Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute myocardial infarction (MI) is defined as death of the myocytes (myocardial necrosis) in a clinical setting consistent with acute myocardial ischemia [ ]. Echocardiography, nuclear imaging techniques, CMR, and CT play an important role in detecting and following patients with acute MI (AMI).
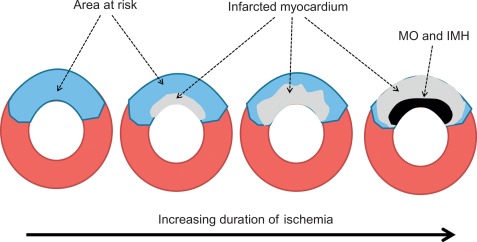
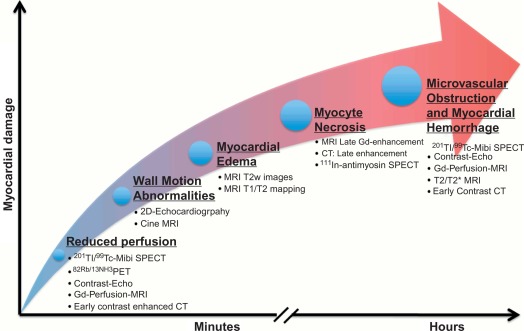
AMI is most often caused by acute coronary artery plaque rupture and thrombosis, but it may also occur due to vasospasm, myocardial supply-demand imbalance with or without associated stable coronary artery disease (CAD), or after revascularization procedures. AMI develops generally when myocardial ischemia lasts for more than 20 min. Because myocardial workload is higher and perfusion is less in sub-endocardial areas, infarcts spread in a wavefront phenomenon from sub-endocardial to epicardial layers with increasing duration of ischemia [ ] ( Figure 10.1 ). Complete transmural necrosis of the ischemic area of risk usually occurs when ischemia lasts longer than 4–6 h, depending on the severity of the coronary artery occlusion, the presence of collateral circulation, preconditioning, and the individual oxygen demand/supply balance. Intermittent durations of ischemia less than 4–6 h result therefore in incomplete mostly subendocardial necrosis, in which part of the area at risk remains viable. This concept is fundamental to current revascularization therapy of AMI. Indeed, modern treatment strategies of AMI aim at opening the infarct-related artery as quickly as possible, in order to reduce the duration of ischemia and to save viable myocardium in the risk area. The importance of saving such viable myocardium was underscored by the recent OAT trial [ ], which demonstrated no benefit of opening the infarct-related artery by PCI in patients having suffered an STEMI more than 3 days ago, likely because in such subacute STEMI the window of action for saving residual viable myocardium had expired. Experimental studies showed that additional myocardial injury may occur at the time of reperfusion or shortly thereafter [ ]. This suggests that the relation between risk area and final MI size for a given duration of occlusion could be still modified at the time of reperfusion by administration of “cardioprotective” drugs; however, clinically, none of such drugs are currently available.
