to 8 hours, neutrophils move into the tissues and can then stay there for up to a few days. In the fetus, as in the adult, the NSP is contained in the marrow, with some portion within the liver and spleen.
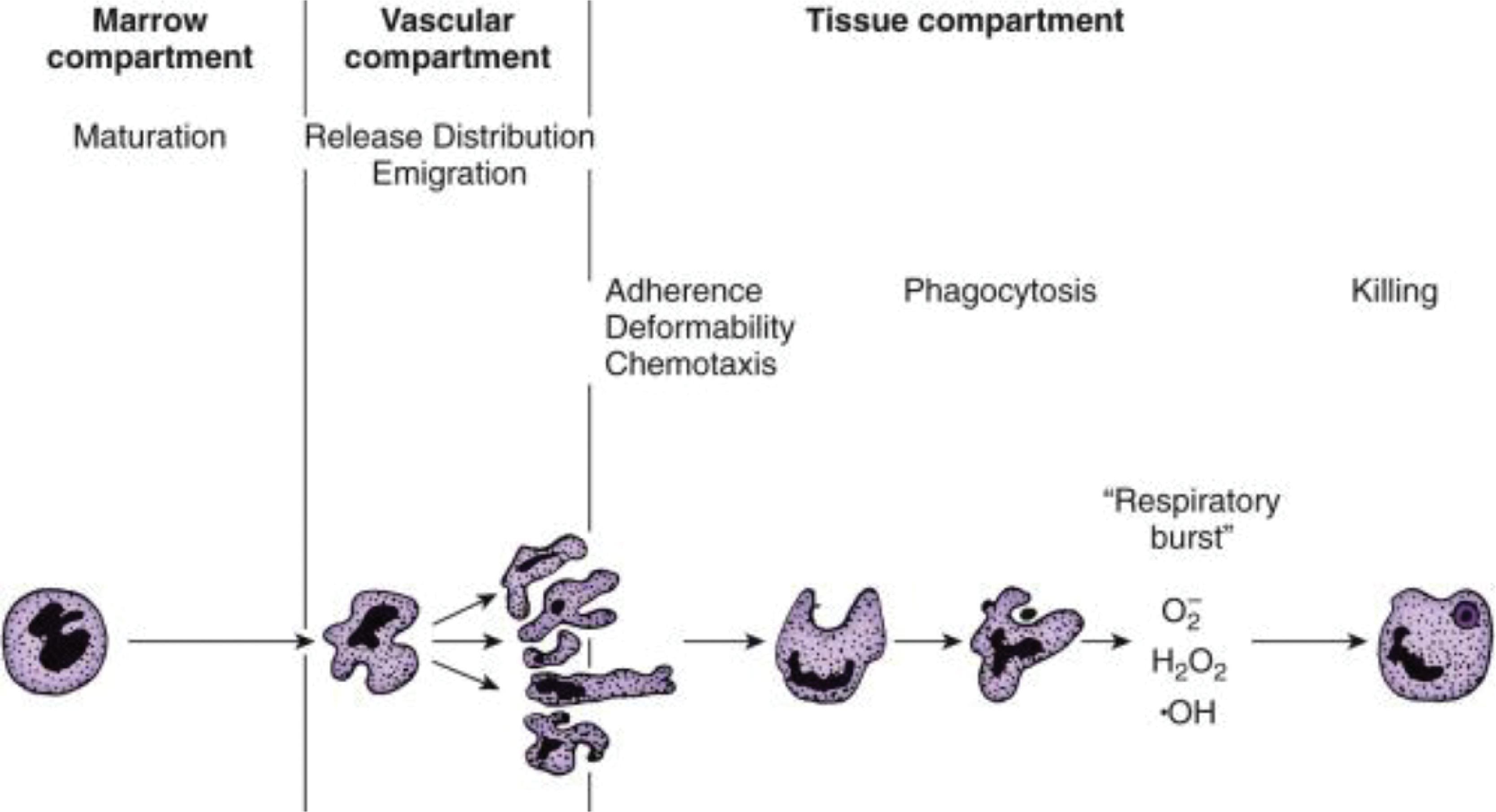
Circulating neutrophils leave the intravascular compartment to enter the tissues in three major steps: margination and rolling on vascular endothelium, attachment to the endothelial cells, and transendothelial migration ( Fig. 56.5 ). In the inflamed tissues, neutrophils are recruited through regional differences in vascular flow and along concentration gradients of chemokines ( Fig. 56.6 , some members in Table 56.1 ), chemotactic tripeptides such as N -formylmethionyl-leucyl-phenylalanine ( N -fMLP), complement fragments (C5a), and leukotrienes (LTB 4 ). Among chemokines, the CXC subfamily with a glutamate-leucine-arginine tripeptide sequence (such as interleukin-8 [IL-8/CXCL8]) are most important. , N -fMLP is an endogenous, chemotactic peptide derived from mitochondria in dying cells that attracts and activates neutrophils and macrophages. It mimics N -formyl oligopeptides released by bacteria and attracts and activates circulating blood leukocytes by binding to specific G-protein–coupled receptors on these cells.
