Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
After reading this chapter, readers will be familiar with the epidemiology of congenital infections such as CMV, HSV, Zika, and SARS-CoV-2 virus.
Risk factors for acquiring these infections are discussed.
Clinical manifestations, diagnosis, treatment, and clinical outcomes are also discussed in detail.
Among the numerous viral pathogens that cause central nervous system (CNS) infections in the neonatal period, herpes simplex virus (HSV) and cytomegalovirus (CMV) are unique in their management. Both have commercially available antiviral drugs that treat the virus, as well as evidence-based data documenting the benefit of antiviral therapy. Neonatal HSV infection primarily is acquired in the peripartum period, whereas congenital CMV infection is the most common viral infection acquired in utero. Utilization of antiviral therapy to improve disease outcomes is influenced by these differences, with antiviral therapy of neonatal HSV disease aimed primarily at improving mortality and antiviral therapy of congenital CMV infections targeting improvement in longer-term audiologic outcomes. Additionally, the extent of data and clinical experience differs between the two viruses, with antiviral treatment of neonatal HSV disease required in all cases, but antiviral management of congenital CMV infection is an option rather than a requirement.
The studies conducted by the National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Antiviral Study Group (CASG) over the past 30 years have defined the benefits and toxicities of antiviral treatment of neonatal HSV and congenital CMV. In conducting controlled investigations of these rare infections, the CASG also has characterized the natural history of infection with these viruses in neonates. These advances in our understanding of neonatal HSV and congenital CMV disease not only provide the foundation for advances in the management of these infections but also establish the scope through which newly recognized congenital and perinatal infections such as Zika and SARS-CoV-2 are appreciated.
Unlike HSV and CMV, which are transmitted person to person, Zika virus is unique among the viruses that cause congenital infections by being mosquito-borne. When a pregnant mother acquires a primary Zika infection, the virus can cross the placenta to infect the developing fetus with devastating consequences, including microcephaly, tremendous brain abnormalities, and even fetal loss. While much about congenital Zika virus infection remains to be elucidated, what we know already draws heavily from existing knowledge of congenital CMV and even rubella disease.
Novel coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It emerged in China in late 2019, and has caused over 200 million infections worldwide, with greater than 4 million deaths. Cases of vertical transmission have been described but are rare, and most infected neonates tend to have no adverse outcomes. Neonatal SARS-CoV-2 infection also can be acquired via horizontal transmission, from direct contact when feeding or via respiratory droplets of symptomatic mothers. There is much still to be learned about SARS-CoV-2 infection, in neonates in particular.
HSV disease of the newborn is acquired during one of three distinct times: intrauterine (in utero), peripartum (perinatal), and postpartum (postnatal). Among infected infants, the time of transmission for the majority (∼85%) of neonates is in the peripartum period. An additional 10% of infected neonates acquire the virus postnatally, and the final 5% are infected with HSV in utero.
CMV infection also can occur at any of these three distinct times (intrauterine, peripartum, and postpartum). Congenital infection, though, is synonymous with in utero acquisition, and is clearly associated with long-term morbidity. In contrast, peripartum transmission can produce acute illness, but rarely if ever results in long-term sequelae. Infection of women both immediately before and during pregnancy puts the fetus at risk for congenital CMV infection. , In utero transmission occurs after primary maternal infection, as is the case with toxoplasmosis, rubella, and Zika (see later text), and also in recurrent infections, including reinfection with a different strain of the virus or reactivation of latent virus.
Zika virus infection in the Americas peaked in 2016, and then declined substantially through 2017 and 2018. Zika virus transmission has been found in all countries in the Region of the Americas except mainland Chile, Uruguay, and Canada, and is also found in Africa and parts of Asia and the Pacific Islands. Congenital Zika, like congenital CMV, is acquired most often in utero. This happens when a pregnant woman acquires Zika for the first time from the bite of an infected mosquito. The resulting primary infection can cross the placenta and infect the developing fetus.
SARS-CoV-2 infection spread throughout the world, starting initially in Wuhan, China at the end of 2019. A global pandemic was declared by WHO on March 11, 2020, and continues at the time of the writing of this chapter. There have been numerous reviews, meta-analyses, and national registries that have collectively evaluated thousands of infected pregnant women with SARS-CoV-2, and in totality found that less than 5% result in congenital SARS-CoV-2 infection. , , Currently, it is unclear what the exact timing of SARS-CoV-2 vertical transmission is. , Congenital SARS-CoV-2 infection can be acquired via vertical transmission (in utero) from an infected symptomatic or asymptomatic pregnant woman to neonate. Neonatal SARS-CoV-2 infection can also be acquired through intrapartum transmission, although it is quite rare. Finally, postpartum transmission seems to be the most common way of acquiring SARS-CoV-2. ,
The following five factors are known to influence transmission of HSV from mother to neonate:
Type of maternal infection (primary vs. recurrent)
Maternal antibody status
Duration of rupture of membranes
Integrity of mucocutaneous barriers (e.g., use of fetal scalp electrodes) , ,
Mode of delivery (cesarean section vs. vaginal)
Infants born to mothers who have a first episode of genital HSV infection near term are at much greater risk of developing neonatal herpes than are those whose mothers have recurrent genital herpes. This increased risk is due both to lower concentrations of transplacentally passaged HSV-specific antibodies (which also are less reactive to expressed polypeptides) in women with primary infection, and to the higher quantities of HSV that are shed for longer periods of time in the maternal genital tract when compared with women with recurrent genital HSV infection.