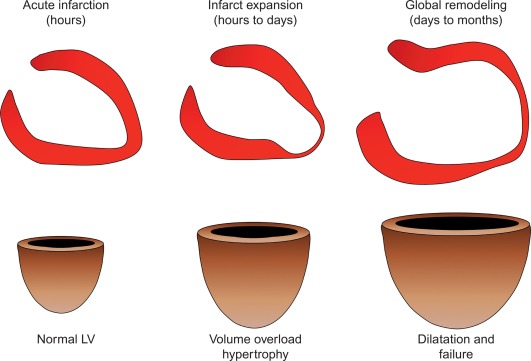
By histology, AMI is characterized by coagulation and contraction band necrosis of myocytes. In addition, AMI leads to the destruction of the extracellular matrix, and in the center of large infarcts, to destruction and occlusion of the microvasculature (the so-called no-reflow phenomenon) [ ], may be complicated by intramyocardial hemorrhage ( Figure 10.2 ). Non-infarcted post-ischemic areas may present with myocyte and interstitial edema. Subsequent to the acute injury is a complex healing process occurring in several different phases both in the infarcted area, the MI border zone, and in remote non-infarcted areas [ , ]. In the infarcted area, after an initial inflammatory phase leading to detersion of dead myocytes and other cellular debris, follows a proliferative phase characterized by neoangiogenesis and myofibroblast proliferation aiming to reconstitute a new extracellular matrix. Finally, MI healing is concluded when mature fibroblasts deposit a solid collagen-based extracellular scar (replacement fibrosis), approximately 4 weeks after the acute injury. During the phase of MI healing, the left ventricle (LV) can undergo significant remodeling ( Figures 10.3 and 10.4 ) caused by different underlying mechanisms: In the first days after infarction, the infarcted area may present significant expansion through sliding of myocytes during the inflammatory phase before collagen deposition has stabilized the extracellular matrix. This occurs particularly in areas where the extracellular matrix and microvasculature have been heavily damaged, such as in areas of microvascular obstruction. At later times, border zone and remote myocardium present left ventricular remodeling also occurring through adaptive hypertrophy of non-infarcted cardiomyocytes and extracellular matrix. These processes can continue for several months after AMI. Unfavorable MI healing and excessive remodeling may result in infarct thinning, ventricular dilatation, functional mitral regurgitation, and ultimately heart failure (HF).
Imaging techniques can detect AMI by various principles ( Table 10.1 ) based on the different phenomena occurring during the ischemic cascade ( Figure 10.5 ). Most often, AMI is revealed indirectly by demonstrating the presence of new regional wall motion abnormalities (WMA) or of new fixed perfusion defects. These features are, however non-specific, since they may occur also in acute other diseases mimicking acute coronary syndromes (ACSs), such as aborted MI with myocardial stunning, Takutsubo cardiomyopathy, myocarditis, etc. Definite detection of myocardial necrosis is allowed by late gadolinium-enhanced (LGE) CMR (and late contrast enhancement on MDCT) in typical subendocardial or transmural location corresponding to the distribution of coronary arteries. Myocardial necrosis can also be detected by 99m Tc-pyrophosphate or 111 In-antimyosin antibody imaging, which are, however, rarely used today because of their inability to early identify the occurrence of the acute event. Fibrosis in chronic scars may also be identified by echocardiography as regions with higher ultrasound reflection [ ].
| WMA | Global LV function | Ischemia | Coronary artery occlusion | Infarcted myocardium | Measurement of infarct size | MVO | Infarct hemorrhage | Area at risk | |
|---|---|---|---|---|---|---|---|---|---|
| Echocardiography | ++ | +++ | Possible with stress | − | − | Indirectly by WMS. EF | ++ with CE | − | Possible with intracoronary CE |
| SPECT | + | ++ | +++ | − | Possible with infarct avid tracers (rarely used) | Indirectly by perfusion defect | − | − | Possible before reperfusion |
| PET | + | + | +++ | − | Indirectly by perfusion defect/FDG defect | − | − | Theoretically possible before reperfusion | |
| MR | +++ | +++ | Possible with stress | + | Late enhancement | Area of LGE | ++ | Possible with T2/T2* T1 map |
Possible by T2 imaging |
| CT | +− | + | Possible with stress | ++ | Late enhancement | Area of late-enhancement | +++ | − |
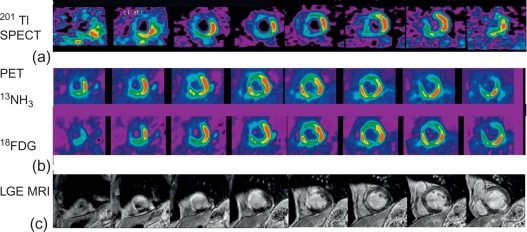
The quantification of MI size ( Figure 10.6 ) is traditionally performed in research studies by measuring Tc-99 m sestamibi defect size [ ]. This approach is, however, only valid for a first AMI. Measurements of ejection fraction or regional wall motion score at 6 weeks or later after AMI provide indirect measurements of MI size with good correlation to sestamibi defect. LGE CMR is currently the most accurate technique for measuring MI size; however, the actual size may depend on the cutoff value of signal intensity on LGE images that is used, and recent data suggests that LGE CMR performed very early after AMI may overestimate MI size [ ] as opposed to measurement performed later than 10 days. By combining LGE and T2-weighted imaging, CMR may also separate acute from chronic MI.
