Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A significant number of deaths and permanent neurologic disabilities occur during the first month of life, the neonatal period. During the first month of life, term infants and particularly preterm neonates are vulnerable to a wide range of adverse events that may cause brain injury. Imaging is used not only to diagnose and understand neonatal brain injury but also to predict the neurodevelopmental outcome.
Because of its portability, cranial ultrasound is usually the first imaging modality to be performed and can be used serially to monitor the evolution of certain injuries. Ultrasound is usually sufficient for evaluation of germinal matrix hemorrhage (GMH) and intraventricular hemorrhage (IVH), serial assessment of ventricular size in hydrocephalus, cystic white matter changes, and severe brain malformations. Ultrasound is less sensitive than computed tomography (CT) or magnetic resonance imaging (MRI) in the detection of small calcifications and is less sensitive than MRI in the detection of hypoxic-ischemic injury and subtle brain malformations. MRI also is excellent for the evaluation of punctate white matter lesions, which are seen in the setting of premature white matter.
Because of the associated ionizing radiation, the use of CT is mainly restricted to instances in which there is a suspicion of intracranial calcifications, lesions that contain fat, acute intracranial hemorrhage, and to evaluate the bones. MRI is the most sophisticated modality to evaluate the neonatal brain, and with advanced imaging techniques such as diffusion-weighted imaging (DWI), functional magnetic resonance imaging (fMRI), and magnetic resonance spectroscopy (MRS), it has the advantage of providing information regarding physiology, function, and metabolism. Arterial spin labeling techniques provide assessment of brain perfusion in the neonate without the use of intravenous contrast material. Intravenous contrast is not routinely used because of the physiologic renal immaturity in the first few days to weeks after birth.
High-resolution images with good tissue contrast are essential for an adequate evaluation of the neonatal brain. Ultrasound provides high-resolution images of the neonatal brain, but it has limitations regarding visualization of deeper structures and the cerebellum. Because increased tissue contrast in CT usually is achieved at the expense of increased radiation dose, CT usually is performed with use of low radiation dose protocols and is reserved for a limited number of situations.
MRI is the imaging modality with the highest sensitivity for differentiating abnormalities from normal brain. Conventional MRI, including T1-weighted, T2-weighted, DWI, and gradient-echo sequences or susceptibility-weighted images, provide the most useful diagnostic information. Sequences should be modified to optimize signal to noise for the neonatal brain. Structurally, the brains of preterm infants and neonates have much higher water content and much lower lipid content compared with brains of older children. With myelination, these compositions invert. Myelination begins along specific tracts in the third trimester and continues well into the postnatal years. Longer echo times are useful for younger patients because of the differences of extracellular lengthening of the T2-relaxation time with decreasing age. Similarly, the T1 relaxation is longer for infants and young children and varies with the magnetic field strength. Longer inversion times of T1-weighted scans provide additional contrast between myelinated and unmyelinated white matter in infants. Finally, the higher water content and lower anisotropy in infants necessitate a lower b-values (600–700 s/mm 2 ) to optimize signal to noise in DWI.
Dedicated neonatal MR head coils improve image contrast and resolution at the smaller field of view optimal for neonatal imaging. These dedicated coils improve gray-white matter differentiation and provide better visualization of the brainstem and posterior fossa. If a dedicated neonatal head coil is not available, coils for small body parts (i.e., knee coil) should be used to improve image quality.
Ultrasound can be performed at the bedside and does not use contrast material or ionizing radiation. The only precaution with ultrasound is prevention of infection, which can be achieved with use of sterile gel and a probe cover. CT scanning time is short but usually requires transportation of the neonate to the scanner. Portable CT scanners may be useful in selected patients. Contrast material is rarely needed and should be avoided in neonates because of their physiologic renal immaturity, which is present in the first few days to weeks after birth.
Because of the magnetic resonance (MR) environment and length of most MRI examinations, MR safety is a particular concern for neonates. Before the MR examination, any patient, including neonates, should be screened for possible cardiac devices, implants, non–MR-compatible leads, or surgically implanted wires. The compatibility of any device must always be verified with the manufacturer. Continuous monitoring and support for respiratory and cardiovascular functions can be achieved with MR-compatible equipment. Thermoregulation, which can be a particular concern for preterm neonates, can be supported through use of MR-compatible incubators and monitored through use of an MR-compatible temperature probe. Special attention should be given for loops in conductive cables, which may cause burns and possibly fires.