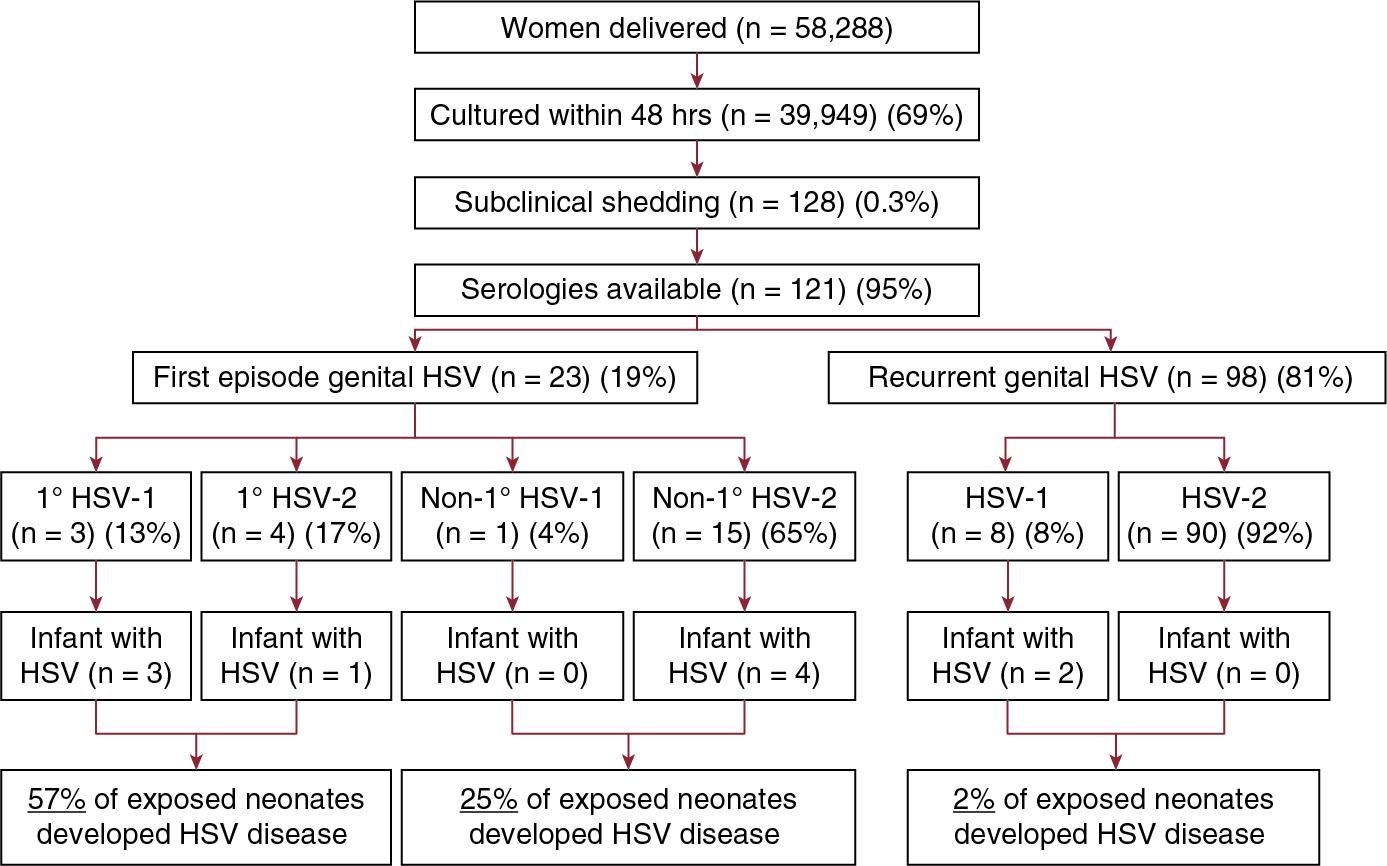
The largest assessment of the influence of type of maternal infection on likelihood of neonatal transmission is a landmark study involving almost 6 women in labor who did not have clinical evidence of genital HSV infection, ∼40,000 of whom had cultures performed within 48 hours of delivery ( Fig. 12.1 ). Of these, 121 women were identified who both were asymptomatically shedding HSV and for whom sera were available for serologic analysis. In this large trial, 57% of infants delivered to women with first-episode primary infection developed neonatal HSV disease, compared with 25% of infants delivered to women with first-episode nonprimary infection and 2% of infants delivered to women with recurrent HSV disease (see Fig. 12.1 ).