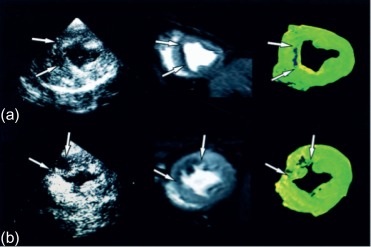
Microvascular obstruction ( Figure 10.7 ) is a phenomenon frequently associated with large infarcts and can be detected as perfusion defects in patients after reperfusion of epicardial coronary arteries. It may be revealed by various imaging techniques, in particular contrast-echocardiography, CMR, and CT. It is fairly specific, but not sensitive of AMI. It persists only for approximately 4–6 weeks after MI, and disappears thereafter, so it can provide additional information on the age of MI. Also, microvascular obstruction was shown to be an important predictor of events and left ventricular remodeling [ , ].
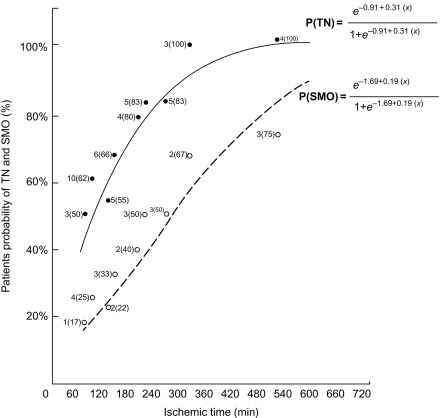
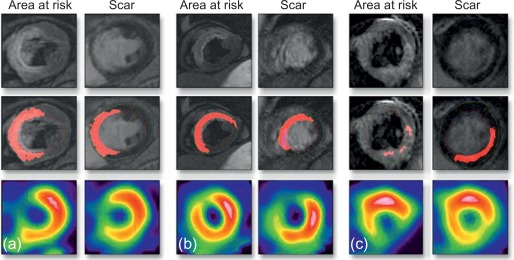
The area at risk of infarcts ( Figure 10.8 ) can be measured by intracoronary contrast echocardiography in the catheter laboratory. It can also be quantified by nuclear imaging if the tracer is injected prior to opening the occluded artery and images are acquired thereafter. These approaches are, however, rarely feasible in clinical practice and remain confined to research studies. A posteriori, that is, after revascularization, the area of edema by T2 weighted MR or non-enhanced T1 mapping provides an estimate of the initial area at risk. This seems to be possible up to several days after the acute event; however, controversy remains on the exact underlying mechanisms. Comparison of MI area and area at risk (AAR) allows an estimate of myocardial salvage [ ] of revascularization therapy.
Imaging techniques play a major role in diagnosis, follow-up, and prognosis of patients with AMI. Clinically, diagnosis of AMI is usually performed by a combination of clinical symptoms (i.e., chest pain or ischemic equivalents such as dyspnea or fatigue), electrocardiographic (ECG) findings such as new significant ST–T wave changes (either ST elevation or depression), development of pathological Q waves, or new left bundle-branch block and detection of rise and/or fall of biomarkers of myocardial necrosis, and in particular of troponin I or T. For the detection of STEMI, the ECG by itself is most often diagnostic, and the decision to reperfuse should not be delayed by imaging studies. Yet in some patients, and in particular in those with non-interpretable ECG such as left bundle block, or paced rhythm, the diagnosis of MI may be uncertain and may benefit from non-invasive imaging techniques to support the diagnosis of AMI. Because of its availability and ease, echocardiography is usually the technique of choice for this purpose. Also in the setting of suspected NSTEMI with normal or borderline ECG, patients may benefit from imaging studies to confirm the diagnosis and estimate the severity of ischemic injury prior to treatment initiation.
