Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In temperate climates, multiple sclerosis (MS) is the most common episodic neurologic illness of early adult years. The process begins as periodic and focal loss of central nervous system (CNS) myelin and the oligodendrocytes (OGCs) that synthesize myelin. Axons that have lost their myelin function imperfectly or not at all. Accordingly, symptoms ensue, and as episodes recur, disability accumulates.
MS is thought to be an autoimmune disease even though no antigens are identified, with certainty, against which a disease-relevant autoimmune response might be directed. As with other autoimmune entities, there is a genetically determined propensity to develop the illness. The strongest positive genetic association is with the class II major histocompatibility complex (MHC) antigen, that is, the human leukocyte antigen (HLA)-DRB1*15:01. HLA-DR alleles present antigenic peptides to CD4 + T cells, pointing to a major disease-promoting role for CD4 + T cells in MS. In contrast, HLA-A*02:01 expression is reduced in MS, indicating a protective role for this, the most commonly expressed class I allele in humans. The less prevalent HLA-A*03:01 class I allele doubles risk for developing MS. HLA-A alleles present antigenic peptides to CD8 + T cells, indicating that CD8 + T-cell–mediated protection is suboptimal in MS . HLA-A*03:01 and HLA-DRB1*15:01 are independent risk factors . Genome-wide studies have identified some 50 additional minor genetic associations. Most have a role in immune system function with a major enrichment in cell surface receptor genes implicated in T-cell activation and proliferation. One third of identified genomic loci overlap with regions associated with one or more other autoimmune diseases.
The prevalence of MS can be as high as 1 in 500 in the overall population. Twenty percent of patients have a blood relative with the disease. In siblings and in children of an affected parent, concordance for MS is 1% to 3%, ruling out simple dominant, recessive, or sex-linked inheritance. Siblings share half their genes. Yet, even among identical twins sharing all their genes, MS concordance is only 25%, indicating that environmental factors have a major role in determining risk for MS.
Epstein-Barr virus (EBV) is acquired in adolescence or early adult years in developed countries, where MS is encountered frequently and where EBV often causes infectious mononucleosis; in less-developed countries, where MS is uncommon, EBV is usually acquired asymptomatically in early childhood. Unlike controls, at diagnosis all MS patients test positive for prior contact with EBV, and a history of frank infectious mononucleosis (always before disease onset) is increased threefold over the general population. EBV is at present the leading candidate environmental trigger for propensity to develop MS. The presence of subnormal vitamin D levels is a possible additional putative environmental factor in MS. This vitamin is an inflammatory response inhibitor and an enhancer of regulatory T-cell function, coupled with the fact that MS is uncommon in regions with high sunlight exposure, the chief inducer of vitamin D synthesis.
Clinical Course. MS usually begins in young adults; peak age at first attack is 30 years, but onset can occur before age 10 years or after age 50 years. MS is two to three times as frequent in women. Eighty-five percent of patients present with a clinically isolated syndrome characterized by subacute loss of neurologic function that will usually worsen over a week or more, stabilize for a time, and eventually recover partially or, quite often, completely. Subsequently, after highly variable intervals, additional episodes, known as relapses, develop. Relapses, having finite spans of a few weeks, are followed by recovery of variable extent and duration. Periods of seeming disease quiescence occur with remissions lasting for months or years. These patients are referred to as having relapsing-remitting multiple sclerosis (RRMS). Symptoms and signs vary from one relapse to the next as additional sites of myelin loss accumulate within the CNS white matter. Sites of myelin loss are called plaques; their locations determine symptoms.
After some years, the character of MS can change. Relapses diminish in frequency, ultimately cease, and are replaced by slow but steady worsening of nervous system impairment referred to as secondary progressive MS (SPMS), distinguishing it from the 15% of cases in which a primary progressive course is present from symptom inception. Primary progressive MS (PPMS) usually begins later in life than RRMS; a female preponderance is less evident. The usual presentation is a slowly progressive myelopathy evolving into paraparesis or paraplegia.