Increasingly, neonates undergoing MRI are scanned during natural sleep (“feed and bundle” procedures). Although acoustic noise and table vibration from the MR scanner may awaken an older infant (i.e., >3 months of age, a developmental stage at which infants normally begin to awaken to startling noises), young infants (<3 months) often tolerate even long MR protocols when imaging is performed during natural sleep. At our institution, we routinely use MR-compatible noise-canceling headphones designed for neonates. When imaging during natural sleep is not possible, neonates and older infants are scanned while sedated. Sedation should be performed only by properly trained and credentialed clinicians.
Birth trauma is more common in vaginal delivery, particularly if forceps or vacuum extraction is used. The most common traumatic birth lesions are extracranial hematomas, skull fractures, osteodiastasis, and subdural hematomas.
The major types of extracranial hematomas are cephalohematoma, caput succedaneum, and subgaleal hematoma (also see Chapter 23 ). In subgaleal hematomas, the hemorrhage accumulates between the epicranial aponeurosis of the scalp and the periosteum ( e-Fig. 30.1 ). The hematoma may enlarge rapidly and lead to potentially life-threatening hypovolemia. Anemia, coagulopathy, metabolic acidosis, renal impairment, and skull fracture have been reported as predictors of poor outcome. Early recognition and management are essential. Upon imaging, extracranial hematomas appear as a hemorrhagic collection overlying the calvarium and crossing the sutures. They may extend underneath the attachments of the occipitofrontalis muscle to the orbital margins anteriorly, the temporal fascia laterally, and the nuchal ridge posteriorly ( e-Fig. 30.2 ).

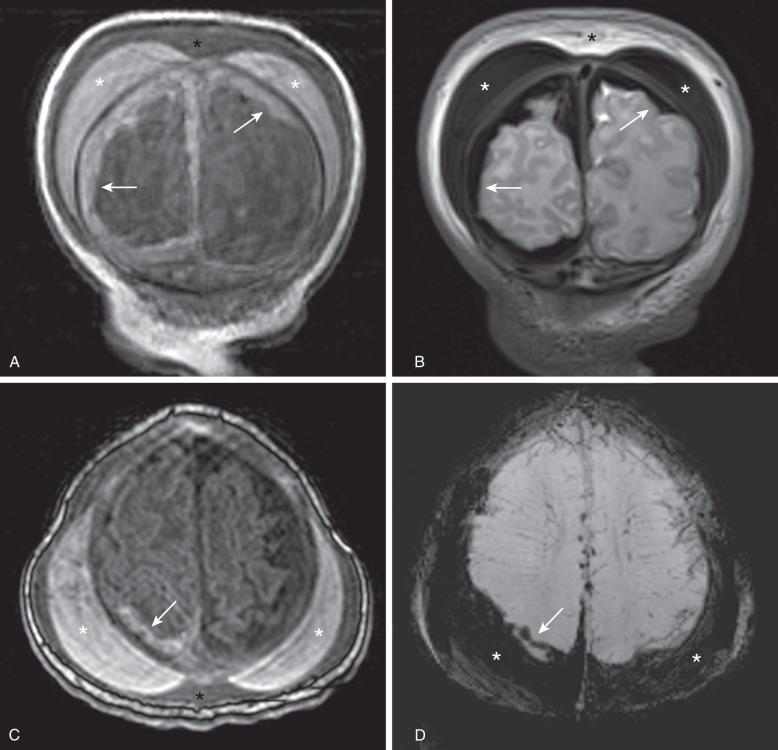
Caput succedaneum is common after vaginal delivery, particularly when vacuum extraction is used. Typically, resolution occurs within a few days without complications. Caput succedaneum represents edema, which may be accompanied by hemorrhage, within the subcutaneous tissues, which are not limited by the cranial sutures (see e-Fig. 30.2 ).