Imaging techniques play an even more important role in detecting complications of AMI . Indeed, the acute phase of MI may be complicated by HF and shock, due to severe left ventricular dysfunction, or mechanical complications such as papillary muscle rupture and acute mitral regurgitation, or left ventricular septal or free wall rupture and tamponade. Echocardiography is the technique of choice for detection of these complications. Patients with AMI can also develop intraventricular thrombi complicated by systemic embolization or stroke. This may be detected by echocardiography, but more sensitively by CMR or MDCT.
The long-term prognosis of patients with AMI is mainly determined by the magnitude size of the initial injury (MI size), by the severity of left ventricular remodeling, and by the extent of myocardial ischemia in the remote areas subtended by the stenosed artery, in particular in patients who have undergone thrombolysis and who have not been fully revascularized ( Figure 10.9 ). In clinical practice, LV ejection fraction (LVEF) and end-systolic volume are some of the most important predictors of prognosis in patients after acute ST- and NSTEMI [ , ]. Indeed, numerous studies have demonstrated that reduced LVEF predicts mortality, both due to HF and to arrhythmia [ ]. Yet because a substantial amount of myocardium after AMI may present reversible injury due to acute stunning, the initial severity of left ventricular dysfunction can overestimate true MI size and the final degree of left ventricular dysfunction. Therefore, the true MI size is more closely estimated by LVEF late (3–6 months) post AMI than by early LVEF. Also, in the acute phase of MI, viability imaging by nuclear imaging, stress echo, or direct MI size measurement by CMR/MDCT can better predict prognosis than severity of contractile dysfunction. The identification of microvascular obstruction and hemorrhage appears to have additional prognostic value.
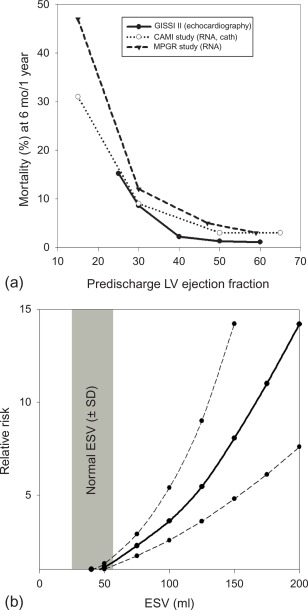
Finally, imaging may also direct treatment . Patients with initial large infarcts and depressed ejection fraction require more clinical attention and benefit from more aggressive treatment with ACE inhibitors and beta blockers. Those who develop large ventricles due to adverse post MI remodeling and who present persistent low LVEF have substantial risk of subsequent development of HF and high risk of sudden cardiac death from ventricular arrhythmia. The MADIT-II trial demonstrated benefit of automated implantable cardioverter defibrillator (AICD) implantation in those patients. Therefore, it is necessary to evaluate the magnitude of remodeling and measurement of LVEF by cardiac imaging, 2–3 months after MI, allowing identification of high-risk patients who may benefit from AICD implantation for primary prevention. Also, nuclear myocardial innervation imaging appears to be a promising approach to identify such patients at high risk of sudden cardiac death after MI; however, this approach has not been validated for treatment selection. Finally, stress imaging techniques play an important role in detecting ischemia due to progression of coronary artery stenosis or due to in-stent restenosis post MI and to direct revascularization therapy in these patients.
Because of its wide availability, and its portability to the emergency room and intensive care unit, echocardiography remains the most commonly used imaging technique in patients with AMI. It allows comprehensive evaluation not only of regional wall motion and global systolic and diastolic function, but also of valve function in patients with AMI. It also allows detection of complications such as pericardial effusion, myocardial and pericardial rupture, and intraventricular thrombus. It is therefore useful not only for the initial diagnosis, but also for the follow-up of patients with AMI, and provides important prognostic information in these patients.