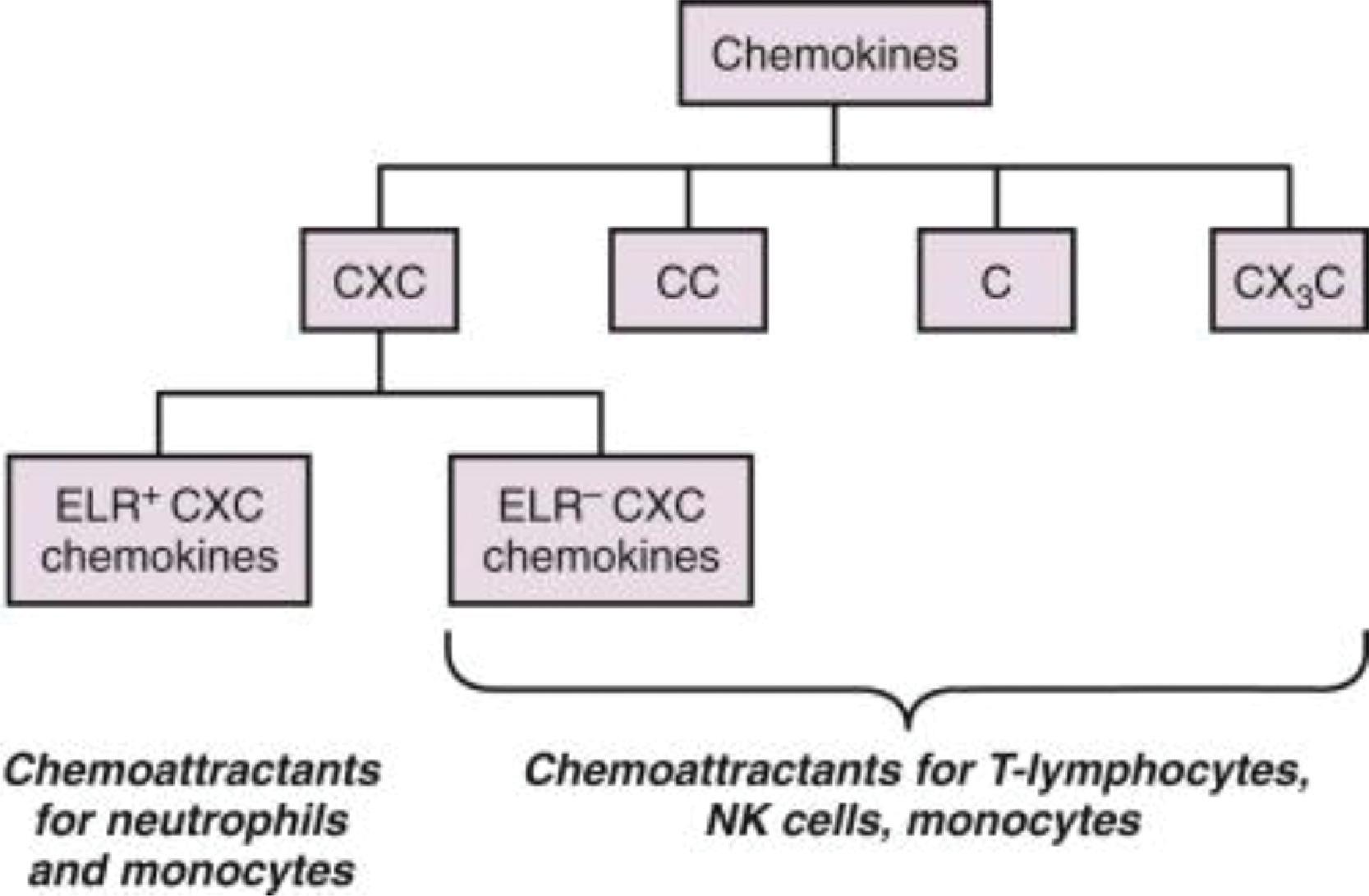
| Chemokines | Leukocytes Recruited | Systematic Name | Chemokine Receptors |
| Growth-related oncoprotein (GRO)-α | Neutrophils and monocytes | CXC-motif ligand (CXCL) 1 | CXC receptor (CXCR) 2 |
| GRO-β | Neutrophils and monocytes | CXCL2 | CXCR2 |
| GRO-γ | Neutrophils and monocytes | CXCL3 | CXCR2 |
| Epithelial neutrophil chemoattractant (ENA-78) | Neutrophils and monocytes | CXCL5 | CXCR2 |
| Granulocyte chemoattractant protein (GCP)-2 | Neutrophils and monocytes | CXCL6 | CXCR2, CXCR1 |
| Neutrophil-activating peptide (NAP)-2 | Neutrophils and monocytes | CXCL7 | CXCR2 |
| IL-8 | Neutrophils and monocytes | CXCL8 | CXCR2, CXCR1 |
| Monokine induced by gamma interferon (MIG) | Monocytes and lymphocytes | CXCL9 | CXCR3 |
| Interferon-gamma-inducible protein 10 (IP-10) | Lymphocytes | CXCL10 | CXCR3 |
| Interferon-inducible T-cell alpha chemoattractant (I-TAC) | Lymphocytes | CXCL11 | CXCR3 |
| Stromal cell-derived factor 1 (SDF-1) | Lymphocytes | CXCL12 | CXCR4 |
| B-cell chemoattractant (BCA-1) | Lymphocytes | CXCL13 | CXCR5 |
| Monocyte chemoattractant protein (MCP)-1 | Monocytes and lymphocytes | CCL2 | CCR2 |
| MCP-2 | Monocytes and lymphocytes | CCL8 | CCR3 |
| MCP-3 | Monocytes and lymphocytes | CCL7 | CCR1, CCR2, CCR3 |
| CCL13 | CCR2, CCR3 | ||
| Macrophage inflammatory protein (MIP)-1α | Monocytes and lymphocytes | CCL3 | CCR1, CCR5 |
| MIP-1β | Monocytes and lymphocytes | CCL4 | CCR5 |
| RANTES (regulated upon activation, normally T-expressed, and presumably secreted) | Monocytes and lymphocytes | CCL5 | CCR1, CCR3, CCR5 |
| Eotaxin-1 | Eosinophils | CCL11 | CCR3 |
| Eotaxin-2 | Eosinophils | CCL24 | CCR3 |
| Eotaxin-3 | Eosinophils | CCL26 | CCR3 |
| Liver and activation-regulated chemokine (LARC) | Monocytes and lymphocytes | CCL20 | CCR6 |
| Thymus expressed chemokine (TECK) | Monocytes and lymphocytes | CCL25 | CCR9 |