Cephalohematomas are rarely associated with complications but may be associated with skull fracture and epidural hematomas. Blood degradation within a hematoma may cause jaundice. Cephalohematomas are subperiosteal hemorrhages confined by cranial sutures. Typically, hematomas increase in size after birth, and a small proportion of them calcify and may cause skull deformity (calcified cephalohematoma).
The most common intracranial hemorrhage in term neonates is subdural hematoma, and most common is asymptomatic in parafalcine location or over the tentorium. The incidence of these small subdural hematomas in neonates has been reported as high as 46% in the first 72 hours after birth. Most subdural hematomas are venous in origin related to tearing of the dura, inferior sagittal sinus, and cortical veins. The typical appearance of subdural hematomas is of an extraaxial collection that may cross under sutures, but respect the dural reflection, such as the falx and tentorium (see e-Fig. 30.2 ). Arterial subdural hematomas caused by arterial bleeding are rare but should be suspected in large hyperacute subdural hematomas. Most lethal cases are related to large infratentorial hemorrhages with compression of the brainstem. Associated small subarachnoid hemorrhages related to tearing of cortical veins are not infrequent. Large subdural hematomas may cause parenchymal infarction as a result of impaired arterial supply and venous drainage, leading to hemorrhagic venous infarction. Large subdural hematomas may interfere with cerebrospinal fluid (CSF) reabsorption and cause hydrocephalus. Subdural hematomas that are not evacuated undergo absorption or evolve into subdural hygromas, which can persist for several months.
Finally, late subdural hematomas after the first week of life may be a presentation of a nonaccidental trauma, in addition to late hemorrhagic disease of the newborn (vitamin K deficiency).
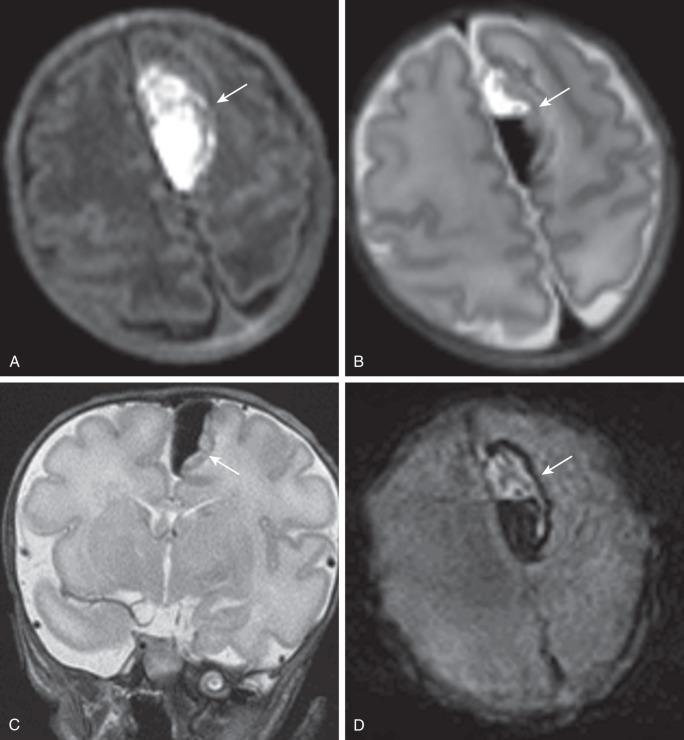
Spontaneous superficial parenchymal and leptomeningeal (subpial) hemorrhage may also occur in otherwise healthy asymptomatic neonates. They are most common in the temporal lobes, adjacent to a suture. The hemorrhage may extend into the adjacent sulci but is confined by the pia matter ( e-Fig. 30.3 ). Mild surrounding decreased diffusion is typically present.
Brain damage in the term neonate is highly variable and depends on the severity and duration of insult. Moreover, the imaging findings vary dramatically in relation to the timing of imaging studies. Imaging before 72 hours may underestimate the severity because of delayed cell death. Therapeutic hypothermia, which typically is applied for 72 hours beginning within 6 hours of life, may further delay this process.