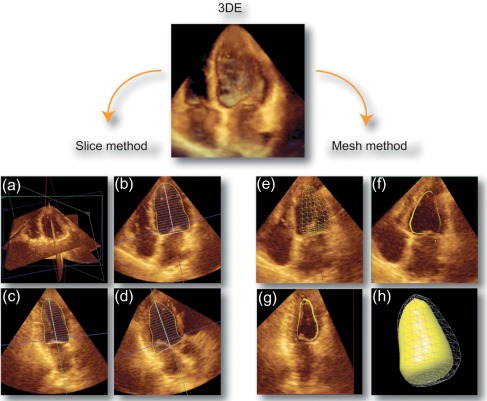
To date, echocardiography offers a multitude of techniques for assessment of patients with AMI. Standard 2D echocardiography still remains the most commonly used imaging technique and allows for the comprehensive evaluation of the dimensions of the left and right ventricles, and of their systolic global and regional function, as well as imaging of the pericardium. Doppler and tissue Doppler imaging of mitral flow patterns allows the evaluation of diastolic function and left ventricular filling pressures. Color , pulse , and continuous wave Doppler imaging allows the evaluation of valve function. Contrast echocardiography enhances discrimination between myocardial tissue and the blood pool by opacifying the LV cavity with contrast agents, which consist of gas-filled microbubbles surrounded by a shell (see Chapter 2 ). Commercially available contrast agents for use are Optison, Definity, and Sonovue. These are referred to as the second-generation contrast agents and allow accurate assessment of regional and global LV function ( Figure 10.10 ). Contrast enhancement is also recommended in patients requiring confirmation or exclusion of LV structural abnormalities and intracardiac masses and for the assessment of myocardial perfusion (discussed in detail in Chapter 2 ) and detection of microvascular obstruction in AMI. Myocardial deformation imaging (MDI) using either tissue Doppler-based or 2-dimensional speckle tracking-based methods ( Figure 10.11 ) (see Chapter 2 ) allows computation of regional and global myocardial strain and strain rate, parameters of regional and global of contractile function, which are believed to have higher reproducibility than 2D-LVEF. 3D echocardiography offers advantages in the assessment of LV volumes and LVEF ( Figure 10.12 ). Unlike 2DE, it eliminates the need for geometric modeling, which is inaccurate in the presence of aneurysms, asymmetrical LVs, and WMA, which commonly occur in patients with AMI. Several studies comparing real-time 3-dimensional echocardiography with widely accepted reference techniques such as radionuclide ventriculography and CMR have demonstrated higher levels of agreement and reproducibility in comparison to 2DE [ ]. Recently, RT3DE with contrast enhancement was found to be comparable to CMR for accuracy and reproducibility of LV volumes and LVEF and superior to unenhanced images [ ].
![Figure 10.11, (a) Strain imaging in a normal subject. Examples of tissue Doppler imaging velocity, strain rate, and strain curves for a cardiac cycle from a subject with normal cardiac function. L, length; V, velocity. Reproduced with permission from Ref. [ 27 ]. (b) Speckle tracking strain by echocardiography diagram of speckle tracking strain from 2-dimensional short-axis echocardiographic images. Figure 10.11, (a) Strain imaging in a normal subject. Examples of tissue Doppler imaging velocity, strain rate, and strain curves for a cardiac cycle from a subject with normal cardiac function. L, length; V, velocity. Reproduced with permission from Ref. [ 27 ]. (b) Speckle tracking strain by echocardiography diagram of speckle tracking strain from 2-dimensional short-axis echocardiographic images.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Myocardialinfarction/10_3s20B978178242282200010X.jpg)
In patients presenting to the emergency department (ED) with possible MI, both the assessment of global [ ] and regional WMA [ ] enhance risk stratification and may facilitate rapid decision making. Diagnostic uncertainty remains even if resting LV function is normal when patients present several hours after chest pain with non-diagnostic ECG, negative troponins, and an intermediate clinical risk score (TIMI score). In this population, stress echocardiography may be potentially useful in risk stratification and rapid discharge of patients with a normal stress echocardiogram. In 839 such patients, stress echocardiography performed within 24 h of admission was able to risk stratify patients during follow-up. In the first year, the event rate was 0.5% and 6.6% in the normal and abnormal stress echocardiography groups, respectively. Over 70% of patients could be discharged within 24 h with a normal stress echocardiogram [ ].