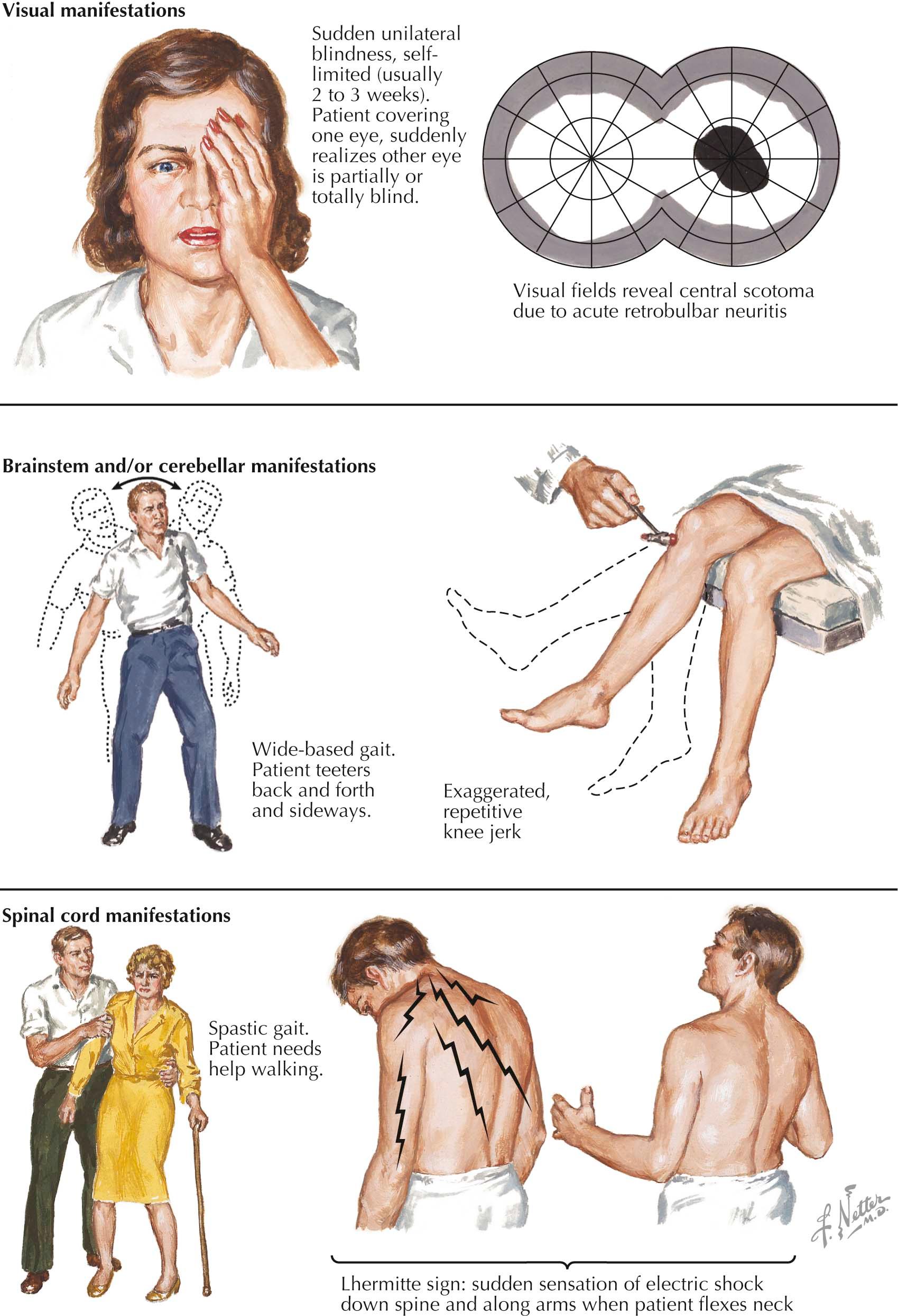
Symptoms and signs of MS vary with the locations of the plaques.