The central pattern of hypoxic-ischemic encephalopathy (HIE) usually occurs with profound asphyxia, when there is an abrupt interruption of the blood supply, depriving the neonatal brain of oxygen and glucose. Highly metabolic structures such as the thalami, basal ganglia, and brainstem are more vulnerable to hypoxia and ischemia, more specifically the ventral lateral thalami, posterolateral lentiform nuclei, posterior midbrain, hippocampi, lateral geniculate nuclei, and perirolandic cerebral cortex ( Fig. 30.4 ). Quadriparesis, choreoathetosis, seizures, mental retardation, and cerebral palsy have been associated with profound asphyxia. Ultrasound and CT have low sensitivity to detect early ischemic changes in the deep structures of the brain. The most common pattern on ultrasound is transient or persistent hyperechogenicity, which may progress to cavitation in the basal ganglia and thalami, particularly in the globus pallidus and ventral lateral nuclei of the thalamus. With more severe insults involving the cortex and subcortical white matter, ultrasound and CT may depict indirect evidence of edema, such as effacement of the sulci, loss of gray-white matter differentiation, and compressed lateral ventricles.
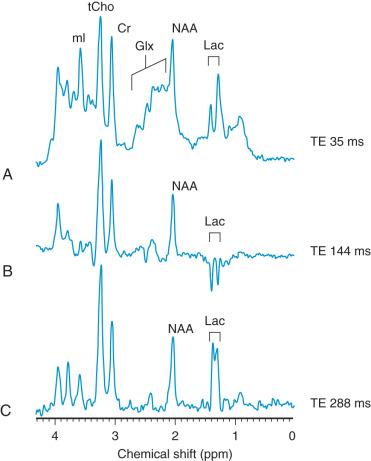
MRI is the imaging modality of choice for neonatal encephalopathy. MRS performed 24 hours after the insult is considered sensitive for hypoxic-ischemic brain injury. Elevated lactate and diminished N-acetyl-aspartate (NAA) are the most common MRS findings in neonates with neurologic and developmental abnormalities ( e-Fig. 30.5 ). Lactate rises after the hypoxic-ischemic event, peaking at 3 to 5 days, whereas NAA starts to decline around the third day. Although a minimally elevated lactate level (lactate/choline ratio <0.15) may be detected in the normal neonatal brain at term, an increased lactate level relative to the total creatine peak in the basal ganglia provides an early indication of brain injury. It has been suggested that resuscitation may rapidly clear lactate and that a secondary increase in lactate may occur after 12 to 24 hours. This may cause false-negative MRS with normal spectra but abnormal outcomes. The metabolites tend to normalize after about day 5, although in some cases abnormal metabolite ratios persist. Persistently elevated lactate levels in the basal ganglia provide prognostic information about the severity of the brain injury and the subsequent adverse neurodevelopmental outcome.
DWI is a sensitive technique for assessment of acute brain injury and shows deep gray matter and perirolandic gray matter lesions before they are seen with conventional MRI. If DWI is performed in the first few hours after the injury, it may underestimate the extent of the injury or even show normal results. Paralleling the clinical presentation of HIE, in which neonates may actually transiently improve before demonstrating a more permanent decline in neurologic functioning because of delayed cell death via apoptotic mechanisms, some patients demonstrate mild brain damage for the first few days and then proceed to demonstrate extensive brain involvement around 5 days after the injury. Apparent diffusion coefficient (ADC) values always evolve over time; they decrease initially after the injury, with the nadir around 3 to 5 days, and increase (via facilitated diffusion) later in the chronic phase. As ADC values increase, a point of transient “pseudonormalization” exists in which injured tissue can be misdiagnosed as “normal.” Conventional MRI findings are usually unremarkable during the first few days but then begin to demonstrate first an increased T1-weighted signal and a decreased T2-weighted signal in the subacute period followed by an increased T2-weighted signal in the more chronic period. Evidence suggests that hypothermia therapy delays the onset of MR changes (in metabolic, diffusion, and conventional imaging) associated with injury and, in particular, delays the onset of pseudonormalization in the ADC signal.