Contrast defects by MCE allow the identification of AAR during arterial occlusion [ ]. Recent work demonstrated the prognostic value of MCE in patients presenting to the ED with chest pain syndromes and non-diagnostic ECGs.
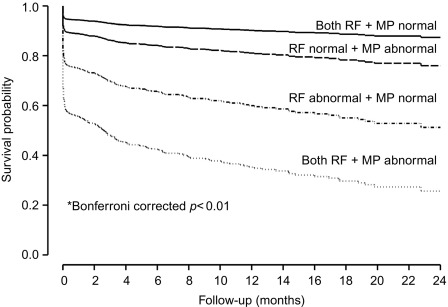
In a large single-center study in over 1000 patients in with chest pain and non-diagnostic ECG [ ], adding regional function assessment by MCE increased the prognostic information of the clinical and ECG variables significantly for predicting early events occurring within 48 h. When myocardial perfusion assessment was added, additional information was obtained. Also, when patients without early events were followed up for a median of 7.7 months, those with normal perfusion and function had excellent prognosis, whereas those in whom both were abnormal had the worst outcome. Intermediate outcome was noted in those with normal perfusion despite abnormal function ( Figure 10.13 ). In another study in 532 patients presenting to the ED, regional function on MCE provided incremental information over modified TIMI scores for predicting intermediate (< 30 days) and late (> 30 days) events. In patients with abnormal regional function, myocardial perfusion further classified those into intermediate- and high-risk groups. The full TIMI score (after troponin levels became available) could not improve on these results at any follow-up time point [ ].
No reflow as assessed by MCE has been found to be an important predictor of LV remodeling, LV dysfunction, and adverse cardiac events in patients with established STMI. Initially, this was demonstrated using intracoronary MCE, where such imaging is performed within the first hour following PPCI. In a study of 199 patients, MCE was performed on average 15 min following PPCI. No reflow occurred in 40% of patients, and this population had higher CK levels than those without MCE no-reflow [ ]. Also in patients with anterior MI, where no-reflow phenomenon identified by this technique was associated with higher incidence of pericardial effusion, early and late HF and increase in LV end diastolic volume during the convalescent period [ ]. Subsequently, these results were also reproduced for intravenous MCE with second- or third-generation contrast agents. In a small number of patients, MCE performed within 24 h of AMI treated by primary PCI (PPCI) was the best predictor of LV remodeling at 1 month over clinical and angiographic parameters, with a sensitivity and specificity of 88% and 74%, respectively [ ]. More recently, a larger multicenter study has produced similar results [ ]. The optimal timing to assess no-reflow by intravenous MCE is at 48 h. Here, the coronary hyperemia has settled and the dynamic character of the microcirculation has abated. One study examined patients at 24 h and 3–5 days after PCI following AMI. Intravenous MCE performed at 3–5 days correlated more strongly with contractile reserve than MCE at 24 h [ ].
Prognosis after AMI depends on residual LV dysfunction, extent and degree of residual myocardial viability, and ischemia (at the site of MI or remote territory).