The duration of rupture of membranes and mode of delivery also appear to affect the risk for acquisition of neonatal infection. A small study published in 1971 demonstrated that cesarean delivery in a woman with active genital lesions can reduce the infant’s risk of acquiring HSV if performed within 4 hours of rupture of membrane. Based on this observation, it has been recommended for more than four decades that women with active genital lesions at the time of onset of labor be delivered by cesarean section. It was not until 2003, however, that cesarean delivery was definitively proven to be effective in the prevention of HSV transmission to the neonate from a mother actively shedding virus from the genital tract. Importantly, neonatal infection has occurred despite cesarean delivery performed before rupture of membranes. ,
Intrauterine infection usually is the result of a susceptible woman acquiring infection from a child in the family or from day care exposure early during her gestation. Multiple studies in Sweden and the United States have shown that the rate of CMV infection is much higher in children who attend day care than those who do not. , Many initially seronegative children become infected with CMV from their day care peers. CMV infection then is transmitted horizontally from child to child, most likely through saliva on hands and toys. , Infected children excrete large amounts of CMV for extended periods of time, exposing parents and other caregivers who may become pregnant.
Maternal shedding of virus directly correlates with the risk of perinatal infection. Infected breast milk and exposure to CMV in the genital tract lead to high rates of peripartum and postnatal CMV transmission. Infants who breastfeed from CMV-seropositive women have an estimated rate of infection between 39% and 59%. The risk is greater when the maternal viral load is higher than 7 × 10 genome equivalents/mL. Excretion of the virus in breast milk is greatest between 2 weeks and 2 months after birth. Infected infants usually begin to excrete CMV between 3 weeks and 3 months after birth. Many of these infants excrete CMV chronically (for years), providing an opportunity to infect caretakers or others in contact with these children.
Zika virus is acquired by a pregnant woman in one of three ways:
The bite of an infected mosquito to a nonimmune pregnant woman
Sexual transmission from a carrier to a pregnant woman
Transfusion of an infected blood product
The prevalence of Zika virus is related to the prevalence of the Aedes aegypti mosquito and the proportion of seronegative hosts. The greatest risk of serious sequelae for the fetus occurs in the first or second trimester but has also been reported in the third trimester. In a case series from Brazil, Zika virus caused adverse outcomes in 55% of infants when maternal infection occurred in the first trimester, in 52% of infants with maternal infection in the second trimester, and in 29% of infants with maternal infection in the third trimester.
SARS-CoV-2 acquisition is possible via in utero transmission, peripartum transmission, or via postpartum transmission. Current data are insufficient to elucidate the effects of SARS-CoV-2 on the fetus in first-, second-, or third-trimester infections. Thus far, reports have been overwhelmingly from pregnant women infected in their third trimester and their fetal and neonatal outcomes in third trimester. , One retrospective cohort study with 882 infected pregnant women showed that a subset of women infected in first and second trimesters had an increased risk for preterm birth and stillbirth, but that a second subset was completely unaffected despite early gestation infection. However, this study did not look at neonatal infections or maternal-to-fetal transmissions. Another retrospective cohort study including 17 hospitals in the United States found that pregnant women with SARS-CoV-2 infection prior to 28 weeks of gestation had an increased risk of fetal or neonatal death or preterm birth at <37 weeks of gestation. A systematic review including 936 neonates born to mothers with SARS-CoV-2 found only 3.2% rate of maternal-to-fetal transmission. This rate was similar to positivity transmission rates in China (2%) where there were stringent precautions for babies born to infected mothers, and in studies done outside of China (3.5%). Interestingly, in a national cohort study from Sweden which included 2323 neonates of infected mothers had only a 0.9% positivity rate, despite allowing rooming in of infant with mom, breastfeeding, and skin-to-skin.
HSV infections acquired either peripartum or postpartum can be classified as (1) disseminated disease involving multiple visceral organs, including lung, liver, adrenal glands, skin, eye, and the brain (disseminated disease); (2) CNS disease, with or without skin lesions (CNS disease); and (3) disease limited to the skin, eyes, and/or mouth (SEM disease). This classification system is predictive of both morbidity and mortality.