The peripheral pattern of HIE usually results from a period of decreased blood supply to the brain (rather than a near total and abrupt interruption) and is thought to develop as a result of a compensatory shunting of blood to vital brain structures, such as the brainstem, thalami, basal ganglia, hippocampi, and cerebellum, at the expense of less metabolically active structures, namely the cerebral cortex and white matter ( Fig. 30.6 ). Therefore the brainstem, cerebellum, and deep gray matter structures generally are spared from injury in mild to moderate hypoxic-ischemic insults. More prolonged insults result in injury to the intervascular border (watershed) zones, which are relatively hypoperfused as a result of this shunting. Neurologic examination varies depending on the severity of the insult, from asymptomatic in mild cases to proximal extremity weakness or spasticity and cerebral palsy in persons who sustain severe insults. Increasing severity of watershed-distribution injury is associated with impaired neurocognitive functioning, including language, visuoperceptual, and executive functioning impairments.

Ultrasound lacks sensitivity in assessing partial prolonged hypoxia-ischemia because it provides poor visualization of the triple watershed zone. CT also is not sensitive to the early changes but may show effacement of the gray-white junction, hypoattenuation with mass effect from acute edema, or hypoattenuation with volume loss in the watershed zone. Similar to central HIE, in the acute phase, MRI can detect lactate and restricted diffusion with corresponding low ADC values in the affected brain regions, which predominantly involve the cortex and underlying white matter along the parasagittal frontal-parietal cortex. With time, increased T2/fluid-attenuated inversion recovery signal and mass effect related to edema may develop. Chronically, gliosis and volume loss mainly involving the deep portion of the gyri result in mushroom-shaped gyri (known as ulegyria), which sometimes is associated with an epileptogenic focus. Atrophic changes and gliosis predominately involve the subcortical white matter in the border zone between the anterior and the middle cerebral arteries, in the parasagittal watershed zone, and in the parietal lobes at the border zone of the three major cerebral arteries, that is, the triple watershed zone.
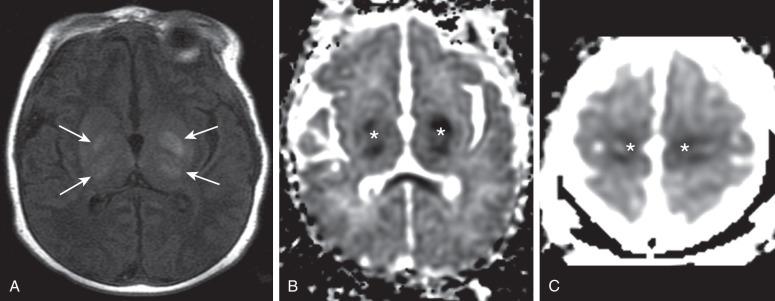
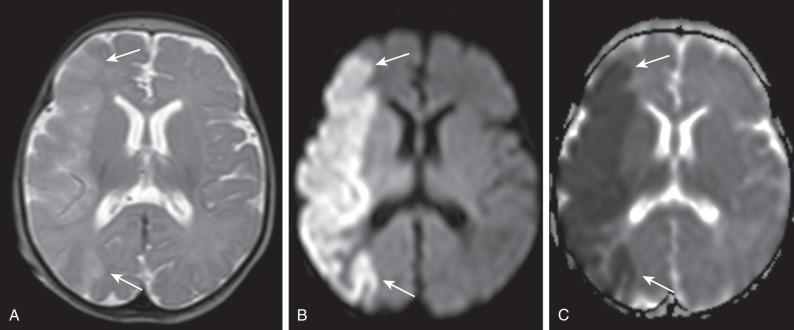
Infarctions involving the vascular territory of a major artery, most commonly the middle cerebral artery, are more common in term than in preterm neonates. Symptoms are subtle and nonspecific, and many neonatal arterial infarcts may be unrecognized until motor or cognitive symptoms develop later in infancy or childhood. In the neonatal period, the most common presenting symptom is a focal motor seizure involving the contralateral limbs, which may secondarily generalize. Infarcts of the anterior and posterior cerebral arteries are underdetected because they may be asymptomatic and difficult to visualize on ultrasound. Several causes have been described, including sepsis, bacterial meningitis, inherited or acquired coagulopathies, and cardiac abnormalities, but often no specific cause is identified. Initial imaging shows a loss of gray-white matter differentiation, which in severe instances may be detected on ultrasound but is most readily visualized on MRI as increased signal on DWI with low ADC values ( Fig. 30.7 ). Later (after 24–48 hours) the edema becomes apparent as increased echogenicity on ultrasound, hypoattenuation on CT, increased signal on T2-weighted MRI, and decreased signal on T1-weighted MRI. Although the high water content of the unmyelinated neonatal brain poses significant challenges for the detection of edema on CT or conventional MRI sequences, loss of gray-white matter differentiation frequently is present. This lack of contrast between the normal brain and the infarction is particularly relevant at 5 to 10 days after infarction when the ADC may be pseudonormalized. With time, ADC values increase (via facilitated diffusion); after a few weeks, volume loss and encephalomalacia become apparent. Hemorrhagic transformation of an ischemic stroke is rare in neonates, but increased signal on T1-weighted imaging along the cortex related to laminar necrosis is not uncommon. Interestingly, acutely after a perinatal ischemic infarct, hyperperfusion as demonstrated on arterial spin label sequences frequently is observed in the regions corresponding to areas of low ADC on diffusion-weighted MRI.