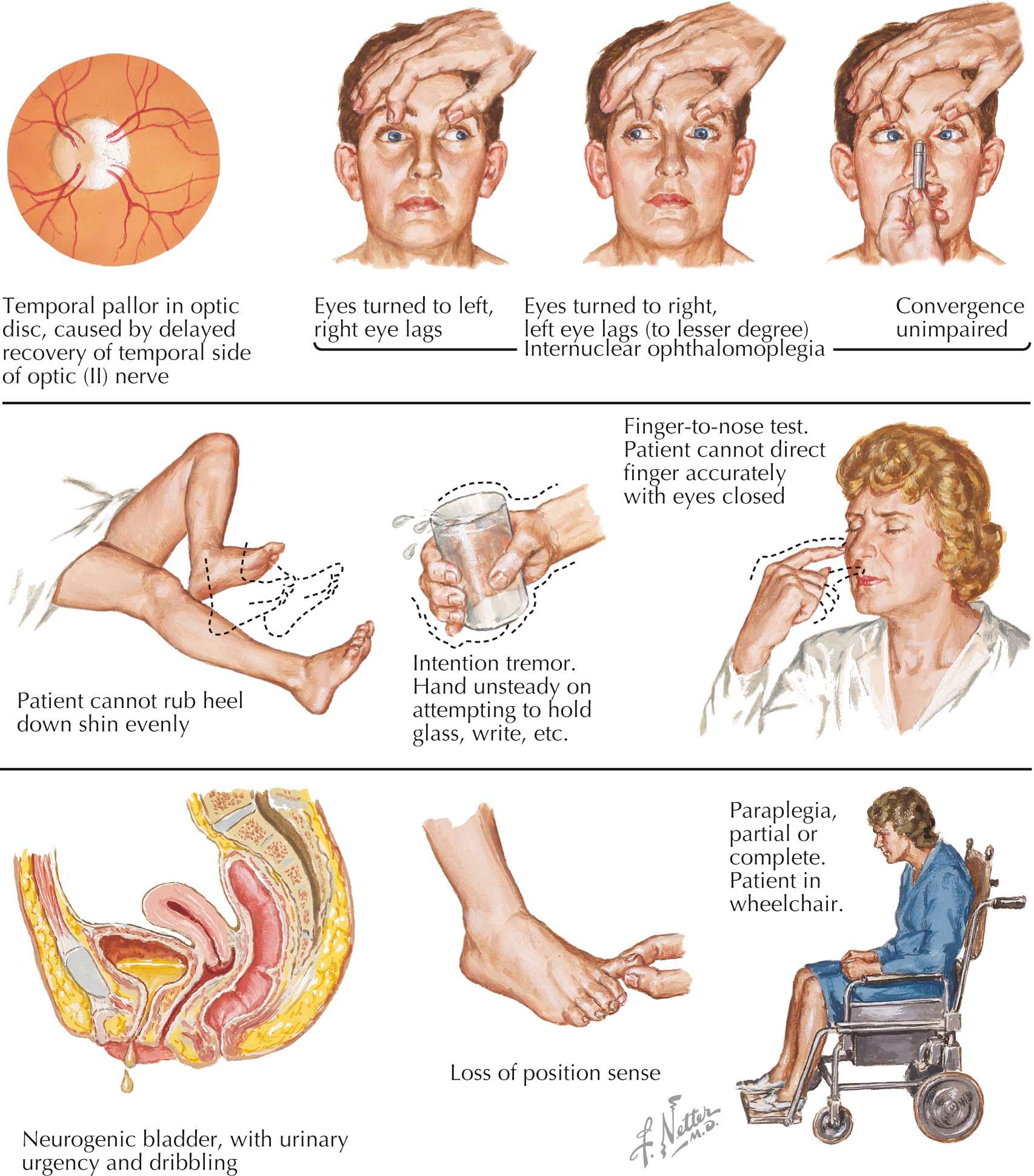
Typically, patients experience relatively abrupt unilateral decrease in central or paracentral vision with pain on movement of the globe; this is a very common MS presentation. At times, symptoms are subtle, with brief episodes of decreased visual acuity provoked by exposure to heat, such as hot showers, followed by prompt resolution. Magnetic resonance imaging (MRI) may show a lesion in the affected optic nerve, and usually, if this is a first attack of MS, other lesions indicative of earlier clinically silent multiple sclerosis activity are seen on brain MRI. Optic neuritis without the concomitant presence of such MRI lesions is seldom an initial sign of MS. Optic disc pallor often develops during recovery.
