Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiomyopathies include various diseases of the myocardium, often chronic or progressive, and associated with cardiac dysfunction. These are rare but serious disorders; only 25% of children survive more than 5 years after the onset of symptoms. Although our understanding of the causes of pediatric cardiomyopathy has advanced, the prognosis has not changed considerably in the past 30 years and is the same in developing and industrialized nations.
Cardiomyopathies may be classified according to the dominant pathophysiology ( e-Box 79.1 ). Four major physiologic forms of cardiomyopathy are described: dilated, hypertrophic, restrictive, and arrhythmogenic. While often discussed as distinct entities, occasionally there is considerable overlap in pathophysiologic features. Dilated and hypertrophic cardiomyopathies are the most common forms in children.
Congestive cardiomyopathy
Hypertrophic obstructive cardiomyopathy
Idiopathic hypertrophic subaortic stenosis
Asymmetrical septal hypertrophy
Nonobstructive hypertrophic cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy
Arrhythmogenic right ventricular dysplasia
Right ventricular dysplasia
Right ventricular cardiomyopathy
Cardiomyopathy may also be classified according to etiology. Inflammatory and isolated familial cardiomyopathies account for the majority of pediatric cases with a known cause. Other specific cardiomyopathies that are less common in the pediatric population include those resulting from metabolic disorders, general systemic diseases, muscular dystrophies, neuromuscular disorders, and sensitivity or toxic reactions (e.g., radiation and chemotherapy). In two-thirds of children with cardiomyopathy, no specific etiology is found.
Because of its availability, lack of radiation use, and portability, echocardiography is the most common method used to classify cardiomyopathy and evaluate cardiac function. With improvements in technology, cardiovascular magnetic resonance imaging (MRI) has become an increasingly powerful tool. With the multiple techniques available, cardiac MRI can be used to assess myocardial morphology and function (cine sequences), with expanding techniques for myocardial tissue characterization, including perfusion, delayed myocardial enhancement, and T1 mapping.
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiovascular disease and has autosomal dominant inheritance. HCM demonstrates quite variable penetrance and expression, but significant disability and even sudden death can occur at any age.
The cellular myocardial architecture in HCM is disorganized, while the characteristic gross morphologic feature is that of hypertrophy. Myocardial wall thickening most commonly manifests in an asymmetric distribution involving the anterior septum, but some patients show symmetric (concentric) involvement of the left ventricle ( ). Furthermore, there can be associated obstruction of the left ventricular outflow tract secondary to septal hypertrophy and systolic anterior motion of the mitral valve.
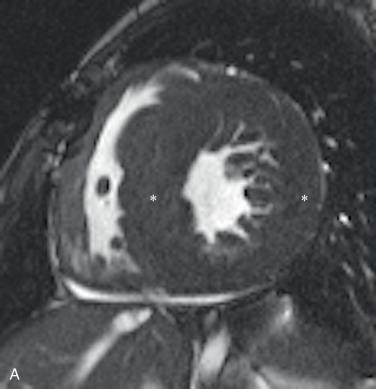
Echocardiography is the primary imaging study used to diagnose and characterize HCM. MRI can provide a more comprehensive evaluation of regions of wall thickening as well as offer more advanced techniques such as assessing for late myocardial enhancement within regions of scarring ( Fig. 79.1 ), perform native T1 mapping of the myocardium, and even analyze cardiac strain parameters.
Dilated cardiomyopathy is the most common form of cardiomyopathy and currently the most frequent indication for heart transplantation. Characterized by dilation and impaired systolic function of the left or occasionally both ventricles, patients typically present with progressive heart failure ( Fig. 79.2 ), up to 75% succumbing or undergoing transplantation within 5 years. While the majority of pediatric cases (66%) are idiopathic, common specific etiologies may include infectious myocarditis, familial disease, and neuromuscular disorders (most commonly Duchenne and Becker muscular dystrophies). Although a frequent cause of dilated cardiomyopathy in adults, coronary artery disease is uncommon in childhood. Children with sickle cell disease may develop ischemic cardiomyopathy related to “sludging microinfarctions” with resulting focal myocardial fibrosis. Most children with dilated cardiomyopathy are diagnosed in the first year of life. Late presentation in childhood, reduced left ventricular shortening fraction or ejection fraction, severe mitral regurgitation, right ventricular failure, and mural thrombus were found to be associated with a worsened prognosis, while those with infectious myocarditis generally fare better.