Neonatal HSV disseminated disease is manifest by hepatitis that can be very severe, disseminated intravascular coagulopathy, and pneumonitis. The mean age at presentation (±standard error [SE]) is 11.4 ± 0.8 days. CNS involvement is a common component of this category of infection, occurring in about 60% to 75% of infants with disseminated disease. Although the presence of a vesicular rash can greatly facilitate the diagnosis of HSV infection, more than 40% of neonates with disseminated HSV disease will not have cutaneous vesicles at the time of illness presentation. , , , Events associated with disseminated neonatal HSV infection that can result in death relate primarily to the severe coagulopathy, liver dysfunction, and pulmonary involvement of the disease.
Clinical manifestations of neonatal HSV CNS disease include seizures (both focal and generalized), lethargy, irritability, tremors, poor feeding, temperature instability, and bulging fontanelle. The mean age at presentation (±SE) is 19.7 ± 1.6 days. Between 60% and 70% of infants classified as having CNS disease have associated skin vesicles at any point in the disease course. , With CNS neonatal HSV disease, mortality is usually the product of devastating brain destruction, with resulting acute neurologic and autonomic dysfunction.
SEM disease is the most favorable of the presenting categories of neonatal HSV infection. By definition, infection in infants with SEM disease has not progressed to multiorgan, visceral involvement and does not involve the CNS. Presenting signs and symptoms can include skin vesicles in approximately 80% of patients, fever, lethargy, and/or conjunctivitis. The mean age at presentation (±SE) is 12.0 ± 2.2 days. There is a high degree of likelihood that, in the absence of antiviral therapy, SEM disease will progress to one of the more severe categories of neonatal HSV infection.
Congenital CMV infection is the most frequent known viral cause of mental retardation, and is the leading nongenetic cause of neurosensory hearing loss in many countries including the United States. It also is the most common congenital infection in humans, with approximately 0.5% of all live births in the United States involving CMV infection (∼20,000 infants per year). CMV can be acquired in utero during any trimester of pregnancy.
Of the fetuses infected, approximately 10% will be symptomatic at birth, and ∼20% of these patients will die in the neonatal period; of the survivors, 90% will have significant neurologic sequelae. The majority of these infants will have sensorineural hearing loss (SNHL), mental retardation, microcephaly, seizures, and/or paresis/paralysis. , These impairments frequently result in spastic quadriplegia requiring lifelong dependence on a wheelchair, along with cognitive and speech impairments that dramatically limit their ability to interact with and function in the world. Between 25% and 40% of all childhood, SNHL is caused by intrauterine CMV infection. Fetuses can be infected with CMV at any point throughout gestation. However, infections occurring earlier in gestation (first or early second trimesters) are more likely to result in severe forms of encephaloclastic injury.
Most infants (∼90%) with congenital CMV infection have no detectable clinical abnormalities at birth (asymptomatic infection), and SNHL develops in about 10% of these children. Because most infants with congenital CMV have asymptomatic infection, approximately 70% of CMV-associated SNHL occurs in this group, even though the likelihood of sequelae in any given asymptomatically infected child is much lower than in a symptomatically infected child ( Table 12.1 ).
| Estimated Number | |
|---|---|
| No. of live births per year | 4,000,000 |
| Rate of congenital cytomegalovirus infection | 1% |
| No. of infected infants | 40,000 |
| No. of infants symptomatic at birth (5%–7%) | 2800 |
| No. with fatal disease (±12%) | 336 |
| No. with sequelae (90% of survivors) | 2160 |
| No. of infants asymptomatic at birth (93%–95%) | 37,200 |
| No. with late sequelae (15%) | 5580 |
| Total no. with sequelae or fatal outcome | 8076 |
CMV-associated SNHL is extremely variable with respect to the age of onset, laterality, degree of the deficit, and continued deterioration of the loss (progression) during early childhood. , , About half of all children with CMV-associated SNHL have normal hearing at birth (delayed-onset SNHL) and therefore will not be detected by newborn hearing screening. Delayed-onset SNHL, threshold fluctuations, and/or progressive loss of hearing are observed in both symptomatic and asymptomatic infections. The age of onset of delayed-onset SNHL can range from 6 to 197 months. However, the median age is 33 and 44 months for symptomatic and asymptomatic children, respectively. , Therefore, neither routine physical examination in the nursery nor newborn hearing screening will identify the majority of children with CMV-associated SNHL at birth.
The natural history of congenitally acquired CMV infection is well described. , , , In contrast, outcomes of perinatally and postnatally acquired CMV infections are less well characterized. It is generally agreed that postnatal acquisition of CMV in term infants does not lead to symptomatology or disease. In preterm infants, initial case reports suggested that perinatally and postnatally acquired CMV infections could produce severe disease. Larger series and case-controlled trials more recently suggest that symptomatic disease in preterm infants is less common than asymptomatic infection, and long-term sequelae are rare. Nevertheless, severe disseminated CMV disease can occur in premature infants, including life-threatening pneumonitis, hepatitis, and thrombocytopenia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here