These are common and tend to occur early. Diplopia is usually caused by a lesion affecting the abducens (VI) nerve. Nystagmus is a common sign but is usually asymptomatic. It is a particularly useful sign when it is pronounced in degree and especially when the primary component is vertical.
Internuclear ophthalmoplegia is a classic MS sign indicating involvement of the medial longitudinal fasciculus. Examination reveals paresis of adduction on lateral gaze and associated nystagmus in the abducting eye. Despite the unilateral loss of adduction on lateral gaze the ability to converge (i.e., bilateral adduction) may be preserved.
Vertigo may be difficult to differentiate from a benign labyrinthitis, although a finding of vertical nystagmus points to a CNS rather than a peripheral cause. Trigeminal neuralgia is sometimes confused with idiopathic tic douloureux, a disease primarily of senior adults. Trigeminal neuralgia occurring in young adults is highly suggestive of MS because it is otherwise most atypical in this age group. Similarly, facial weakness may be mistaken for Bell palsy.
This occurs in about 50% of patients. Symptoms include poor balance, intention tremor, dysarthria and, when ataxia is extreme, titubation. Cerebellar symptoms can be severely disabling.
Typically occurring early with paresthesias and dysesthesias, often described as constricting or swollen sensations, these symptoms indicate posterior column demyelination in the cervical spinal cord, an area that may be affected early in MS. A hemicircumferential bandlike patch of numbness, usually midtrunk, is frequent but can also be seen with transverse myelitis or spinal cord mass lesions. Often patients forget to mention a very important, clinically useful phenomenon, namely the Lhermitte sign. The physician needs to inquire about this symptom because patients seldom volunteer this information. The Lhermitte sign is typified by momentary electric shocklike sensations shooting or radiating down the arms, back, or legs, precipitated by neck flexion. However, the Lhermitte sign is not diagnostically specific; other posterior cervical spinal cord lesions can provoke it. Examination often reveals diminished vibration and position sense.
This causes muscle fatigue, stiffness, spasticity, and weakness. Hyperreflexia, clonus, and the Babinski sign are frequently elicited. Clonus is a form of movement marked by contractions and relaxations of a muscle occurring in rapid succession. Clonus is most often elicited at the ankle. The Babinski sign is an extension of the great toe and abduction, or fanning, of toes two to five instead of the normal flexion response to plantar stimulation.
Urinary frequency and urgency suggest a hyperreflexic neurogenic bladder. Constipation and sexual dysfunction are also frequent complaints.
Inordinate fatigability is common and can be overwhelming. Demyelinated axons require far more energy to conduct nerve impulses than properly insulated axons; thus conduction may fail with effort. For example, a limp may replace a seemingly normal gait after walking some distance, only to disappear after a rest period. The inefficiency of demyelinated axons worsens as body temperature rises, thus short-lived symptoms, including a transient limp, can be provoked by summer heat, taking hot showers, by fever, or may occur in the late afternoon, when the normally modest diurnal upward body temperature swing peaks, as does MS-related lassitude.
MS patients may experience ill-defined pain, presumably neuropathic in origin. However, one must always seek out other pathophysiologies before presuming that MS is the cause.