Intraparenchymal hemorrhage occurs infrequently in full-term neonates. Most parenchymal bleeding in the term infant involves the thalamus, is associated with IVH, and develops in infants at risk for venous thrombosis. The most documented cause of intraparenchymal hemorrhage is cerebrovenous sinus thrombosis, followed by coagulopathy, infection, hypoglycemia, vascular malformation, tumor, and genetic disorders. A hemorrhagic and thrombotic screening is the first step in any neonate with intracranial hemorrhage. Additionally, confirmation of vitamin K administration and examination of alloimmune antibodies in the mother's blood should be obtained. Although hemorrhage may occur anywhere in the term neonatal brain, the thalamus and the choroid plexus are the most common locations. Rarely, hemorrhages occur in areas of residual germinal matrix (GM). Blood degradation products without surrounding edema or hydrocephalus with chronic blood products in the first days of life are suggestive of intrauterine hemorrhage, which are most commonly caused by maternal factors such as anticoagulants, vasogenic agents (including illicit drugs), diabetes, and trauma. Less commonly, fetal conditions such as arteriovenous malformation (AVM and vein of Galen malformation), congenital brain tumor, and genetic disorders may lead to prenatal hemorrhage.
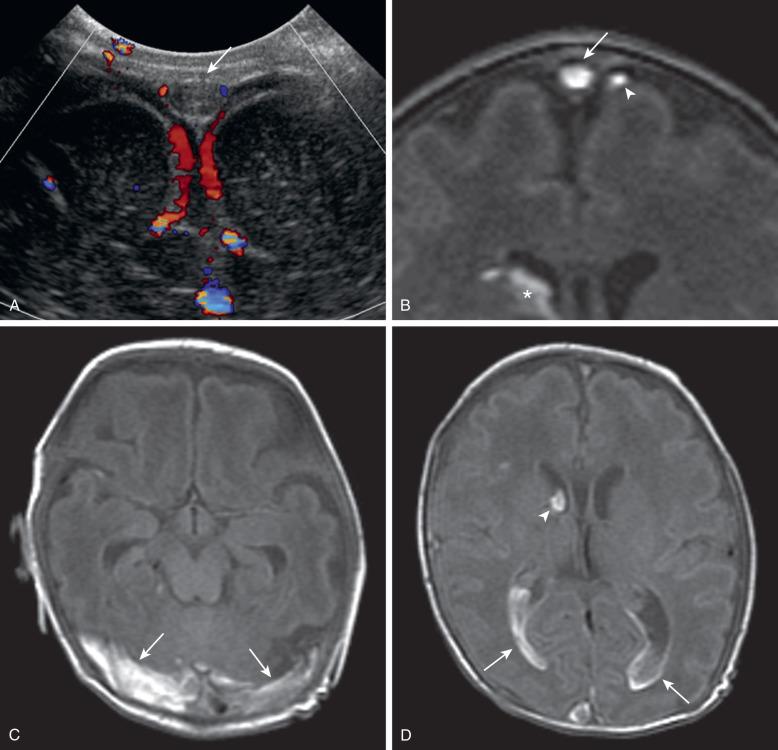
Neonatal cerebrovenous sinus thrombosis (CVST) is a rare but devastating condition. It may be a result of trauma, increased hematocrit, sepsis, dehydration, cardiac failure, and thrombotic disorders such as factor V Leiden. The impaired venous drainage commonly results in cytotoxic edema, vasogenic edema, and parenchymal hemorrhage, and in more severe cases acute or chronic hydrocephalus. Although venous infarction can occur anywhere in the brain, the most characteristic locations are the thalami, resulting from thrombosis of the straight sinus and vein of Galen, and the bilateral parasagittal cortex and subcortical white matter, resulting from thrombosis of the superior sagittal sinus. Diffuse cerebral swelling may be a result of extensive CVST.
