Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The detection of residual myocardial viability in a patient with regional or global severe left ventricular (LV) dysfunction is of clinical importance to plan the therapeutic strategy because revascularization of dysfunctional but viable myocardium may improve LV function. Several imaging techniques have been shown to be successful in detecting myocardial viability; these include LV angiography using appropriate interventions, perfusion scintigraphy, positron emission tomography (PET), and echocardiography. More recently, cardiovascular magnetic resonance (CMR) has gained widespread acceptance as a technique to identify viable myocardium and distinguish it from myocardial necrosis and scar. This chapter reviews the current knowledge of how these techniques can be used in humans to guide clinical decision making and predict recovery of function after revascularization of dysfunctional myocardium.
All scientific papers on the value of imaging techniques for detecting myocardial viability use the well-known statistical terms of sensitivity, specificity, and the predictive values. The sensitivity of a test is commonly defined as the number of true positives divided by the sum of true positives and false negatives. In other words, the sensitivity of a test is the number of diseased persons with a positive test divided by the total number of diseased persons. Common sense might suggest that scar tissue and, hence, absence of viability might indicate the presence of disease. The total number of diseased people or segments would thus be the number of people with scar or segments without recovery of function. The presence of viability would be synonymous with a healthy state (relatively speaking), and the total number of viable, healthy people or segments would appear as the denominator in the formula for calculating specificity. In the viability literature, however, sensitivity indicates the ability of a test to identify viable myocardium, and specificity is an indicator of how well the test performs when it comes to detecting scar. Positive and negative predictive values are used accordingly. This needs to be borne in mind when it comes to the interpretation of test results reported later in this chapter.
Myocardium is commonly defined as viable if it shows severe dysfunction at baseline but recovers function with time either spontaneously (myocardial stunning) or following revascularization (hibernating myocardium). Clinically, stunned myocardium may be found in patients with early reperfusion of an infarct-related artery. If there is no residual high-grade stenosis, blood flow at rest will be normal and the myocardium will recover spontaneously after a few days. Patients with hibernating myocardium often present with severe triple vessel disease, globally depressed LV function, and prominent dyspnea but often surprisingly little angina. This type of dysfunction is often more chronic, and previous myocardial infarction may or may not be reported in the history. Pathology may reveal regions of transmural scar, regions with predominantly subendocardial scar, and regions with mixtures of scar and viable myocardium.
Severe wall thinning is the hallmark of transmural chronic myocardial infarction. However, wall thinning is the end result of infarct healing, and it may take up to 4 months before the remodeling process is completed. In contrast to the severe thinning of chronic transmural scar, the best example for which is the thin-walled anterior LV aneurysm, acute and subacute transmural infarcts may not yet have reached the stage of thinning because local infarct remodeling is incomplete. In contrast with transmural myocardial infarction, which may or may not appear thinned, depending on infarct age, healed nontransmural infarcts usually do not develop severe thinning. Some thinning may be observed, however, depending on the degree to which the endocardially located infarct extends throughout the wall. Therefore the finding of preserved myocardial wall thickness in diastole in a patient with a known chronic infarct that is more than 4 months old will likely represent nontransmural infarction with a substantial rim of viable myocardium surrounding the endocardial scar. If the infarct is more recent than approximately 4 months, preserved end-diastolic wall thickness cannot be used to distinguish between viable and nonviable myocardium.
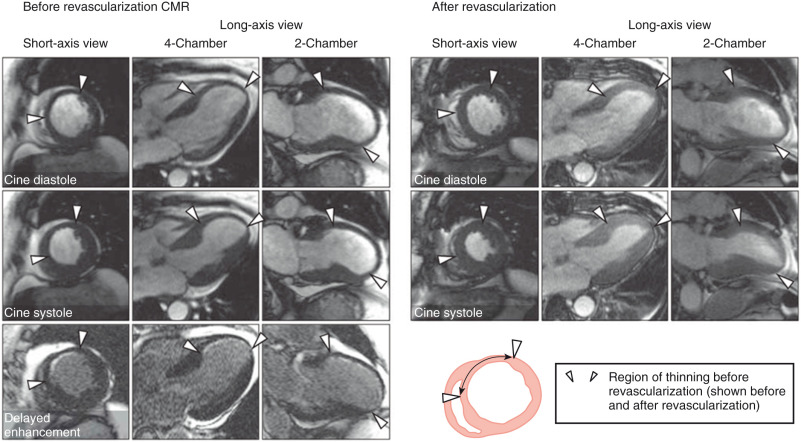
Patients with small subendocardial infarcts may, however, also present with regional wall thinning despite the presence of substantial amounts (>50% of wall thickness) of viable myocardium. In a case described by Kim and Shah, diastolic thickness of the anterior wall on a long-axis late gadolinium enhancement (LGE) CMR image measured only 5 mm. However, the subendocardial rim of scar was only 1.5 mm of total wall thickness, indicating the presence of substantial amounts of viable myocardium. Indeed, recovery of the myocardium occurred following revascularization. Shah et al. systematically looked at more than 1000 consecutive patients who had LGE CMR for viability assessment and found that 19% of them had regional wall thinning. Within these regions, the extent of scarring was 72%. However, 18% of thinned regions had only limited scar burden (≤50% of total extent). Among patients with thinning undergoing revascularization and follow-up cine CMR ( n = 42), scar extent within the thinned region was inversely related to regional and global contractile improvement. End-diastolic wall thickness in thinned regions with limited scar burden increased from 4.4 mm to 7.5 mm after revascularization ( P < .001) with resolution of wall thinning ( Fig. 21.1 ). Thus regional wall thinning may be possible in myocardium that has only little subendocardial scarring probably because of ventricular remodeling, and full recovery of function may occur following revascularization. The ratio of viable to total myocardium (viable plus nonviable) irrespective of wall thickness in the dysfunctional region may therefore be more accurate than end-diastolic wall thickness in predicting functional improvement.