Occurring at some time in 50% of MS patients, episodes of depression sometimes antedate overt disease onset. The frequency of depression is threefold of that encountered in the overall population. Cardinal features are anger, frustration, irritability, anxiety, and frank panic attacks.
Mildly deficient short-term memory frequently develops early. This can progress to substantial cognitive deficits in later years.
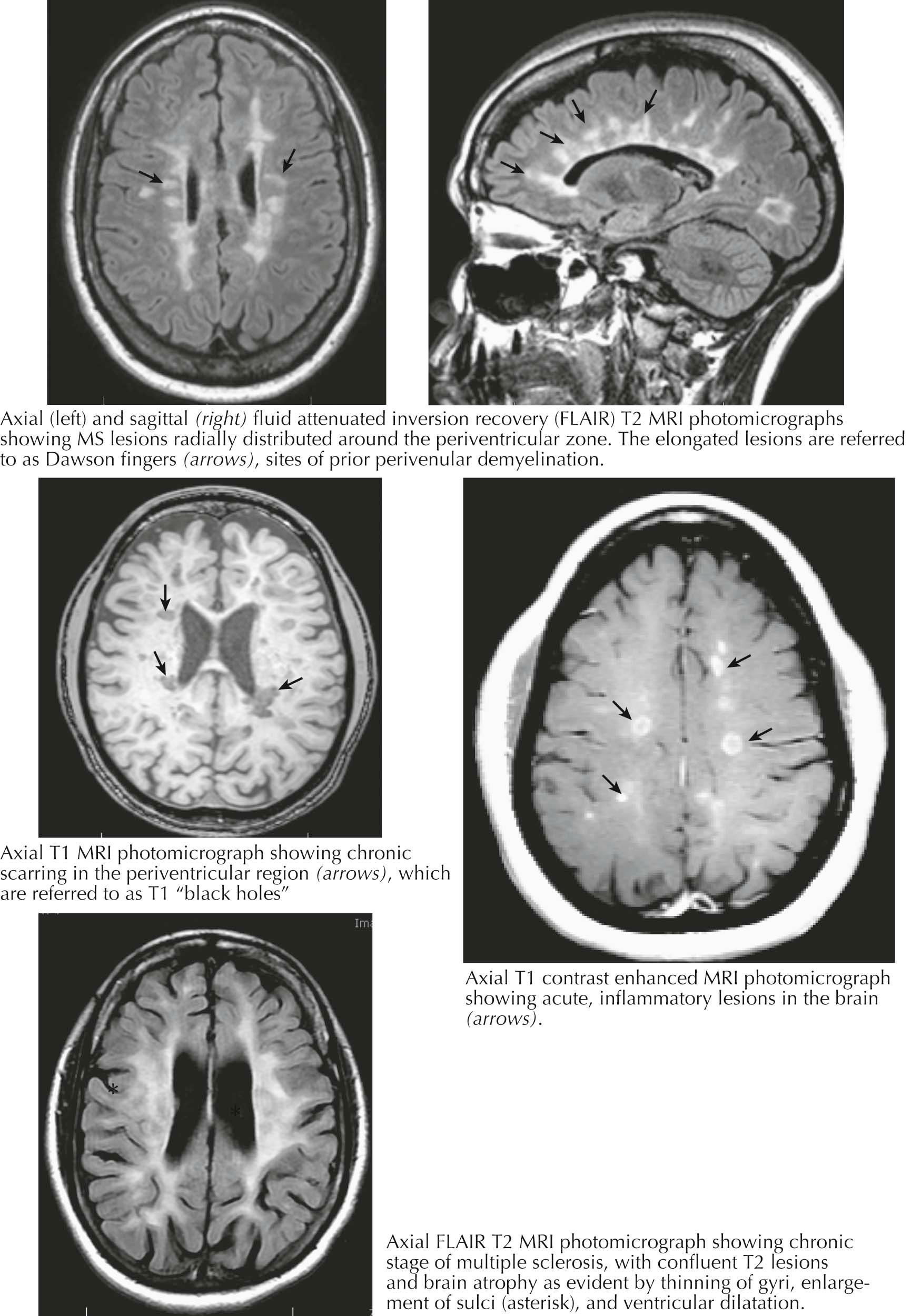
Formerly, a firm diagnosis of MS required evidence of dissemination of clinically detected deficits in both space and time. This was achieved by documentation of two attacks, each lasting more than 24 hours (absent fever or infection), with each attack typical of an acute demyelinating event and with the requirement that there be objective clinical evidence of a lesion in the second episode at a site anatomically distinct from that documented in the first episode. Such a second event might not occur for years or even decades.
