Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A 22-year-old woman, 154 cm tall and weighing 44.2 kg, presents with a 4-month history of myasthenia gravis and mild generalized weakness (Myasthenia Gravis Foundation of America’s class IIA). The diagnosis is confirmed by the patient’s rapid improvement after the administration of intravenous edrophonium chloride and by the presence of antibodies to acetylcholine receptors (12.3 nmol/L; reference value <0.25 nmol/L). Nerve conductions and electromyography studies were normal, but the repetitive stimulation of a nerve demonstrated decrements of the muscle action potential. Magnetic resonance imaging identified an abnormal thymus gland. The patient is scheduled for transcervical-sternal thymectomy. Preoperatively, she took pyridostigmine 60 mg orally three times a day and had plasmapheresis. Results of her preoperative pulmonary function tests were as follows: forced vital capacity (FVC), 2.79 L/second (79% of predicted); maximum expiratory flow at 50% of FVC, 3.2 L/second (68% of predicted); and forced midexpiratory flow between 25% and 75% of FVC, 3.03 L/second (77% of predicted). Anesthesia was induced with fentanyl and propofol and maintained with a thoracic epidural block supplemented with propofol and 70% nitrous oxide in oxygen. Tracheal intubation was performed under topical laryngotracheal anesthesia (4 mL 4% lidocaine). No neuromuscular blockers were used. She required mechanical ventilation for 12 hours postoperatively.
The plasticity of the neuromuscular transmission is dependent on a coordinated mechanism involving (1) synthesis, storage, and release of acetylcholine from the presynaptic motor nerve endings at the neuromuscular junction; (2) binding of acetylcholine to nicotinic receptors on the postsynaptic region of the muscle membrane, with consequent generation of the action potential; and (3) rapid hydrolysis of acetylcholine by acetylcholinesterase enzyme present in the synaptic cleft.
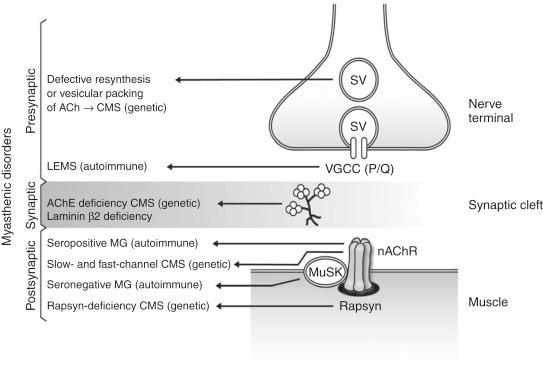
Autoimmune or genetic defects at the presynaptic region, synaptic basal lamina, or postsynaptic structure of the neuromuscular junction can compromise the safety margin of neuromuscular transmission. This can result in a diverse array of myasthenic disorders ( Fig. 14.1 ). Fluctuating muscle weakness and fatigability are the main characteristics of myasthenic disorders ( mys, meaning “muscle”; aesthenia, meaning “weakness”). Myasthenic disorders affect the motor system only. Sensory and autonomic functions are not impaired. The exception is Lambert-Eaton syndrome, a myasthenic syndrome in which a significant minority of patients have autonomic dysfunction. Myasthenic disorders can be classified into three main categories: myasthenia gravis, congenital myasthenic syndromes, and Lambert-Eaton myasthenic syndrome ( Tables 14.1 and 14.2 ).
| Site | Disorder | Type | Cause | Morphology | Clinical Features | Management |
|---|---|---|---|---|---|---|
| Presynaptic | Lambert-Eaton myasthenic syndrome | Autoimmune | Antibodies target voltage-gated calcium (Ca 2+ ) channels at motor nerve terminal and possibly another presynaptic component (synaptotagmin), leading to reduction in ACh release | Normal ACh contents and NMJ architecture | Approximately 60% of patients have paraneoplastic response, often in association with small cell lung carcinoma Weakness and fatigability |
With malignancy, successful treatment can lead to marked improvement in symptoms 3,4-Diaminopyridine blocks prejunctional potassium (K) channels to (1) prevent K efflux, (2) increase action potential duration, (3) prolong activation of voltage-gated Ca 2+ channels, and (4) increase intracellular Ca 2+ stores and ACh release Pyridostigmine potentiates response to 3,4-diaminopyridine Often, plasmapheresis or IV immunoglobulin provides transient improvement |
| Choline acetyltransferase deficiency | Genetic | Choline acetyltransferase mutations cause insufficient ACh resynthesis | Number of nAChRs and end-plate structure are normal | Autosomal recessive inheritance Characteristic apneic attacks along with myasthenic symptoms |
AChE inhibitors | |
| Synaptic | AChE deficiency | Genetic | Mutations in the gene encoding the collagenic tail subunit of the enzyme anchoring AChE in the synaptic cleft decrease the expression or the catalytic efficacy of the enzyme | Absent or reduced AChE activity (by histochemical staining) Secondary loss of nAChR and postsynaptic region degeneration |
Autosomal recessive disease with variable phenotypic expression Moderately severe, generalized weakness and scoliosis with restrictive lung disease are common |
Due to deficiency of AChE enzyme, patients do not benefit from anticholinesterase therapy |
