Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ubiquitous in nature, fungi may be opportunistic organisms in compromised hosts or primary pathogens in immunocompetent individuals. Commensal fungi reside in the human body as part of the resident microbiota and cause opportunistic infections. Diagnosis of fungal infections relies on assessment of several factors, including geographical exposure, underlying host condition, clinical manifestations, and diagnostic accuracy of laboratory tests such as culture, anatomic pathology tests, immunologic assays, and molecular tests.
A perspective on the importance of fungi in their natural environment is provided, and their differences in relation to other microorganisms are highlighted. Prevalent and emerging fungal infections are discussed, and a review of nomenclature changes in medical mycology is listed with the understanding of continued uncertainty regarding phylogenetic relationships between fungal taxa. Preanalytical, analytical, and postanalytical procedures for the laboratory diagnosis of fungal infections are reviewed, with emphasis on fungal morphology and issues that require close communication with the clinical team. Common histopathologic and cytologic features related to anatomic pathology are discussed. Given the suboptimal sensitivity, specificity, and relatively long turnaround time for culture-based detection and identification of fungi, a review of nonphenotypic methods such as mass spectrometry, immunologic assays, and molecular tests is provided. Finally, the principal features of fungal infections are summarized, including patient populations affected, clinical manifestations, relevant diagnostic approaches and tests, and therapeutic issues related to antifungal resistance.
Our understanding of fungi is evolving thanks to rapidly expanding data from genome studies on both well-known pathogens such as the microsporidia , and new niches that reveal hitherto unknown fungi. Previously known as nonmotile, aerobic eukaryotes that possess a chitin-rich cell wall, this kingdom of life includes motile members, strict anaerobes, and fungi without a cell wall. Despite the differences among fungal organisms, a common feature is the inability to photosynthesize and the need for heterotrophic feeding (i.e., use of organic compounds for carbon and energy source). Fungi acquire nutrition through saprobic, symbiotic, or parasitic lifestyles. Fungal saprobes feed on dead organic remains—mainly plants—and as nature’s major recyclers, they are indispensable for life on earth.
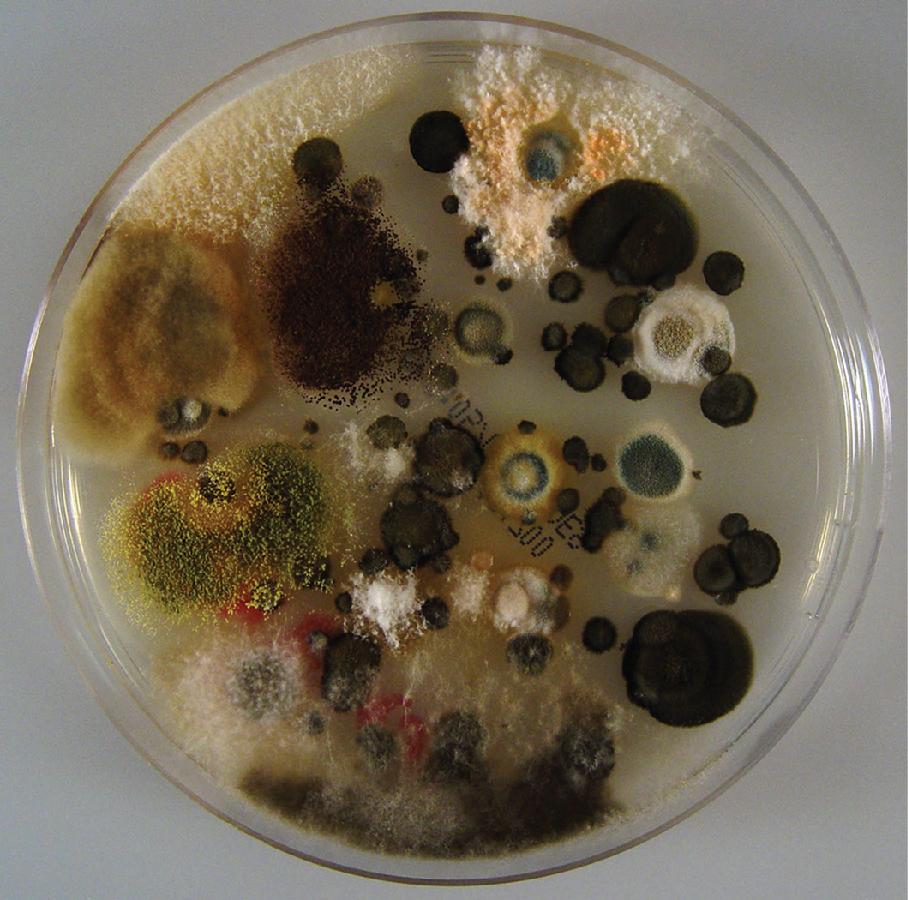
The ubiquitous presence of fungi in soil, water, and on both living , and decaying plants , is reflected in atmospheric air ( Fig. 87.1 ). Fungi produce conidia and spores for dispersion of the species. These propagules, which can be as small as 2 μm, are aerosolized from the growing fungus and transported over short or long distances—sometimes even across continents. Fungal particles are found in concentrations of less than 20 to over 10 5 colony-forming units (CFUs)/m 3 outdoors and are carried with air currents, fomites, and people into buildings where concentrations are generally below 10 3 CFUs/m 3 . , , The fungal species identified in ambient air vary with the methodology used. , Thus the commonly used culture-based techniques miss nonculturable fungi and may lead to overestimates of rapidly growing species at the expense of slowly growing ones. Molecular-based studies show that known human pathogens such as Alternaria , Aspergillus, and Cladosporium are among the 10 most common indoor fungi in most parts of the world. ,
Fungi cause disease in humans via three mechanisms, which are studied by different academic and clinical fields. Mycotoxicology encompasses the study of mushroom poisons and mycotoxins in microfungi (mainly molds). The domains of immunology and allergology are concerned with fungal allergies, and fungal infections are the subject of clinical mycology. With rare exceptions, these fields do not overlap in clinical or laboratory practice. Allergic bronchopulmonary aspergillosis is an exception; the disease mechanism is allergic in nature due to colonizing Aspergillus in the lower airways, and Aspergillus is often cultured from respiratory tract specimens.
The natural role of fungi as recyclers and thus their omnipresence explains a few characteristics by which they differ from other microorganisms of medical importance. Fungal infections in humans are neither vector-borne nor sexually transmitted, and interhuman transmission is rare except for commensals of the resident microbiota that are acquired early in life from other humans and the few anthropophilic dermatophytes. Moreover, unlike any other pathogen group, they can infect every tissue of the body, including the hair. Fungi resemble other organisms in that transmission may be zoonotic and outbreak-related, and their geographical distribution is not uniform. Also, like other pathogens, they infect humans of all ages, and many produce more severe infections in compromised hosts than healthy ones.
The total number of fungal species on Earth is unknown but according to recent estimates it might exceed 3 million. About 120,000 species are known to date and more than 500 have been reported to cause human infections. A mere handful of fungi have adapted to human beings. Candida species colonize mucous membranes and the skin, and the anthropophilic dermatophytes infect only humans. Few fungi are primary pathogens whereby disease is caused irrespective of the host’s underlying conditions. Most are opportunists that take advantage of weakened defenses to cause infections. Fungi are therefore less prevalent as human pathogens than other organisms, and while infections of mucous membranes and keratinized tissues are common in the general population, severe invasive infections are mostly seen in immunocompromised patients. It is perhaps for these reasons that fungal infections have received less attention on the global scale than infections caused by bacteria, parasites, and viruses.
Recently, emphasis has been placed on fungal infections through surveys and organized efforts that have highlighted their impact on public health and the economy. The results indicate that non–toxin-related fungal diseases affect over 25% of the world’s population. Hair, skin, and nail infections are by far the most common, affecting about 1.5 billion people, many of whom are children. , The next most common infections are mucous membrane candidiasis, mainly vaginal candidiasis, which is common in women of childbearing age. While the aforementioned are usually benign, they do carry measurable morbidity and societal cost.
Respiratory tract infections and fungal allergy–related conditions are of moderate to high severity and constitute the third most common disease group. The global burden of respiratory tract infections is estimated at 3 million cases of chronic pulmonary aspergillosis , and more than 700,000 cases of invasive aspergillosis and Pneumocystis pneumonia combined. Allergy-related conditions comprise about 11 million cases of allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. , Invasive and life-threatening yeast infections due to Candida and Cryptococcus follow respiratory tract infections in frequency and are believed to occur in more than 900,000 people worldwide every year. , Invasive fungal infections carry high mortality and are believed to cause over 1.6 million deaths per year, mostly in patients with HIV/AIDS or other immunocompromising or chronic underlying conditions. , It is estimated that the top ten invasive fungal infections cause more deaths in the world than tuberculosis or malaria. In addition, fungi with restricted regional distribution are important causes of morbidity. Examples include life-threatening endemic mycoses due to dimorphic fungi; keratitis, which can lead to loss of vision; and lower limb mycetomas that all too often require an amputation of the infected leg because of poor response to antifungals.