MRI has greatly enhanced the ability to establish a diagnosis early in the course of MS. This modality is noninvasive, reproducible, and sensitive to the presence or absence of disease and to both clinically evident and clinically silent changes in lesion burden. These features permit its use as a follow-up procedure to assess change.
An MRI is highly sensitive for the presence of disseminated white matter lesions. Gadolinium (GD) enhancement provides insights into active (enhancing) lesions because GD crosses into the CNS parenchyma only at sites of blood-brain barrier (BBB) leakage, whereas established inactive lesions do not show GD enhancement. New lesions often have central GD enhancement, whereas a ringlike or arcuate enhancement may be seen with reactivation at the margin of an existing plaque.
MRI may permit diagnosis during an initial clinical occurrence if the study demonstrates evidence of one or more lesions with dissemination in space and time, as illustrated by the simultaneous presence of GD-enhancing and nonenhancing lesions of typical configuration and location (periventricular, juxtacortical, infratentorial, or spinal cord). Diagnosis can also be established if a new T2 or GD-enhancing lesion, often clinically silent, appears on a follow-up MRI.
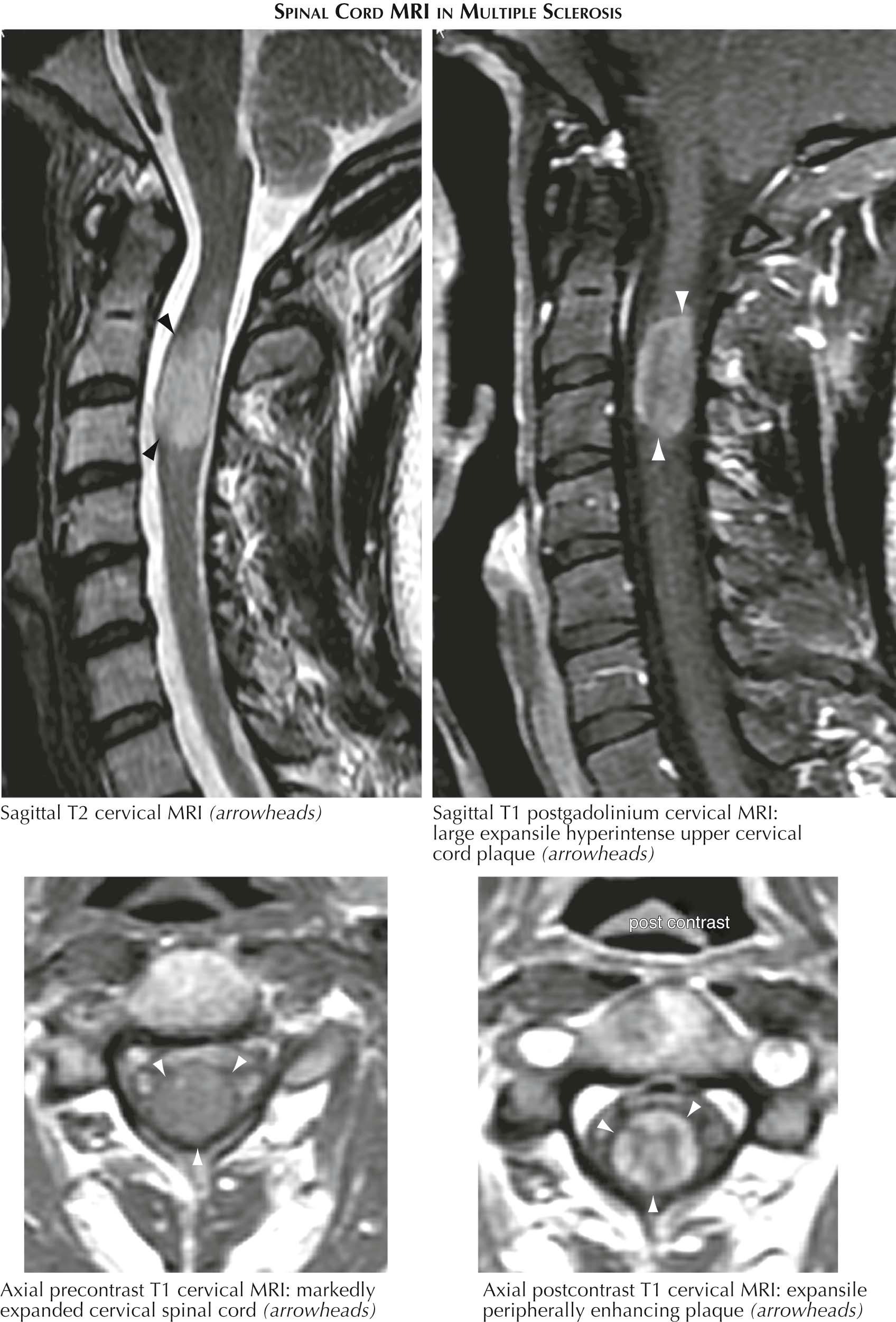
Classic MRI findings include so-called Dawson's fingers, consisting of multiple well-demarcated ovoid perivenular plaques with their long axes situated perpendicularly within the corpus callosum and extending upward from the roof of one or both lateral ventricles, often as a fore and aft row situated parallel to the midline. Additional preferential plaque location sites include subcortical white matter, the middle cerebellar peduncles, the pons, and the medulla beneath the floor of the fourth ventricle. Spinal