The diagnosis of CVST may be achieved by demonstrating the thrombus or the lack of normal venous flow in the venous sinuses. Doppler ultrasound may detect superior sagittal sinus thrombosis in neonates, but it has limited sensitivity in evaluating the remainder of the venous system ( Fig. 30.8 ). Serial Doppler ultrasound is an easily performed and inexpensive alternative for monitoring sinovenous thrombosis. CT without contrast may demonstrate a hyperattenuating sinus with a large thrombus but has limited value for small thrombi. False-positive results can occur with unenhanced CT because of relative hyperattenuation of normal neonatal blood due to the high hematocrit and the adjacent hypoattenuating nonmyelinated brain tissue. Moreover, hyperattenuating subdural hematomas that are present in up to a third of the neonates can be misinterpreted as a thrombus. The demonstration of filling defects using CT venography is confirmatory of CVST. Beam hardening artifact from dense bone adjacent to the dural sinus and normal arachnoid granulations may mimic a filling defect. The normal dural sinus and veins have flow voids on unenhanced MRI. Phase-contrast MR venography and two-dimensional time-of-flight MR venography are specific for flow and are valuable in demonstrating obstruction. High signal on unenhanced MRI or lack of flow-related signal on two-dimensional time-of-flight MR venography can be related to thrombus or slow flow. Thin-slice images oriented perpendicular to the flow in the sinus with an abnormal signal is usually sufficient to correct for this “artifact.” MR venography and contrast-enhanced MRI are complementary to unenhanced MRI to show the filling defect and to differentiate venous thrombosis from adjacent subdural hematoma. The best unenhanced conventional MR sequences for detection of sinus venous thrombosis are spin echo T1 and proton density images (see Fig. 30.8 ). Although full anticoagulation is controversial in the neonatal period, CVST is a medical emergency, and a definite diagnosis should be confirmed as soon as possible. When the aforementioned diagnostic modalities are inconclusive, digital subtraction angiography is the gold standard.
Most vascular malformations are silent during the neonatal period but may be diagnosed prenatally or incidentally. A full description of all the major vascular malformations is beyond the scope of this chapter. Instead, we will focus on those that more commonly manifest in the neonatal period: cerebral (pial) vascular malformations, aneurismal malformation of the vein of Galen, and malformation of the dural sinuses.
AVMs are abnormal thin-walled vessels that connect arteries to veins without intervening capillaries. Most cases of AVM manifest later in childhood or in early adulthood. Neonates with an AVM tend to present with systemic cardiac manifestations or seizures related to parenchymal hypoperfusion. Macrocephaly and hydrocephalus related to abnormally increased venous pressure and spontaneous hemorrhage are less common manifestations of AVMs in the neonatal period.
In symptomatic patients, early diagnosis and emergency endovascular embolization is important because progression to atrophy and leukomalacia may occur rapidly. Unenhanced CT is useful to detect acute hemorrhaging, but it lacks the sensitivity necessary to detect the underlying vascular malformation. CT angiography (CTA) involves radiation exposure but provides exquisite anatomic detail of a nidus (if present), feeding arteries, draining veins, and the presence of possible aneurysms. MR angiography (MRA) is excellent for the detection of hemorrhage and to delineate the AVM, although smaller vascular malformations may go undetected. The flow void seen in the tangled vessel of the nidus has been described as a “bag of black worms.” High-resolution T1-weighted sequences are important to define the location of the nidus. Three-dimensional (3D) time-of-flight MRA has slightly less special resolution than does CTA, but it does not expose the neonate to radiation. Time-resolved MRA has less special resolution than 3D time-of-flight (TOF) MRA and CTA, but it may provide sufficient information to differentiate feeding arteries and draining veins.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here