Despite technological leaps in most aspects of health care sciences over the past decades, health care systems are still challenged with the diagnosis and treatment of invasive fungal infections. Autopsy studies epitomize the current situation. Infections, including those of fungal etiology, were the second most commonly misdiagnosed conditions in intensive care unit patients, and 75% of invasive fungal infections detected at autopsy in patients with hematologic malignancies were undiagnosed before death.
The aim of this chapter is to provide laboratory professionals with relevant information about fungal pathogens and infections and to offer practical guidance for the diagnostic process. A number of atlases and manuals covering the diagnosis of fungal infections are available. Documents relevant to laboratory diagnosis and management are provided in Table 87.1 . Many readers may encounter “new” terms that are specific to mycology, so this chapter includes a glossary of common terms ( Table 87.2 ), as well as a list of abbreviations ( Table 87.3 ).
| Fungi and Infections | Title |
|---|---|
| Yeasts | |
| Candida (invasive and mucosal infections) |
|
| Candida (intra-abdominal infections) |
|
| Candida (invasive infections) |
|
| Candida (invasive infections) |
|
| Candida auris |
|
| Candida (invasive infections) |
|
| Candida (invasive infections) |
|
| Rare yeasts |
|
| Molds | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Various Fungal Groups in Invasive, Mucosal, and Superficial Infections | |
| Invasive fungal infections, allergic bronchopulmonary aspergillosis, aspergilloma |
|
| Invasive fungal infections |
|
| Invasive fungal infections |
|
| Invasive fungal infections |
|
| Invasive fungal infections |
|
| Invasive fungal infections |
|
| Invasive fungal infections |
|
| Aspergillus, Candida, Coccidioides, Cryptococcus, Histoplasma |
|
| Paracoccidioides |
|
| Blastomycosis, Histoplasmosis, and Coccidioidomycosis |
|
| Pneumocystis jirovecii |
|
| Superficial and invasive infections due to bacteria, fungi, parasites, and viruses |
|
| Malassezia (cutaneous) |
|
| Terms and Definitions |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Abbreviation | Full Term |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
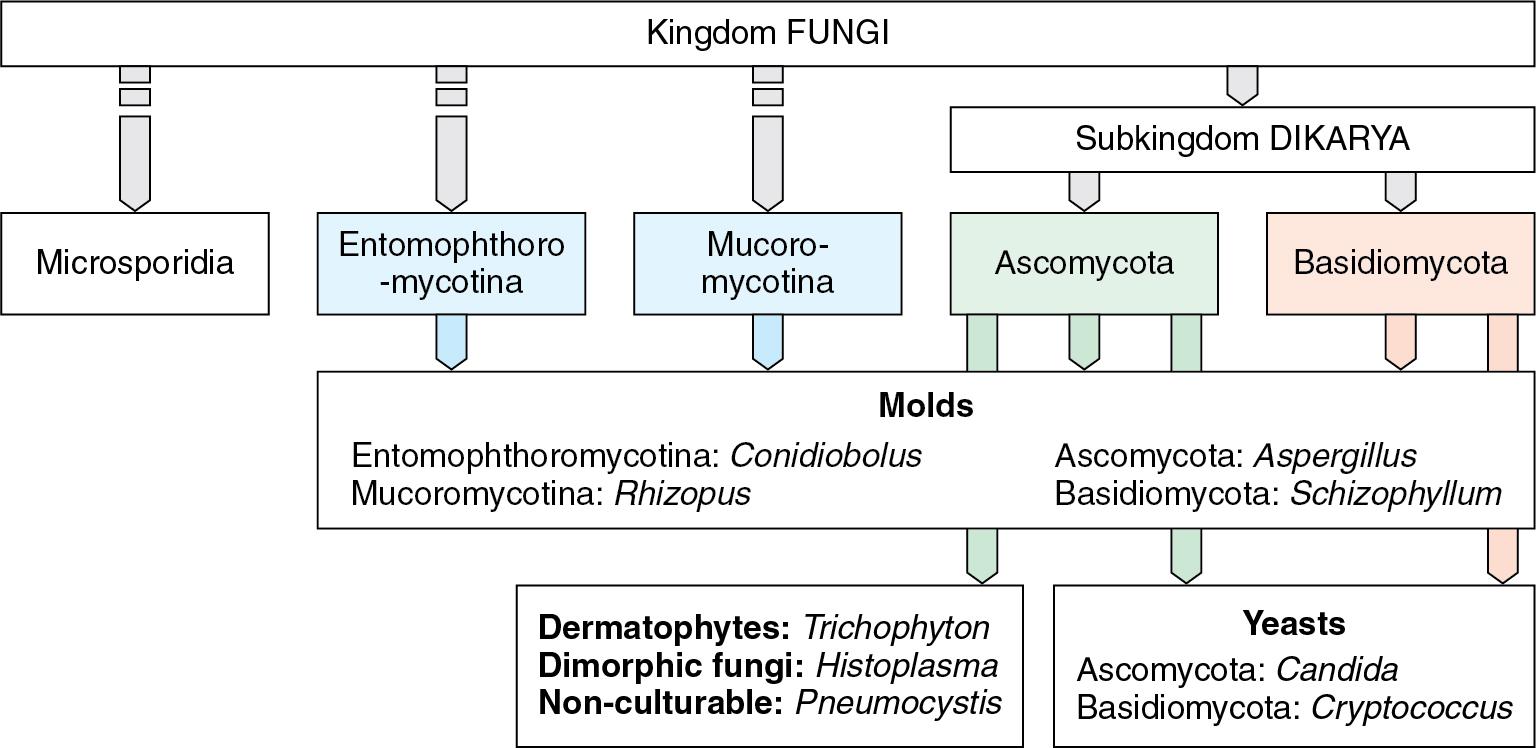
Taxonomy aims to reflect the evolutionary or phylogenetic relationship of organisms. Until recently, this science was based on phenotypic characteristics, such as morphology, but the advent of genomic studies has provided new insights into phylogenetic relationships, including the existence of species complexes comprising multiple genetically distinct but morphologically similar species. , Thus the taxonomic classification and nomenclature of fungi are changing and will continue to be refined over the years to come. Table 87.4 provides examples of fungal species that have been renamed subsequent to newer knowledge. In the practice of clinical mycology, results of taxonomical studies are used to name organisms to genus or species levels. Because fungal species that belong to different taxa may share similar morphologies, growth behaviors, and, in some instances, ecologic niches, they may be combined into informal but practical groups, such as yeasts, molds, dermatophytes, and dimorphic fungi. Fig. 87.2 provides a simplified view of the current taxonomic classification and informal, mostly morphologic, groups.
| Previous Name(s) | Current Name |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Many fungi are capable of both sexual reproduction and asexual propagation. These two life cycles produce morphologic forms, called teleomorph for the sexual state and anamorph for the asexual state, that are often so different that they may not be recognized as the same species. For this reason, fungi have traditionally been assigned two distinct names, for the teleomorph and the anamorph forms, which were regulated by the International Code of Botanical Nomenclature that permitted a dual nomenclature for pleomorphic fungi. However, this provision was abolished in 2011 in favor of “one fungus, one name” because molecular methods have now replaced morphology for classification. Furthermore, to better reflect the purpose of the Code, it was simultaneously renamed the International Code of Nomenclature for algae, fungi, and plants. The transition to a one-name system is ongoing and although some changes have occurred already (see Table 87.4 ), others will take time. In this chapter we reference the currently accepted names, where changes have occurred, and otherwise the names in common clinical use (typically the anamorph name).
Microsporidia are now considered a separate phylum in the kingdom Fungi . Their nomenclature, however, is still governed by the International Code of Zoological Nomenclature, which explains the absence of the suffix -mycota in the phylum name.
Fungi inhabit a variety of ecologic niches in humans, nonhuman hosts, and the environment. The ecologic niche of each organism defines, in part, the manner in which it leads to disease. For instance, certain Candida spp. are commensals or colonizers of various parts of the human body. Candida infections generally result from compromised defenses at the particular body site or system, such as a break in the skin, a surgical procedure involving a breach of the gastrointestinal (GI) tract, or immunosuppression. Candida spp. are thus considered opportunistic pathogens. Opportunistic infections may be classified as endogenous when infections are due to organisms that are typically found in the human microbiota, or exogenous when infections are acquired from outside the body. The natural environment is the main source of exogenous infections (e.g., a compost pile releasing Aspergillus fumigatus conidia). Knowledge of the ecologic niche of fungi, on the human body or in the environment, helps the clinician to determine the most likely pathogen causing disease.