cord plaques originate at the meningeal surface and expand inward. Spinal cord plaques, as with plaques elsewhere, need not be symptomatic. Gray matter lesions within the cerebral cortex can be abundant in MS but are usually underappreciated because they are poorly visualized by standard MRI techniques. Rarely, white matter lesions can be ominously large, so-called tumefactive MS, and may even on occasion require biopsy to exclude glioma.
Up to 40% of time-spaced serial MRI scans demonstrate new GD-enhancing lesions in untreated patients with RRMS. Most such lesions are clinically silent. The finding indicates a several-fold higher level of disease activity than can be appreciated by considering overt clinical relapses alone. In contrast, the majority (60% plus) of randomly obtained MRI scans do not show current activity; this illustrates the presence of relative disease quiescence much of the time. However, this clinically inactive hiatus is intermittently interrupted by episodic pulses of MRI-detected or clinical MS disease activity. The frequency of MRI-positive newly active lesions is reduced by up to 80% by current treatments. GD-positive lesions are uncommon in progressive MS.
The validity of a diagnosis of MS based on the MRI criteria described above is established for RRMS in adults in Western countries. Criteria for a diagnosis of PPMS still require at least 1 year of disease progression plus two of three of the following criteria: (1) evidence for dissemination in space (DIS) from spinal cord into the brain based on at least one T2 lesion, not necessarily new, in a location characteristic for MS; (2) evidence for DIS in the spinal cord based on two lesions in the spinal cord; and (3) cerebrospinal fluid positive for oligoclonal bands, a positive immunoglobulin G (IgG) index (see later), or both.