As for other techniques, LV function assessed by echocardiography strongly predicts outcome following AMI [ ]. Several studies have shown that contrast-enhanced determination of LV volumes and function are more accurate and reproducible than non-enhanced echocardiography using CMR as the gold standard [ , ]. In a post-AMI study by Lim, LVEF determined by contrast-enhanced echocardiography with low-power real-time imaging was found to be more accurate compared with unenhanced echocardiography [ ]. The importance of accurate assessment of LVEF is not only for risk stratification, but also accurate delivery of life-saving, invasive treatments.
In patients with AMI, DSE allows for detection of myocardial ischemia and viability in regions with abnormal resting function (see Chapter 11 ) by four different responses. (1) biphasic response—an increase in contractile function and wall thickening at low dose, with deterioration of systolic function with higher dose ( Figure 10.14 ), indicating both viability and ischemia; (2) uniphasic—an improvement in function at low dose that persists or further improves into high dose, indicating myocardial viability; (3) worsening of resting wall function, indicating ischemia; or (4) no change in function, indicating scar. The biphasic response has the higher predictive value (72%) for improvement of regional recovery [ ] than uniphasic response, since this latter pattern can be present not only in jeopardized myocardium, but also in subendocardial scar or remodeled myocardium with no flow-limiting stenosis. Although the interpretation of wall motion is subjective, and there is significant intra- and interobserver variability with DSE, in the clinical setting, the diagnostic accuracy of DSE is good with 85% sensitivity and 79% specificity for the identification of regional functional recovery [ ] after AMI.
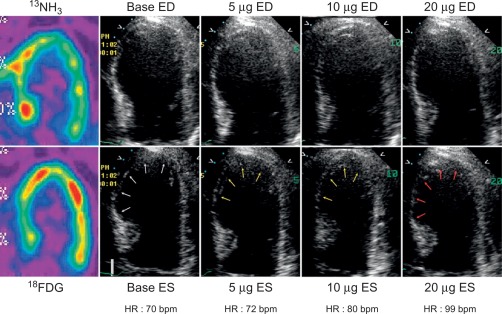
Adding tissue Doppler deformation measurements with DSE was shown to provide additional benefit over wall motion in predicting functional recovery [ ]. Hanekom showed that the addition of strain rate imaging enhanced the sensitivity of dobutamine echocardiography (82% vs. 73%) for assessing myocardial viability, without affecting the specificity [ ]. Few studies have examined the role of speckle tracking during stress testing.
Deformation imaging has also been shown to predict myocardial viability at rest. Zhang performed tissue Doppler strain rate measurements and contrast-enhanced CMR to assess the extent and transmurality of scar in the immediate post MI period. Peak myocardial deformation by SRI was able differentiate transmural from non-transmural MI, and thereby the extent of non-viable myocardium [ ]. In 147 AMI patients, global longitudinal strain was able to predict viability and LV recovery with sensitivity 86% and specificity 74% [ ].
The assessment of viability assumes that viable myocardium requires a preserved micro-vasculature. This can be accurately assessed by MCE, with its excellent spatial resolution ( Figure 10.15 ). The absence of myocardial contrast enhancement on MCE should indicate areas that lack viability, and tissue necrosis. This was verified in 20 patients with ischemic cardiomyopathy undergoing CABG, in which MCE was performed within 24 h of surgery. Myocardial signal intensity (an index of myocardial blood volume) closely correlated with microvascular density and capillary area on pre-procedural MCE. An inverse correlation was demonstrated between the degree of myocardial fibrosis, and myocardial intensity [ ]. The role of MCE in predicting regional and global wall motion recovery after AMI is well documented. In 96 AMI patients with a patent infarct related artery, failure of myocardial replenishment within 10 cardiac cycles during high-power imaging resulted in lack of recovery of function in 84% of the time [ ]. Similarly in patients with recent AMI and occluded infarct-related artery (IRA), MCE accurately evaluates collateral blood flow and contractile reserve [ ]. In a study by Janardhanan, 50 patients underwent low power continuous MCE 7–10 days after AMI. Myocardial segments that did not replenish within 10–15 cardiac cycles following microbubble destruction showed significantly less contractile reserve after revascularization compared with those segments that replenished. Contrast intensity measured in this way had a predictive value of 90% for the presence of contractile reserve. The absence of contrast enhancement predicted the absence of contractile reserve in 90% of cases ( Figure 10.6 ) [ ].