The majority of fungi in human infections are opportunists. Immunocompromised states that increase susceptibility to opportunistic fungi include innate or acquired immunodeficiency (e.g., HIV/AIDS) and medication-induced immunodeficiency (e.g., corticosteroid use or other immunosuppressive therapies). Other host factors that may increase a person’s susceptibility to infection include extremes of age, diabetes mellitus, pregnancy, surgery, prolonged hospital stays, traumatic injury, and the presence of intravascular catheters. Some fungi are primary pathogens and can cause disease in immunocompetent hosts. Primary pathogens include dimorphic fungi such as Coccidioides, Histoplasma, Blastomyces, and Emergomyces species. Infections caused by primary pathogens may be subclinical, mild and self-limiting, or they may result in disseminated, severe disease. Dissemination and severity vary according to host factors and the number of infectious organisms to which the person is exposed. Immunocompromised persons typically develop more severe disease from primary pathogens compared to immunocompetent ones.
Systemic fungal pathogens, which lead to disease in immunocompetent persons, are often located in geographically restricted (endemic) areas. On the other hand, fungi leading to opportunistic infections are typically found worldwide. One exception is the opportunistic fungus Talaromyces (formerly Penicillium ) marneffei , an endemic dimorphic pathogen restricted to Southeast Asia. Knowledge of the geographical distribution of various fungi helps to guide differential diagnosis, selection of appropriate diagnostic tests, and choice of therapy.
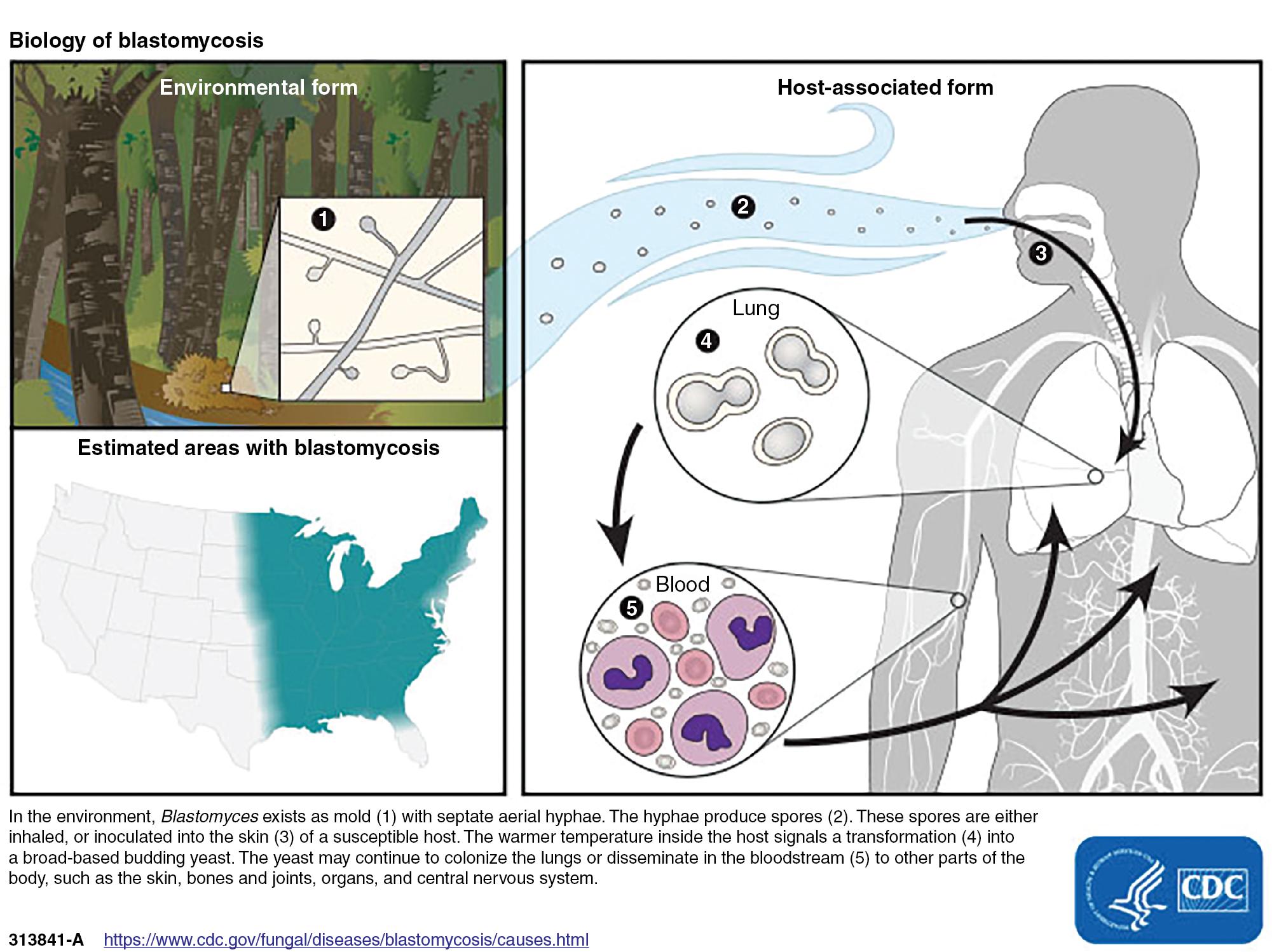
Most of the thermally dimorphic fungi are either geographically restricted or significantly more prevalent in some regions compared to others and are therefore often referred to as “endemic.” Blastomycosis is endemic in the southeastern and south-central areas of the United States that border the Mississippi and Ohio Rivers ( Fig. 87.3 ). The endemic region extends to areas of Canada along the Great Lakes and the St. Lawrence River. Originally thought to be restricted to North America, blastomycosis has also been reported since the 1950s throughout Africa, primarily in South Africa. A few case reports of autochthonous (nonimported) blastomycosis have originated from India, Israel, and Saudi Arabia, and Australia (reported as Emmonsia parva ).
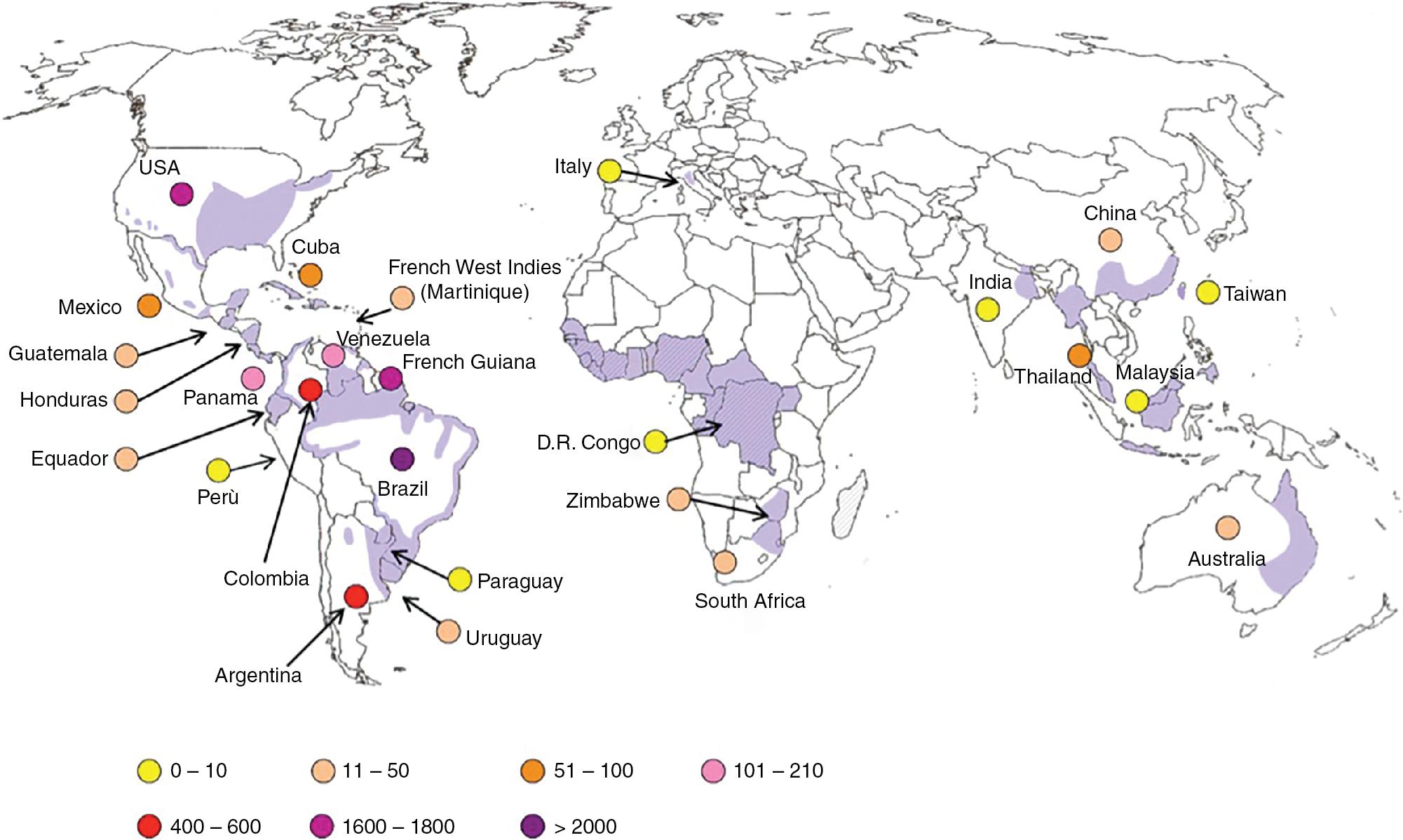
Geographical areas endemic for histoplasmosis overlap with blastomycosis in the United States and include states bordering the Mississippi and Ohio River valleys, extending into the St. Lawrence River and south to the Rio Grande River on the Mexican border. Histoplasma capsulatum was recently recognized as four distinct species with distinct but partially overlapping geographic distributions; H. capsulatum sensu stricto comprises isolates from Panama (and presumably Central America), while Histoplasma suramericanum comprises isolates predominantly from South America, and Histoplasma mississippiense and Histoplasma ohiense account for the majority of cases in North America, with endemic foci in the Mississippi and Ohio River valleys, respectively. The worldwide distribution of histoplasmosis is extensive, although the precise causative species, as relates to the above, remains to be assessed. Brazil and French Guiana are highly endemic for histoplasmosis. Areas of lower endemicity include the remainder of Central America, South America, islands in the Caribbean, portions of South and Southeast Asia, Australia, and Africa ( Fig. 87.4 ). In addition to H. capsulatum “sensu lato,” two further varieties exist in Africa, H. capsulatum var. duboisii, and H. capsulatum var. farciminosum (not a human pathogen). Further changes to the nomenclature of this group of pathogens are likely.