| Cutaneous T-cell-attracting chemokine (CTACK) | Monocytes and lymphocytes | CCL27 | CCR10 |
| T-cell-directed CC chemokine (TARC) | Monocytes and lymphocytes | CCL17 | CCR4 |
| Macrophage-derived chemokine (MDC) | Monocytes and lymphocytes | CCL22 | CCR4 |
| Dendritic cell-specific chemokine (DC-CK1) | Monocytes and lymphocytes, dendritic cells | CCL18 | Not known |
| Epstein-Barr virus–induced molecule 1 ligand chemokine (ELC) | Monocytes and lymphocytes, dendritic cells | CCL19 | CCR7 |
| Secondary lymphoid tissue chemokine (SLC) | Monocytes and lymphocytes, dendritic cells | CCL21 | CCR7 |
| Fractalkine | Monocytes and lymphocytes, dendritic cells | CX3CL1 | CX3CR1 |
In inflamed tissues, alterations in the endothelial surface, with increased leukocyte-binding receptors, and increased gradients of the CXC motif-bearing chemokines (see Fig. 56.5 and Table 56.1 ) promote neutrophil emargination. This process is mediated through a process of repetitive binding and release of selectins (L-selectin on neutrophils, E- and P-selectin on endothelium; Fig. 56.7 ). Rolling neutrophils slow down and attach to endothelium through the binding of β 2 -integrins to cognate receptors on endothelial cells. The β 2 -integrins expressed on neutrophils include the leukocyte function-associated antigen 1 (LFA-1) (α L β 2 , CD11a/CD18), CR3 (Mac-1, α M β 2 , CD11b/CD18), and p150, 95 (α X β 2 , CD11c/CD18), which bind endothelial receptors such as the intercellular adhesion molecule 1 and 2 (ICAM-1 and ICAM-2) and vascular cell adhesion molecule 1 (VCAM-1). LFA-1 binds to both ICAM-1 and ICAM-2, whereas Mac-1 and p150, 95 bind exclusively to ICAM-1. These neutrophils undergo activation on the endothelial surface and migrate through the capillary/venular wall, a process that involves the platelet-endothelial cell adhesion molecule (PECAM1, CD31), the integrin-associated protein (CD47), and other junctional molecules ( Fig. 56.8 ).