| Laminin β 2 deficiency | Genetic | Mutations in the gene encoding laminin β 2 subunit | Immature hypoplastic nerve terminals, which remain encased by cytoplasmic processes of the Schwann cell Moderate simplification of postsynaptic folds and intact expression of the endplate acetylcholinesterase |
Autosomal recessive inheritance | Ephedrine and albuterol (but not pyridostigimine) appear to improve neuromuscular transmission via an unknown mechanism | |
| Postsynaptic | Myasthenia gravis: seropositive or seronegative | Autoimmune | Antibodies to nAChRs Antibodies to MuSK |
End-plate regions have simplified architecture with smaller folds and marked reduction in nAChR (approximately 30% of that in normal NMJ) | Age at onset of myasthenic symptoms is earlier in MuSK antibody–positive patients Neck muscles are commonly involved in MuSK antibody–positive patients, and limb muscles in MuSK antibody–negative patients |
Preoperative optimization by plasmapheresis and continued pyridostigmine therapy Patients are extremely sensitive to NDMRs Response to SCh and mivacurium depends on butyrylcholinesterase activity, which is expected to decrease after plasmapheresis or pyridostigmine |
| Reduced expression of nAChR or rapsyn deficiency | Genetic | Mutations in nAChR or in rapsyn decrease expression of nAChRs | Changes in end-plate regions are similar to those seen with autoimmune MG | Autosomal recessive inheritance Patients exhibit myasthenic symptoms from birth or infancy Facial malformations are common in rapsyn deficiency |
Response to anticholinesterase is incomplete Combined therapy with 3,4-diaminopyridine (which increases ACh release) is beneficial |
|
| Slow-channel congenital myasthenic syndromes | Genetic | Kinetic defects and/or gain-of-function mutations in nAChR cause lengthy nAChR opening and excessive Ca 2+ influx with postsynaptic degeneration | Postsynaptic degeneration with loss of nAChRs; AChE is normal | Usually dominant inheritance Selective weakness in cervical, scapular, and finger extensor muscles; variable weakness in other muscles |
Open channel blockers (quinidine, fluoxetine) normalize slow-channel mutant opening durations No response to AChE medications Avoid SCh because it can worsen excitotoxicity |
|
| Fast-channel congenital myasthenic syndromes | Genetic | Mutations in nAChR markedly reduce binding affinities, resulting in rapid ACh dissociation from binding sites, reducing the rate of channel opening, and increasing its closure rate | NMJ structure normal; density of nAChRs normal or decreased | Autosomal recessive inheritance Moderate symptoms from birth to infancy Partial response to AChE inhibitors |
Combination treatment with 3,4-diaminopyridine and AChE |
| Myasthenia Gravis | Lambert-Eaton Myasthenic Syndrome | Congenital Myasthenic Syndromes | |
|---|---|---|---|
| Cause | Autoantibodies targeting nAChRs or MuSK | Autoantibodies targeting presynaptic voltage-gated (P/Q) calcium (Ca 2+ ) channels or synaptotagmin | Genetic mutations of presynaptic, synaptic, or postsynaptic proteins Dominant or recessive inheritance (no antibodies against nAChRs, MuSK, or P/Q type Ca 2+ channels) |
| Associated conditions | Thymic lymphoid follicular hyperplasia present in 70% of MG patients Thymoma present in 12% of MG patients (paraneoplastic autoimmune response) Associated autoimmune conditions include thyrotoxicosis, systemic lupus erythematosus, rheumatoid arthritis, and pernicious anemia |
60% of LEMS patients have paraneoplastic autoimmune response Small cell lung carcinomas express voltage-sensitive Ca 2+ channels; antitumor antibodies to these channels cross-react with prejunctional voltage-gated Ca 2+ channels at the NMJ to impair ACh release |
|
| Target location | Postsynaptic | Presynaptic | Presynaptic, synaptic, or postsynaptic component of NMJ |
| Dysautonomias | Absent | Present in approximately 30% of patients (dry mouth, impotence) | Absent |
| Improvement in muscle strength | After rest | After exercise | After rest |
| Antibody transfer | From myasthenic mother to fetus, causing neonatal MG Injecting healthy animals with MG IgG causes signs of MG |
IgG from LEMS patients can block Ca 2+ channels, inhibiting muscle contraction | Antibodies are not present |
| Electromyography (response to 30–50 Hz stimulation) | Fade | Facilitation | Fade |
| Effect of plasmapheresis | Transient | Transient | No effect |
| Anticholinesterases | Effective in managing symptoms | Minimal therapeutic value | Minimal therapeutic value |
| Response to 3,4-diaminopyridine | No effect | Significant improvement in symptoms | Effective in fast-channel congenital myasthenic syndromes |
| Response to succinylcholine | Resistant | Sensitive | Variable, not recommended due to potential hyperkalemic response in slow-channel mutations |
| Response to nondepolarizing neuromuscular blockers | Sensitive | Sensitive | Sensitive |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here