Emergomyces , a recently described genus related to Emmonsia and Blastomyces causing emergomycosis, occurs globally with the first known case dating back to 1992 (as an undescribed Emmonsia species, now Emergomyces canadensis ) and contains five known species, E. pasteurianus, E. africanus, E. canadensis, E. orientalis, and E. europaeus. E. pasteurianus appears to be the most geographically diverse, with cases reported from Italy, Spain, France, the Netherlands China, India, Uganda, and South Africa; E. africanus has been reported from South Africa and Lesotho; E. canadensis has been reported from Canada and the United States; E. orientalis has been reported from China; and E. europaeus has been reported from Germany. See also Table 87.57 .
| Features | Emmonsia crescens Blastomyces parvus | Emergomyces spp. (Formerly Emmonsia spp.) , , , | Lacazia loboi |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a Microscopy of fresh specimen (usually after staining) and cytologic or histologic examination when appropriate.
Coccidioides spp. extend from the southwestern United States into the deserts of Mexico, and parts of Central and South America are also endemic ( Fig. 87.5 ). Highly endemic areas include Tucson and Phoenix, Arizona, as well as the San Joaquin Valley in California. Recent cases and environmental isolations in Washington state indicate an expanding geographic range of C. immitis.
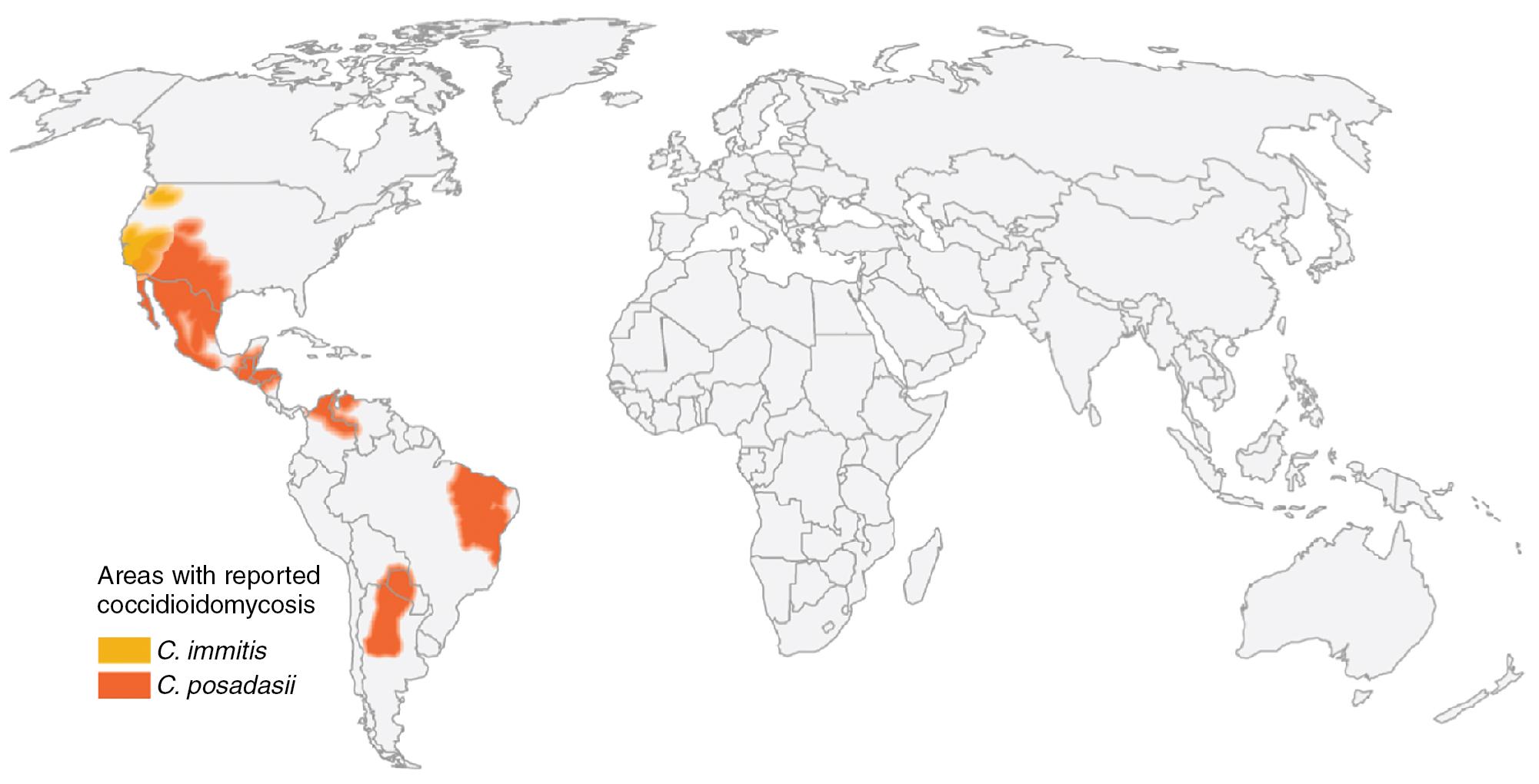
Paracoccidioidomycosis is a rural disease endemic in South America, primarily in Brazil, Colombia, Argentina, and Venezuela, as well as Central America and Mexico. The highest prevalence of cases has been reported from Brazil, especially southern Brazil.