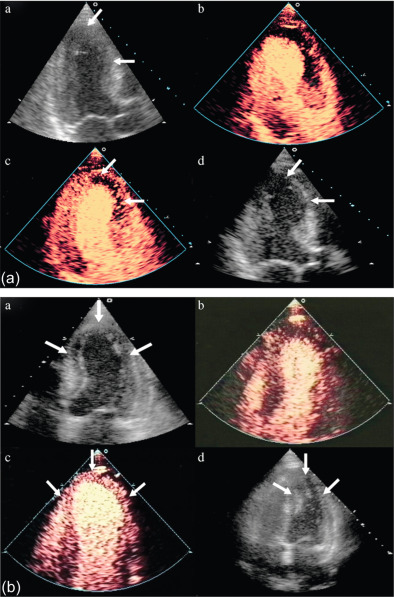
There is excellent agreement between MCE and DSE in predicting LV functional recovery [ ]. Senior et al. assessed the incremental value of MCE in addition to low-dose dobutamine for the assessment of viability post MI [ ]. The presence of contrast enhancement in segments with no contractile response resulted in improvement in function compared with segments with no contrast enhancement. MCE thus predicted viability in this group of patients with no contractile response to dobutamine. Perfusion abnormalities appear earlier in the ischemic cascade, followed by wall motion abnormality during stress testing, making perfusion more sensitive.
Although LVEF is a powerful predictor of outcome, the extent and intensity of the perfusion defect is a superior predictor of recovery of LV function. This underscores the fact that perfusion, not wall motion abnormality, accurately differentiates stunned from necrotic myocardium. MCE performed 7–10 days post MI predicts the transmural extent of AMI as assessed by CMR [ ], and predicts cardiovascular outcomes immediately following AMI [ ]. 99 patients post thrombolysis underwent MCE at 7 ± 2 days and were followed up for 46 ± 16 months. The extent of residual myocardial viability was an independent predictor of cardiac death and cardiac death or AMI. A Contrast Defect Index of < 1.86 and < 1.67 predicted survival and survival or absence of re- current AMI in 99% and 95% of the patients, respectively [ ].
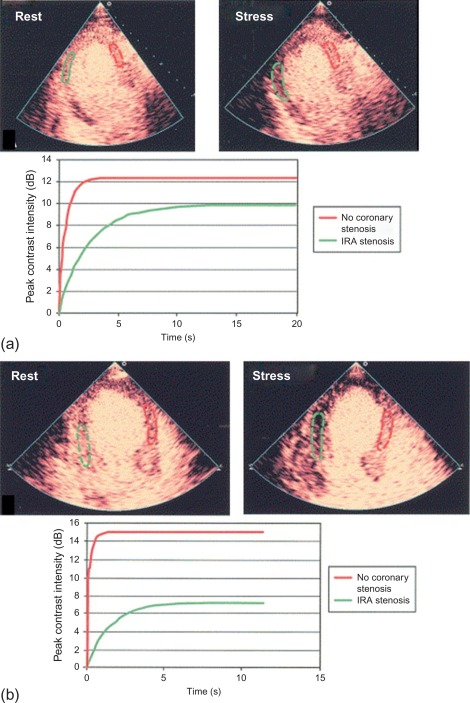
The accuracy of vasodilator MCE for detecting flow-limiting coronary artery stenosis in suspected coronary artery has been confirmed in several studies. A 73-patient study demonstrated the ability of Dipyridamole MCE to detect residual IRA stenosis and presence of remote flow-limiting CAD in patients with recent STEMI and thrombolysis ( Figure 10.16 ). Sensitivities for the detection of > 50% IRA stenosis and MVD were 88% and 72%, respectively [ ].
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here