A well-known feature of viable myocardium is augmented contractility in response to a suitable stimulus. Such stimuli include sympathomimetic agents or postextrasystolic potentiation. In contrast, necrotic or scarred tissue will not respond to such stimulation. Today, the most widely used mode of stimulation is to infuse low doses of dobutamine up to 10 µg/kg/min. If a contractile reserve can be elicited, the responsive myocardium will usually recover function after appropriate revascularization. However, it appears that there is also some spontaneous improvement in the response to dobutamine over the course of infarct healing after reperfused myocardial infarction, which may affect the accuracy of this viability marker after myocardial infarction.
Irreversible myocardial damage occurs after approximately 30 to 120 minutes of ischemia. Very early changes can be observed by electron microscopy; these changes include intracellular edema and swelling of the entire cell, including the mitochondria. The sarcolemma ruptures, and there is free exchange between the extracellular and intracellular compartments. In some infarcts, light microscopy reveals changes just a few hours after the onset of ischemia; these changes are most pronounced at the periphery of the infarct. After 8 hours, there is edema of the interstitium, and infiltration of the infarct zone by neutrophils and red blood cells becomes evident. Small blood vessels undergo necrosis, and karyolysis of muscle cell nuclei can be observed. Plugging of capillaries by erythrocytes is most pronounced in the center of the infarct. If reperfusion can be achieved at an early stage, the resulting infarcts contain a mixture of necrosis and hemorrhage within zones of irreversibly injured myocytes.
Myocardial edema is associated with prolonged magnetic resonance (MR) relaxation times, and this leads to characteristically increased signal intensity on MR images, which are sensitive to such changes. By using modern T2-weighted pulse sequences, the edema associated with acute infarcts can be depicted, and this can be used to differentiate between acute and chronic infarcts.
A feature of the central necrotic region within a myocardial infarct is intracapillary red blood cell stasis. Plugging of the capillaries leads to tissue hypoperfusion. This hypoperfusion is primarily related to the resulting reduced functional capillary density rather than reduced microvascular flow rates. This decrease in functional capillary density results in a prolonged washin time constant. This lack of reperfusion despite restoration of flow in the epicardial vessel is known as the no-reflow phenomenon . When the myocardium is imaged by CMR early after injection with gadolinium (early gadolinium enhancement), no-reflow zones appear dark in comparison with the surrounding rim regions of the infarct.
Gadolinium chelates are commonly used as CMR contrast agents. These metabolically inert molecules are distributed extracellularly, and they shorten both T1 and T2 relaxation times. Rupture of myocyte membranes leads to an increased volume of distribution of CMR contrast agents with a corresponding increase in the effective voxel concentration of such agents. Thus a higher concentration of gadolinium contrast agents leads to a more pronounced shortening of relaxation times. CMR images are usually T1 weighted (because the RR interval is approximately 800 ms, which corresponds to the T1 value of myocardium), and this will result in a higher signal intensity of infarcted as compared with normal tissue once the contrast material has fully penetrated the infarct region. The time–concentration curve of MR contrast agents in infarct tissue does not correspond to that in blood or normal tissue kinetics. Thus, while early hypoenhancement of infarcted regions after injection of contrast material is caused by delayed contrast penetration, late enhancement in infarction is due to both increased volume of distribution and slow contrast washout. The enhancement pattern that is seen will depend on regional differences in tissue washin/washout kinetics, as well as the time after injection of contrast when the image is acquired. LGE has now been extensively validated in animal and human studies, and with improved imaging sequences (notably the use of inversion recovery to null signal from normal myocardium), the signal-to-noise ratio of enhanced to unenhanced tissue is dramatically higher than with previous sequences, at approximately 500%. This has led to greatly improved image quality and a substantial increase in use of the technique. In animal experiments, the area of LGE has been shown to correlate closely with areas of infarction, and for the first time in vivo, high-quality imaging of the distribution of scar is possible.
The primary energy reserve in living myocardial cells is stored in the form of creatine phosphate and adenosine triphosphate (ATP). Depletion of total myocardial creatine, creatine phosphate, and ATP follows severe ischemic injury, as shown in biopsy samples obtained from patients during cardiac surgery or necropsy. Using P magnetic resonance spectroscopy (MRS), it is possible to measure the myocardial content of phosphocreatine and ATP. 1 H-MRS has a higher sensitivity than 31 P-MRS and has the ability to detect the total pool of phosphorylated plus unphosphorylated creatine in skeletal and cardiac muscle. MRS is currently not used clinically and is not discussed further in this chapter.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here