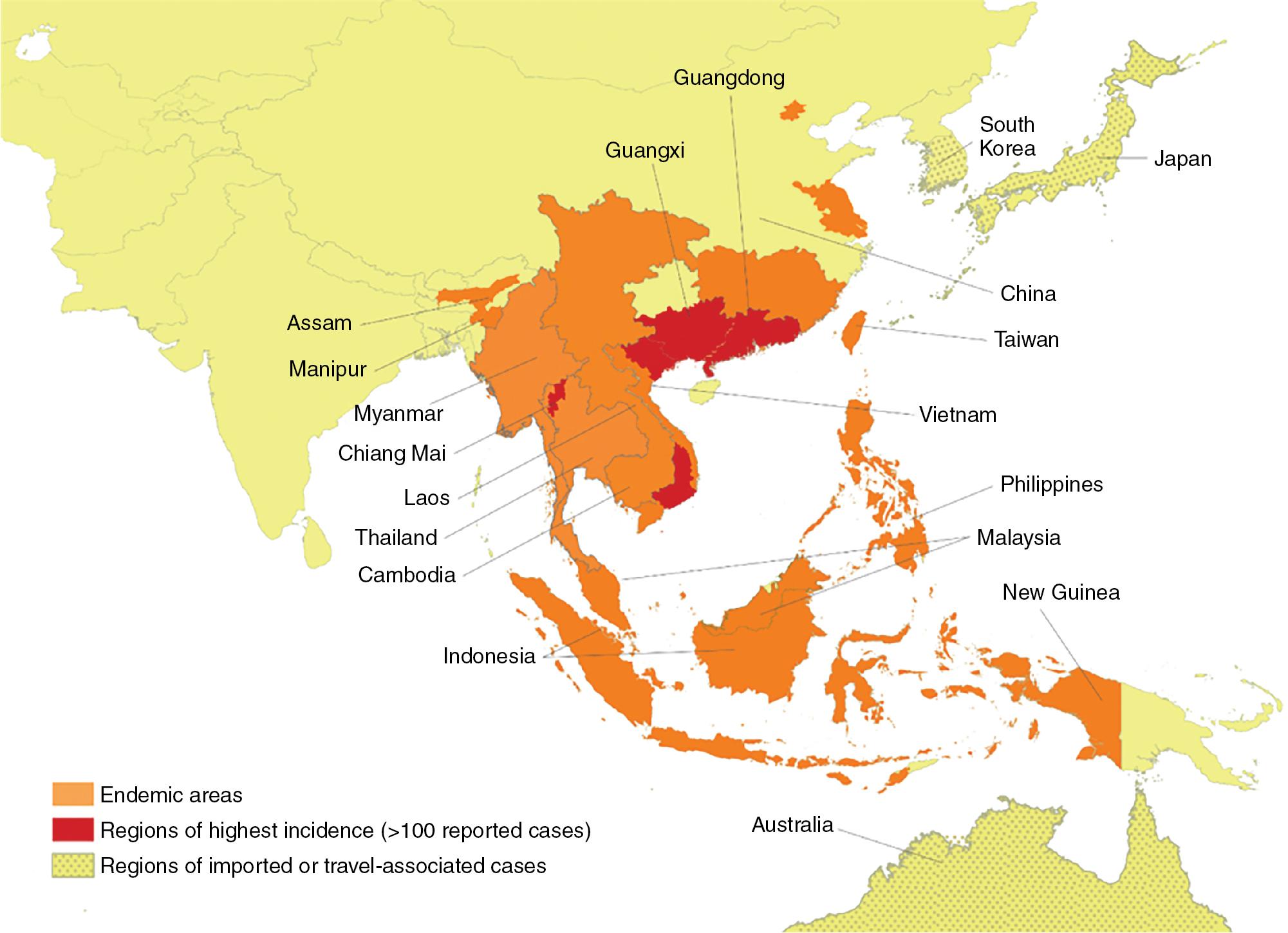
T. marneffei is restricted to Southeast Asia ( Fig. 87.6 ). Infections due to this organism have been reported with highest frequency from northern Thailand and Southwest China (including Hong Kong), and endemic areas also include Malaysia, Taiwan, Vietnam, and northeast India. In the mid-1990s, talaromycosis was the third most common opportunistic disease recognized in HIV-positive individuals, after extrapulmonary tuberculosis and cryptococcal meningitis, and it still remains a highly diagnosed disease.
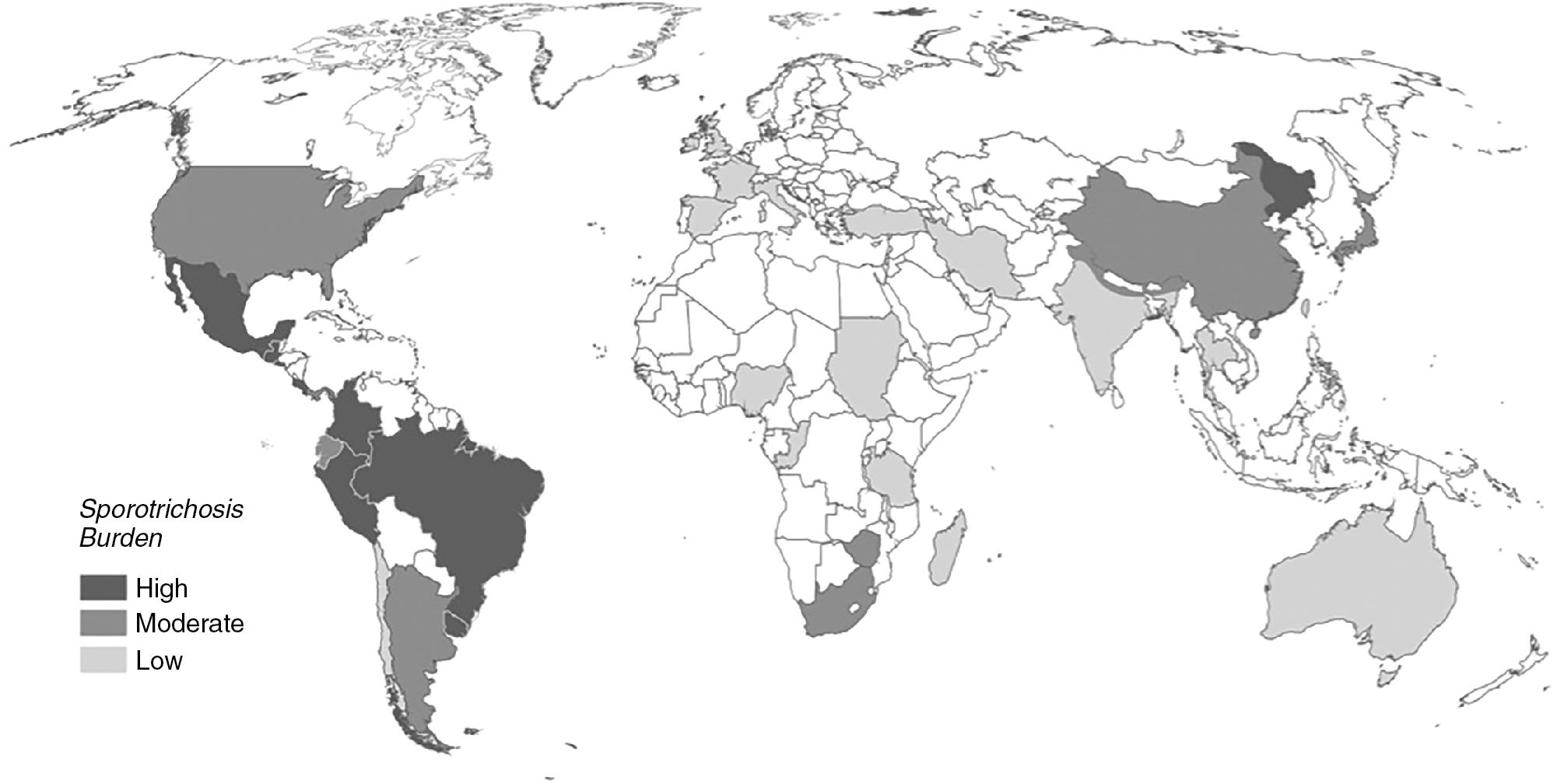
Sporothrix schenckii complex is found worldwide ( Fig. 87.7 ). The burden of disease is highest in areas of South America, Central America, and China. It is common in the central highlands of Mexico and is hyperendemic in Brazil where it causes both cat-related zoonoses ( Sporothrix brasiliensis ) and infections following environmental traumatic inoculations ( S. schenckii sensu stricto), and in the Andean mountainous region of Peru.
The yeasts Candida spp., Malassezia spp., and Cryptococcus neoformans , and the unicellular fungus Pneumocystis jirovecii are globally distributed. Although initially considered geographically restricted to tropical and subtropical areas of Africa, Australia, North America, and South America, Cryptococcus gattii has been increasingly reported in temperate parts of the world. Globally distributed molds include Aspergillus spp., Fusarium spp., and members of the order Mucorales . Scedosporium apiospermum infections occur worldwide, whereas infections due to Lomentospora prolificans have been reported more often in Australia, northern Spain, and southern United States. Most of the melanized (dematiaceous) molds that cause phaeohyphomycosis and chromoblastomycosis have a global distribution, although the latter occurs mainly in tropical and subtropical regions of Central and South America, the Caribbean countries, and Africa. , Mycetoma is a disease of the tropics and subtropics, but it is occasionally reported from temperate zones such as the Appalachian area of the United States. , Sudan appears to have the highest incidence of mycetoma in the world.
The changing geographical distribution of dermatophytes is linked to changes in human migration, the popularity of domestic animals, and public health efforts of various countries to decrease dermatophytic disease. Distributions of various dermatophytes worldwide include the following: ,
Microsporum canis : major dermatophyte in central and southern Europe; uncommon cause of tinea capitis in the United States
Trichophyton rubrum : major cause of tinea pedis and tinea unguium worldwide
Trichophyton interdigitale: second to T. rubrum in dermatophyte infections of the foot
Trichophyton tonsurans : leading cause of tinea capitis in the United States
Other less common dermatophytes include Nannizzia gypsea (formerly Microsporum gypseum ), Nannizzia nana (formerly Microsporum nanum ), Trichophyton verrucosum , and Epidermophyton floccosum
Distributions of geographically restricted dermatophytes include the following:
Microsporum audouinii : Asia and Africa
Trichophyton violaceum : second most common cause of tinea capitis in Europe and United Kingdom; most common cause of tinea capitis in India
Microsporum ferrugineum : Asia, Russia, Eastern Europe, and Africa
Trichophyton concentricum : South Pacific and South America
Trichophyton schoenleinii : Asia and Africa
Fungal infections that have recently appeared within a population or a particular geographic area may be referred to as emerging fungal infections. These may include new or previously unrecognized fungal pathogens (e.g., Candida auris ), known fungal pathogens that have expanded their geographic range (e.g., C. gattii and C. immitis ), or those with new pathogenicities or antifungal resistances (e.g., multi-azole resistant A. fumigatus ). The emergence of C. auris and multiazole-resistant A. fumigatus is of significant concern globally and is described below.