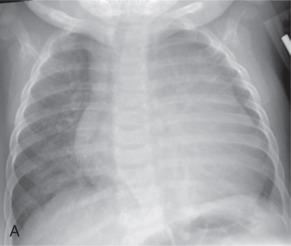
Cardiac MRI can be helpful in characterizing the presence of myocardial edema, late enhancement, ventricular function, myocardial mass, and valvular regurgitation.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is the most arrhythmogenic form of human heart disease, characterized by progressive fibrofatty replacement of myocardium, most commonly involving the right ventricle (RV). Approximately 50% of affected individuals have one or more identified mutations encoding desmosomal proteins, with substantial variation in genetic penetrance and clinical manifestations. ARVC is also related to the rare autosomal recessive cardiocutaneous disorders Naxos disease and Carvajal syndrome. Remodeling of intercellular gap junctions is found diffusely in ARVC, including areas not exhibiting myocyte degeneration and fibrofatty replacement. These changes may play a role in the onset of ventricular arrhythmias and sudden death, with symptoms often manifesting earlier in athletic adolescents and young adults. Fibrofatty myocardial infiltration and wall thinning of ARVC can be visualized on T1-weighted spin echo magnetic resonance (MR) sequences but has low sensitivity and specificity compared with right ventricular volumes and function. Additional reliable MR findings include abnormal right ventricular regional or global wall motion and aneurysms, most frequently involving the RV free wall, inflow, and outflow tracts. Late gadolinium enhancement may also be present. Biventricular involvement has been identified on MRI in more than half of ARVC cases, and while less common, left dominant forms of the disease are increasingly recognized, with involvement of the left ventricle (LV) posterolateral wall most frequent, where the changes can be potentially mistaken for myocarditis.
Restrictive cardiomyopathy (RCM) is the least common pediatric cardiomyopathy and continues to carry the worst prognosis and transplant-free survival despite advances in therapy. RCM is characterized by increased myocardial or endomyocardial stiffness leading to incomplete ventricular relaxation with decreased diastolic filling, while preserving normal or near-normal systolic function. Endomyocardial fibrosis is one of the most common etiologies of restrictive cardiomyopathy in children and adolescents. A wide variety of genetic or acquired causes of infiltrative as well as noninfiltrative myocardial diseases may also be involved in restrictive cardiomyopathy pathogenesis. However, the majority of pediatric cases remain idiopathic. Marked atrial dilation contrasting with the normal or small-sized ventricles is pathognomonic on imaging. Chest radiographs demonstrate cardiomegaly with pulmonary venous congestion in up to 90% of the cases. Cardiac MRI will reflect the same anatomic findings, while additionally allowing real-time evaluation of regional and global ventricular function, calculation of ventricular volumes, and quantitative assessment of blood flow across the atrioventricular valves. Structural myocardial abnormalities, usually fibrosis, can be detected on delayed postcontrast inversion recovery MRI or T1 mapping. A variable degree of increased LV myocardial thickness is present in up to 34% of patients indicating a “mixed” restrictive/hypertrophic phenotype, which has been found to be associated with a better prognosis than “pure” restrictive cardiomyopathy.
Constrictive pericarditis presents with similar restrictive physiology and should be differentiated from restrictive cardiomyopathy given the different management and prognosis. Increased pericardial thickness (>4 mm) with calcifications with or without contrast enhancement on computed tomography (CT) or MRI as well as a tubular configuration of the compressed ventricles are usually present in constrictive pericarditis, in contradistinction to restrictive cardiomyopathy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here