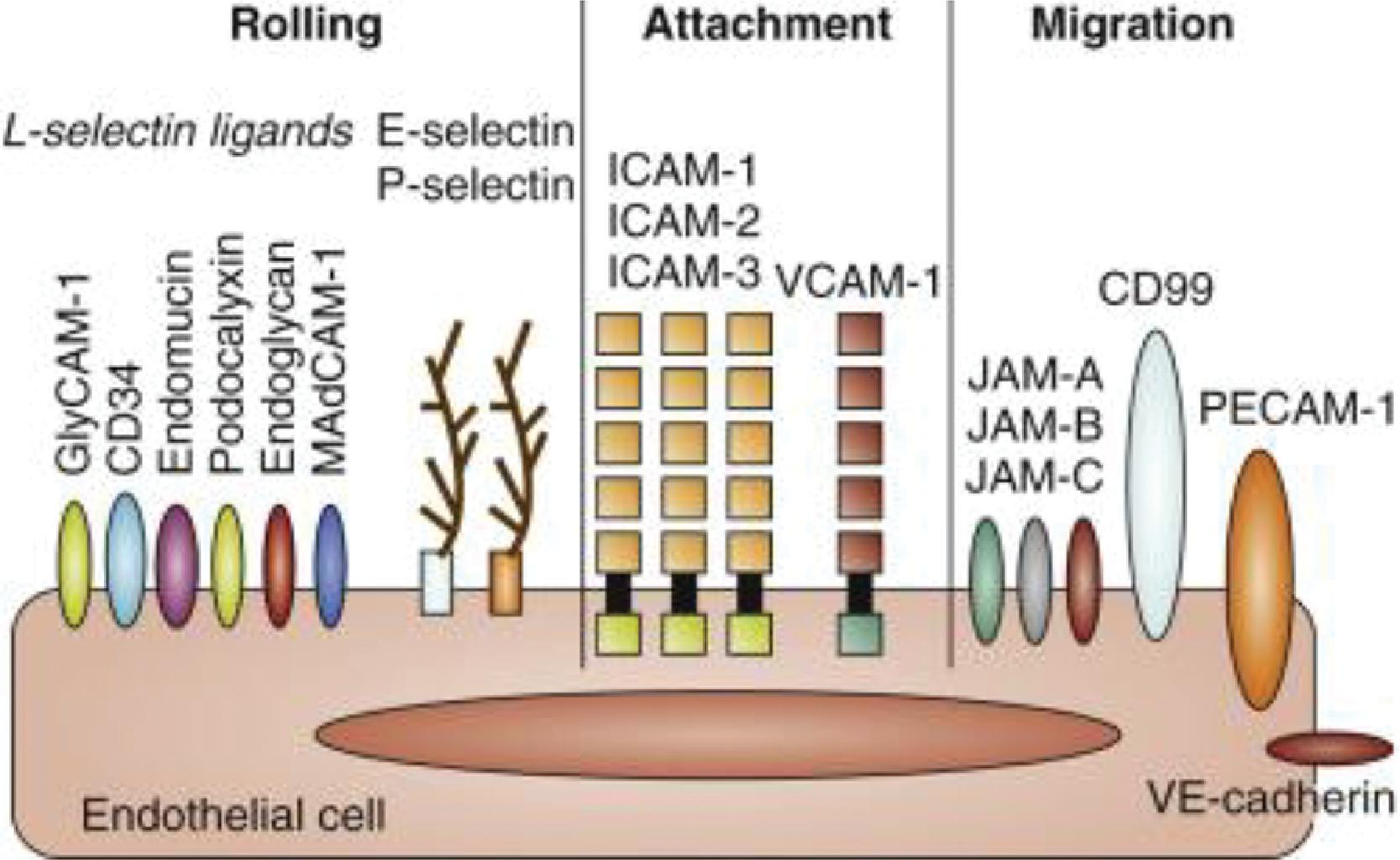
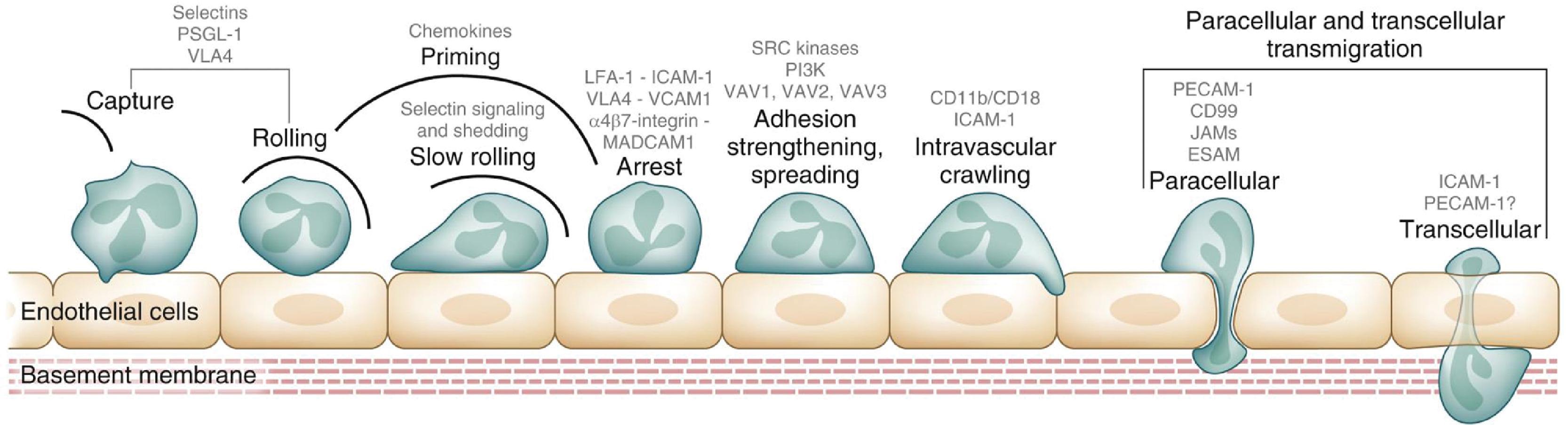
Compared with neutrophils from adults, neonatal neutrophils adhere less avidly to the endothelium. Neonatal neutrophils have lower selectin expression than adults, which might be further reduced by perinatal stress such as in birth asphyxia. In addition, neonatal neutrophils display defective shedding of L-selectin. These properties impair neutrophil rolling on the endothelial surface and thereby the tissue recruitment of these cells. Neutrophil-endothelial adherence and neutrophil transmigration is also limited in neonates due to a developmental deficiency of mac-1 (CD18/CD11b), one of the β 2 integrins. The transendothelial migration of neutrophils is also limited due to less deformability of neonatal neutrophils.
Once outside the blood vessel, neutrophils migrate (see Fig. 56.5 ) along concentration gradients of chemoattractants such as IL-8 and other chemokines, f-MLP, and C5a. These chemotaxins bind high-affinity G-protein–coupled receptors on the leukocyte surface. Bacterial products may be more potent chemoattractants than host chemokines. Neonatal neutrophils migrate slower than do adult neutrophils until 2 to 3 weeks after birth and achieve normal chemotaxis only by 40 to 42 weeks of postconceptional age or later. , Although minor infections may enhance chemotaxis in neonates, the migratory responses of neonatal neutrophils may become further depressed during systemic gram-negative sepsis. ,
Neonatal neutrophils bind various chemoattractants normally, but chemoattractant-induced membrane depolarization, calcium transport, and sugar uptake are less efficient. These chemotactic defects occur because neonatal neutrophils may have a larger, poorly motile neutrophil subpopulation, impaired calcium mobilization, and aberrations in intracellular signaling pathways such as NF-κB activation. , Lower Mac-1 expression can also impede chemotaxis due to impaired neutrophil interaction with the extracellular matrix. , Inability to effectively direct neutrophils to the bacterial source contributes to the neonatal vulnerability to septicemia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here