C. auris is a multidrug resistant yeast with a propensity to cause nosocomial infection that has emerged globally in the past decade. The first report of C. auris was from an ear in Japan in 2009, although retrospective testing of culture collections traced it back to 2006 in South Korea.
Phylogenetic analyses suggest that C. auris is closely related to Candida haemulonii , Candida duobushaemulonii , and Candida pseudohaemulonii and exists as four clonal clades that have emerged simultaneously and independently of one another. These are denoted as the East Asian clade, South Asian clade, South American clade, and South African clade. Additionally, a single isolate representing a potential fifth clade was identified from Iran in 2018. The reasons leading to the rapid and concurrent emergence of these clades remain poorly understood but may be related to the increasing use of antifungal agents, both clinically and in the environment, climate change, as well as the organism’s inherent thermo- tolerance and salt tolerance. , C. auris isolates are associated with resistance to one or all three major classes of antifungals; approximately 90% of isolates are resistant to fluconazole or other azoles, up to 30% are resistant to amphotericin B, and 5 to 10% are resistant to the echinocandins.
Managing infection and preventing transmission of C. auris is difficult due to challenges in obtaining accurate identification. Biochemical yeast identification systems such as API 20C AUX, ID32C, Vitek 2, RapID, BD Phoenix, and Microscan may not identify all strains of C. auris , or may lead to misidentification because C. auris is not represented in all databases. , Currently, both Bruker Biotyper and VITEK matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) in vitro diagnostic (IVD) and research use only (RUO) databases, in addition to publicly accessible curated databases such as MicrobeNet ( https://cdc.gov/microbenet/ ), have the ability to accurately identify the four clades of C. auris from culture. , , , Sequencing of the D1/D2 region of the 28S rRNA gene or the internal transcribed spacer (ITS) regions of the ribosomal DNA (rDNA) can be used to reliably identify C. auris . , , It is probable that cases of C. auris may be missed because it is uncommon for laboratories to routinely identify yeasts to the species level from nonsterile sites such as urine and respiratory specimens. Species-level identification of all yeasts (using a reliable identification method) may be warranted for patients exposed to a health care facility with known C. auris transmission, or when the patient is suspected to have a C. auris infection.
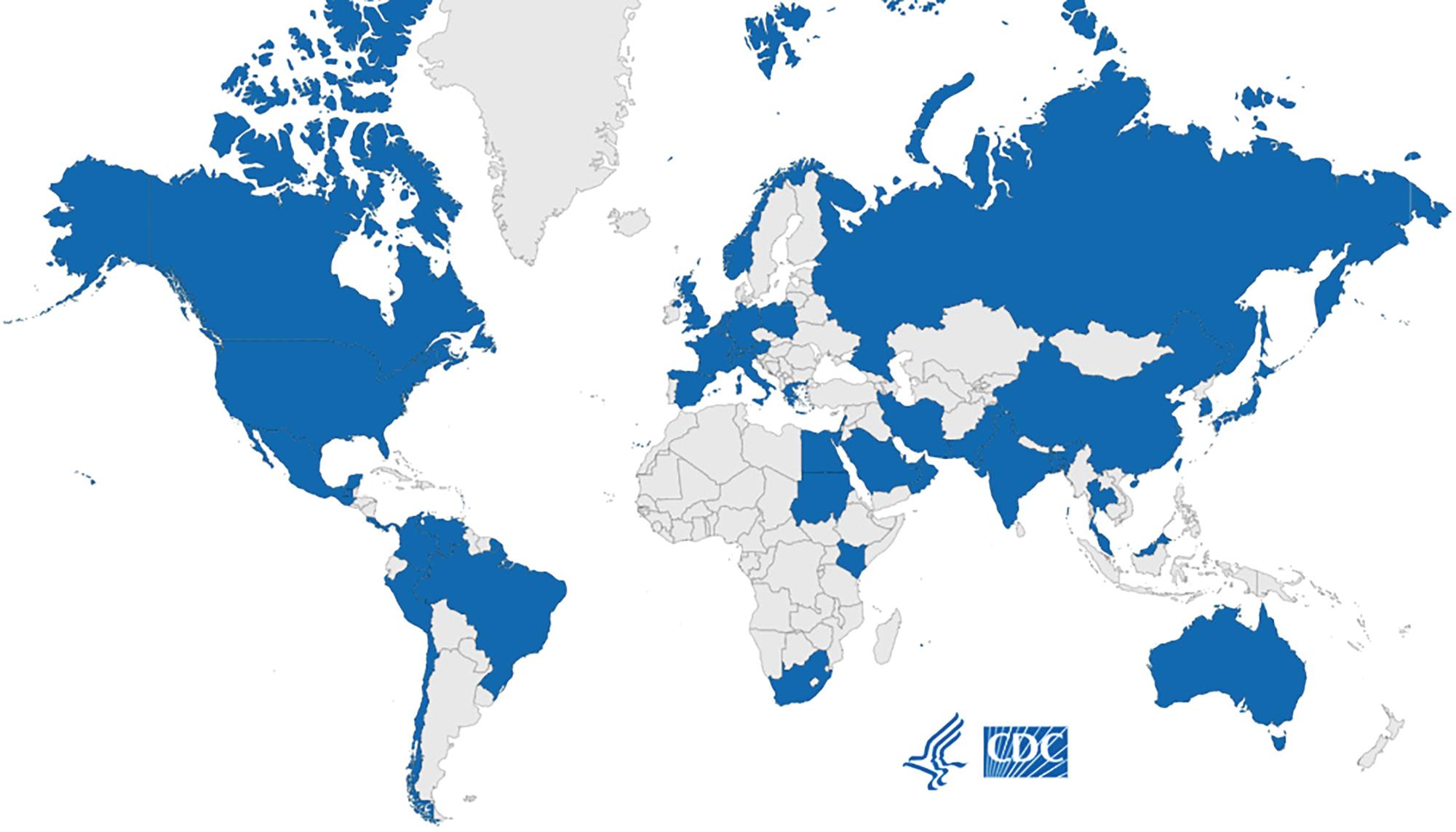
Unlike other fungal pathogens, C. auris has a propensity for nosocomial spread leading to large health care outbreaks around the world and eradication difficulties. Studies have shown that it can survive on surfaces for several weeks, easily colonizes skin, and can be transferred through skin contact/shedding and fomites such as hospital room surfaces and mobile equipment including reusable medical devices. , C. auris has been reported in about 40 countries across all inhabited continents ( Fig. 87.8 ).
Resistance to medical triazoles in A. fumigatus is an emerging problem for individuals at risk of aspergillosis. Those with triazole-resistant invasive aspergillosis have 21% higher mortality 6 weeks after diagnosis compared with triazole-susceptible infection. Resistance may be acquired through exposure of patients to triazoles during therapy or through selective pressure from nonmedical triazole fungicides which are used in agriculture. Triazole-resistant A. fumigatus isolates found in agricultural soil are typically associated with multi-triazole resistance and appear to be the primary cause of the recent emergence.
Triazole resistance in A. fumigatus is associated with mutations in the azole target, 14-α sterol demethylase (cyp51A), with or without tandem repeat mutations in the promoter region of the CYP51A gene. Triazole resistance driven by clinical exposure during triazole therapy tends to be sporadic and may involve combinations of cyp51A mutations at the following residues, among others: G54, F46, G138, M220, Y431, G448. Multi-triazole resistance driven by agricultural azole use is associated with specific residue mutations accompanied by tandem repeats (TR) in the CYP51A promoter. To date three predominant mutations have been identified: TR 34 /L98H, TR 46 /Y121F/T289A, and TR 53, although others have been induced in simulated agricultural settings.
Reported resistance rates vary widely between countries and hospitals. A multi-center prospective surveillance study covering 22 centers in 19 countries during 2009 to 2011 reported azole resistant A. fumigatus isolates from 11 of 19 countries, with resistance rates between 0 and 26.1% (overall prevalence 3.2%) among participating centers.
Isolates possessing the TR 34 /L98H, TR 46 /Y121F/T289A, and TR 53 mutations have been found in Australia, Belgium, China, Colombia, Denmark, France, Germany, India, Iran, Japan, Kuwait, Portugal, South Korea, Spain, the Netherlands, Taiwan, Thailand, Turkey, United Kingdom, and the United States. Since many cases of aspergillosis are culture-negative, the rates of resistance are likely underreported. Furthermore, incomplete surveillance is likely the reason why some countries have not yet identified resistant isolates. The Netherlands and the United Kingdom appear to have the highest rates of triazole-resistant isolates and the use of imidazole or triazole fungicides is very high in the Netherlands.
Azoles are used both pre- and postharvest in agriculture to prevent food spoilage and assist in plant cultivation. Over time, it has become clear that these activities have contributed significantly to the recent emergence of multi-triazole resistant A. fumigatus in the environment. , Compost containing residues of azole fungicide appears to facilitate sexual reproduction in A. fumigatus where resistance mutations are likely to develop. By-products from the compost then serve as a vector for further environmental spread. Resistant isolates have been recovered from hospital environments, flowerbeds, cultivated plants, seeds, and compost. , , In contrast, isolates recovered from untreated soil or compost have not been triazole resistant. , The export of plant bulbs from the Netherlands to other countries has been identified as a vector for international spread of resistant isolates.
Early and accurate diagnosis of fungal infections is the most critical factor for guiding clinical management and improving patient outcomes. This, however, remains challenging because clinical symptoms and radiologic signs of fungal infections are nonspecific. Laboratory diagnostics have significantly advanced over recent years with the advent of highly sensitive molecular platforms that enable rapid detection and identification of fungi directly from clinical specimens and the availability of MALDI-TOF MS that can provide accurate species-level identification of cultured organisms within minutes. , Despite these exciting developments, however, the gold standard for definitive diagnosis of invasive infections still relies on culture of the organism and/or histopathologic detection. Because of the nonspecific nature of invasive infections and the wide variety of test systems available, stratification of patients according to their risk of acquiring an invasive disease should guide test selection to maximize diagnostic yield and minimize the consequences of unnecessary administration of empiric antifungal therapy.
Superficial fungal infections are much more prevalent than invasive ones. Most superficial infections are benign and caused by commonly recognized fungi. However, in some instances, infections may involve fungi that are rarely encountered in the laboratory. Because of the vast spectrum of potential pathogens in superficial and invasive infections alike, every unusual fungus should be evaluated carefully when an infection is suspected and identified by conventional methods in combination with advanced technologies such as MALDI-TOF MS or molecular methods, when possible. This section addresses the clinical laboratory tools available for the diagnosis of fungal infections.
The clinical mycology and anatomical pathology laboratories both play equally important yet distinct roles in the diagnosis of invasive fungal infections. The clinical mycology laboratory provides faster turnaround time for rapid stains and immunologic and molecular assays, with results often available within a day of specimen receipt. The primary advantage of the clinical mycology laboratory, however, is the ability to culture a potential pathogen which can help provide meaningful results via organism identification and antifungal susceptibility profiles for guiding clinical decisions. The availability of a cultured isolate also provides the potential for strain typing for epidemiologic and lineage investigations (most recently evident for C. auris ). A disadvantage of fungal culture, however, is that it may take days or weeks to finalize. Regardless, it is important to remember that all findings for a suspected fungal infection must be considered holistically within the clinical context of the patient, taking into account the potential for recovery of laboratory contaminants, as well as distinguishing between colonization and disease states.
In contrast, the anatomical pathology laboratory provides insight into disease progression and degree of tissue damage, and it can thus provide definitive diagnosis of invasive infection. In general, stains used in anatomical pathology are more sensitive for detecting fungal elements compared with some of the commonly used stains in the clinical mycology laboratory. Although culture and/or molecular testing is required for the identification of most fungi, histopathology enables the etiologic diagnosis of infections caused by the dimorphic fungi and nonculturable organisms such as P. jirovecii and Rhinosporidium seeberi. ,
A clear diagnostic differential with appropriate test selection must be established so that specimens are split appropriately between the clinical mycology and anatomical pathology laboratories. Once sent to anatomic pathology, specimens are fixed in formalin, eliminating organism viability for culture.
It is important that both clinical teams and laboratories are aware of proper safety and handling procedures for the transport of infectious substances. An infectious substance is defined as a microbiological culture, biological product, patient specimen, genetically modified organism, or clinical waste. Briefly, infectious substances are divided into two categories, A and B, according to the risk of developing a severe disease upon exposure. In the clinical setting, the majority of infectious substances are classified in the “less severe” category B. Coccidioides species in culture form is the only fungus classified as category A.
Almost all patient specimens and fungal cultures should be handled in a biosafety level 2 facility in a class II biosafety cabinet. Cultures of most of the dimorphic fungi, however, should be processed and handled in a biosafety level 3 facility. , Laboratory-associated infections due to subcutaneous inoculation and/or organism inhalation have been documented for all the dimorphic fungi. , Clinicians who suspect infection with a dimorphic fungus should alert the laboratory immediately to ensure appropriate handling and safety. Laboratory-acquired infections have also been reported for Cryptococcus spp. and dermatophytes. Other biosafety considerations regarding risk assessment, laboratory facilities, and personnel are described in the CLSI document M54-A.
Appropriate preanalytical specimen management, which includes specimen collection, transport, storage, and rejection criteria, is critical for optimizing diagnostic accuracy. Specimen quality impacts the interpretation of test results, so it is essential that physicians select and collect specimens from appropriate body sites, following specified guidelines, and transport them to the laboratory in a timely manner. A thorough understanding of laboratory requirements through training and education, good communication between laboratory and clinical staff, and/or easy access to laboratory references via a website or portal assist in optimizing preanalytical processes. Poorly and/or improperly collected specimens may lead to erroneous or misleading results that will directly impact patient care and outcomes including therapeutic decisions, length of stay, hospital and laboratory costs, and laboratory efficiency. , , , Laboratories should also ensure compliance with specific preanalytical criteria that are mandated by regulatory and accrediting agencies in their respective jurisdiction.
The recovery of fungi is dependent on the collection of a good-quality specimen at an adequate volume that will allow for specimen concentration and/or pretreatment to maximize sensitivity. Collection from the active site of infection is best, preferably before initiation of antifungal therapy. For invasive disease, and when possible and appropriate for the anatomical site, a deep specimen such as a biopsy or bronchoalveolar lavage (BAL) is preferred over superficial collections. Biopsies should be obtained after appropriate tissue debridement. The sensitivity of culture results for sputum and urine is maximized when first-morning specimens are obtained. The use of sterile swabs with transport media should be limited to cases of suspected yeast infection of the mucous membranes (e.g., conjunctiva, mouth, and vagina) or infections of the scalp and ear. Anaerobic transport media or anaerobic containers are generally not recommended, and package inserts should be referred to for information about fungal viability prior to their use. For sampling of keratinized tissues, the area should first be disinfected with 70% alcohol and air-dried in order to minimize bacterial and environmental mold contamination. Hair and beard stubs should be pulled or plucked so the root is collected with the stubs. Because hair and beard infections initiate in the skin, skin scrapings should also be obtained. , Glabrous skin should be sampled by scraping the infected area, especially the leading edge of the expanding lesion. Sampling of nails is dependent on the type of infection. For nail dystrophy without concomitant paronychia (tissue inflammation adjacent to a nail), the specimen is collected by clipping, scraping, or curetting the nail and nailbed, including the junction of diseased and healthy nail (site of active fungal growth); all material should be submitted for testing. In the presence of concomitant paronychia, which is typical of Candida onychomycosis, the proximal and lateral edges of the nailbed should also be sampled.
Routine blood cultures have limited sensitivity for the detection of fungemia. Automated broth-based blood culture systems, such as BacT/ALERT (bioMérieux, Marcy l’Etoile, France), VersaTREK (Thermo Scientific Microbiology, Oakwood Village, OH), and BACTEC (Becton Dickinson, Sparks, Maryland) can detect most Candida spp. and sometimes Fusarium and Scedosporium/Lomentospora spp. The Wampole Isolator system (Alere, Orlando, FL), which is based on blood lysis and centrifugation, followed by inoculation onto appropriate fungal media, is not automated but is recommended for improved recovery of molds due to white blood cell lysis (although diagnostic yield is still low). Analytical performance and time to positivity may also vary among systems. To improve culture sensitivity, two to three sets of blood culture bottles should be sampled from different venous access sites at a blood volume specified by the manufacturer (often 10 mL for adults and smaller volumes for children). , When disseminated Histoplasma infection is suspected, bone marrow specimens should be collected. Detection of antibodies or antigens in blood and of antigens in respiratory and urine specimens can also be useful for the detection of some invasive fungal diseases.
Mycologic tests should not be performed on urinary catheters, lochia, vomitus, or colostomy discharge. Stool specimens are typically not acceptable for fungal culture except in cases of suspected GI wall invasion due to disseminated Mucorales disease or GI basidiobolomycosis. In the case of suspected intestinal infection by microsporidia, stool specimens may be tested by molecular analysis or appropriate stains.
Specimens submitted for fungal culture should be collected in the appropriate sterile and leak-proof container and transported to the laboratory at room temperature immediately or within 2 hours of collection. Specimens should not be allowed to desiccate or be exposed to extreme temperatures (<10 or >37 °C); dermatophytes are particularly sensitive to cold temperatures.
Specimens should be rejected if they have been improperly collected (inappropriate site and/or container), delayed in transport to the laboratory (generally by more than 72 hours), or leaking. Appropriate laboratory quality assurance parameters should be in place to ensure best practices are followed for appropriate specimen identification and patient safety (further details are provided in Carey et al.).
A variety of stains and media are used for the isolation, detection, and identification of fungi from clinical specimens. A summary of stains used in the clinical laboratory and their mechanism of action is provided in Table 87.5 . While it can sometimes be useful, the Gram stain is not the optimal method for detection of fungi in clinical specimens because yeasts and molds may stain poorly.
| Name | Mechanism of Action | Application | Comments |
|---|---|---|---|
| Stains Used for Direct Examination of Patient Specimens a | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stains Used for Histologic Section of Tissue Specimens | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stain Used for Fungal Cultures | |||
|
|
|
|
a Stains apply to direct microscopy methods but not to histologic staining of paraffin-embedded tissue specimens.
Although most bacteriology media can support the growth of fungi, the potential for bacterial overgrowth limits their utility for fungal culture and isolation. Additionally, morphologic descriptions of fungi are often based on growth from specific fungal media. Primary media are often enriched for the cultivation of fungi directly from clinical specimens and contain antibiotics to inhibit the overgrowth of bacteria. Secondary media are used to stimulate the production of fungal structures to aid in organism identification. The availability of commercially prepared fungal media has assisted greatly in media standardization.
In general, fungal media consist of four main categories, as shown in Table 87.6 . Table 87.7 outlines the common media used for the primary and secondary isolation of fungi in the clinical mycology laboratory.
| Media Category | Examples of Media |
|---|---|
|
|
|
|
|
|
|
|
| Name | Purpose |
|---|---|
| Primary Media for Direct Plating of Patient Specimens a | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Secondary Media for Fungal Identification | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a Performance (for colony yield and growth rate) and colony morphologies may vary between agars of the same type (e.g., Sabouraud dextrose agar) because of differences in agar composition among commercialized agars.
b Emmons modification of Sabouraud dextrose agar (less dextrose and higher pH than the original formula) enhances the recovery of many pathogenic fungi.
Processing for fungal culture varies with the specimen type and volume. , All processing that involves infectious aerosols or splashes must be performed in a class II biosafety cabinet. Table 87.8 provides suggestions for media selection, pretreatment procedures, and incubation conditions for patient specimens. Agar slants or plates may be used; selection is often dependent on test volume and incubator space. Agar plates must be incubated in gas-permeable bags or sealed with gas-permeable tape to prevent dehydration during incubation (2 to 4 weeks). The laboratory is responsible for defining specimen retention time and appropriate specimen storage. Hair, skin scraping, and nail specimens should be kept at room temperature before testing; all other specimens should be refrigerated.
| Source | Pretreatment, Suggested Set of Media, a and Incubation Conditions b | Comments |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a Some institutions include buffered charcoal yeast-extract medium in the set of media for blood, respiratory tract, sterile fluid, tissue, and wound specimens to enhance the recovery of Nocardia species, which are slow-growing aerobic and filamentous bacteria whose clinical manifestations sometimes mimic fungal infections. SAB medium, included in several suggested sets above, also enables growth of Nocardia species.
b Incubation time may need extension to 6 to 8 weeks when blastomycosis or histoplasmosis is suspected.
c Variable concentrations of cycloheximide may inhibit some fungal species in genera such as Aspergillus , Aureobasidium and other melanized fungi, Candida , Fusarium, and Scedosporium .
To maximize culture sensitivity, large-volume liquid specimens (>2 mL) should be concentrated by centrifugation at 2000 × g 10 minutes; , the sediment should then be used to inoculate media. Highly viscous specimens (e.g., sputum and abscesses) may be digested with mucolytic agents prior to specimen concentration. These agents also assist in the removal of bacteria that may be present in the specimen. Recovery of Aspergillus species and other non- Candida fungi from sputa is affected by the media used and the volume of inoculated material. An inoculum of 100 μL or more of sputum plug or digested sputum per plate has a much higher yield than conventional inoculation methods (e.g., dipping a swab or loop directly into the sputum). ,
Direct inoculation should be performed for fluids up to 2 mL, hair, skin scrapings, nails, abscesses, tissues, and swabs from mucosal surfaces. Granules present in abscess specimens are indicative of a mycetoma and should be washed in saline and crushed for culture and microscopic examination. Nail clippings should be minced by the use of a scalpel or pulverized (e.g., with a nail homogenizer) prior to examination and plating. Hair, skin scrapings, and nail particles should be inoculated onto four or five spots spread out over the agar. Keratinous material must be digested in 10 to 30% potassium hydroxide solution (KOH) before microscopic examination to facilitate the visualization of fungal elements. Corneal scrapings may be directly inoculated onto media in the physician’s office and subsequently sent to the laboratory. Mincing of biopsies (rather than grinding) is recommended for fungal recovery to help preserve hyphal structure and organism viability; , the Mucorales are particularly sensitive to crushing. In contrast, tissue specimens from patients with suspected Histoplasma infection should be ground to help release the intracellular organisms. Special consideration must be taken for specimens obtained from patients with suspected Malassezia infection because oil must be added to the media before plating. Specialized media for Malassezia isolation are available (e.g., Dixon’s agar).
Malassezia species require oil, tween, or Dixon’s agar for growth
Mucorales fungi are easily destroyed by crushing and grinding
Growth rate of fungi is slower than standard incubation times
Fungus may be nonculturable (i.e., submitted in fixative)
Fungus may be sensitive to cycloheximide in culture medium
Poor specimen collection from dystrophic nails; distal nail part may contain dead dermatophytes or contaminating molds that inhibit dermatophyte growth
Poor culture recovery due to antifungal effect
Direct microscopy of clinical specimens is an important process in diagnostic clinical mycology. Fungal elements, when present, may be detected within minutes (compared with days or weeks for culture), and their morphology helps to narrow the differential diagnosis. In certain cases, direct microscopy provides definitive diagnosis of the disease etiology (e.g., the presence of spherules indicates coccidioidomycosis). Microscopy also provides the opportunity to better differentiate colonization, tissue invasion, and contamination compared with culture by assessing the position of the organism in relation to inflammatory cells or other tissue elements (e.g., the intracellular location within histiocytes indicates an infectious state). Because direct microscopy can detect nonviable organisms, artifacts such as fibers, lymphocytes, and fat droplets may occasionally be mistaken for fungi ( Fig. 87.10 ). Results should therefore always be correlated with clinical and other laboratory findings, when possible.
The sensitivity of direct microscopy depends in part on appropriate sampling from the infected site and the quality of the specimen. In cases of insufficient specimen volume, priority should always be given to culture (over smears), with the exception of dermatologic specimens, for which microscopy is the test of choice, and infections with Mucorales fungi because they are easily damaged by crushing during specimen processing.
Anatomic pathology is important for understanding the pathophysiologic process of fungal infections by enabling visualization of tissue damage caused by fungi and thus serves as the ultimate confirmation of fungal disease. With a few exceptions, fungi cannot be identified to the genus or species level solely by their anatomic pathologic appearance in paraffin-embedded biopsy specimens or cytology smears. Unless a feature specific to a certain fungus is observed (e.g., conidial head of Aspergillus in specimens from sinuses or lung cavities where the fungus is exposed to air), the anatomic pathology report should include a description of the fungal elements (e.g., thin septate hyphae with 45-degree-angle branching) and, if possible, a differential diagnosis of various fungi with similar appearance. For instance, if a few septate hyphae with parallel walls are seen in tissue without the typical dichotomous branching of Aspergillus species, the pathologist and/or microbiologist may consider conveying to the clinical team a differential that includes Aspergillus and other morphologically similar fungi (e.g., Fusarium in the context of a severely immunocompromised patient). The use of phrases that imply unequivocal identification (e.g., “septate branching hyphae consistent with Aspergillus” ) should be avoided. An overview of fungal hyphae seen in tissues is provided in “At a Glance: Approach to Assessment of Hyphae in Anatomic Pathology-Derived Tissue.” Anatomic pathology reports should refer to microbiologic reports if cultures were submitted.
Box 87.1 highlights points that are important to consider when examining surgical pathology or cytology tissue.
The nature of the host inflammatory response to microorganisms is important in formulating a differential diagnosis (e.g., neutrophilic response to Candida or pyogranulomatous inflammation in blastomycosis).
It is helpful to obtain a history of the immune status of the host (e.g., neutropenic status) in order to guide cytologic or histopathologic interpretation.
A reticle (eyepiece micrometer), commonly used to measure the size of various structures in cultured fungi, enables precise measurements of fungal elements in clinical specimens. Alternatively, sizes of fungi in clinical specimens can be approximated by using nearby red blood cells (approximately 7 μm in diameter) or other cell types of known sizes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here