MS is uncommon in children, and diagnosis at present requires a second attack to distinguish it from acute disseminated encephalomyelitis (ADEM), a distinct demyelinating disorder that is almost always monophasic (see later). MRI features that strongly favor the likelihood that a first attack of demyelinating illness will subsequently prove to be MS include presence of one or more T1-weighted hypointense lesions (black holes) indicative of prior CNS white matter damage or presence of one or more periventricular lesions. The likelihood for MS is further increased when both are present. In children younger than 11 years, MRI lesions are larger and more ill-defined than in teenagers or adults so that shape, size, and lesion frequency do not discriminate reliably between MS and ADEM, although a diffuse bilateral lesion pattern favors ADEM.
The reliability of MRI criteria currently accepted as valid in Western countries remains to be established in Asia and Latin America. The higher frequency of neuromyelitis optica (NMO) and NMO-spectrum disorders and the lower frequency of MS in these geographic regions raise concerns. Both NMO and MS usually relapse. Symptomatic brain lesions at disease onset do not exclude NMO (see later). It is important to clearly distinguish between MS and NMO because of their different clinical courses, prognoses, underlying pathophysiology, and the negative response of NMO patients to at least some of the MS disease-modifying therapies. Findings particularly suggestive of NMO are a myelopathy with MRI-detected spinal cord lesions that are central in location and span more than three spinal segments, and optic neuritis that is bilateral, severe, painless, and associated with a swollen optic disc or a chiasmal lesion. These findings warrant prompt testing for the aquaporin-4 autoantibody found in most patients with NMO, but by no means in all.
From time to time, lesions that appear to be typical for MS turn up unexpectedly on MRI scans of persons with no clinical symptoms suggestive of MS, no history of them, and no findings on examination. The MRI has usually been obtained for reasons unrelated to MS, such as headaches or head trauma. Several groups have followed such persons with this so-called radiologically isolated syndrome (RIS) for a decade or more. Many RIS cases have gone on to develop radiologic progression with new, enlarging, or GD-enhancing, but still asymptomatic, lesions. A smaller proportion have converted to clinically apparent MS. MS usually has a preclinical phase with asymptomatic lesions acquired in the past detected on MRI scans obtained at the time of a first clinical event. It is also agreed that treatment of a first MS attack is more effective than when treatment is delayed. As a counterbalance, it is well known that a forme fruste of MS exists. There are numerous reports of MS being found at autopsies of elderly persons with no known neurologic deficit during life.
Thus the conundrum: should one treat based on new asymptomatic lesions seen on an MRI scan? Can probability to develop overt MS be predicted? It has been proposed that presence of cervical spinal cord lesions shifts the odds for early appearance of clinically definite MS dramatically upward. If so, the absence of a cervical spinal cord lesion shifts the odds downward. The issue of when to treat remains open.
One unusual radiologic imaging feature of MS is the presence of a large tumefactive lesion. Clinical features vary with the specific anatomic location of the lesion and may be atypical for MS. There may be cognitive abnormalities, mental confusion, aphasia, agnosia, seizures, ataxia, hemiplegia, and visual field defects. Median age at onset is about 37 years (8-69), and there is a slight female preponderance. Tumefactive lesions may be found in patients with an established diagnosis of MS, but when they present as a single, ominous-appearing, space-occupying lesion in patients experiencing a first neurologic event, there may be considerable diagnostic difficulty. Tumefactive lesions are larger than 2 cm in diameter, with an open-ring–enhancing edge, an edematous surround, and frequently, depending on their size, a mass effect. These imaging features may mimic a brain tumor, an abscess, other inflammatory disorders, vasculitis, or granulomatous disease and may lead to brain biopsy. Histologic examination reveals hypercellularity, confluent demyelination, foamy macrophages full of myelin debris intermingled with reactive large-bodied astrocytes, relative axonal preservation, and variable perivascular and parenchymal lymphocytic infiltration.
Tumefactive lesions are commonly treated with intravenous methylprednisolone. Most patients respond favorably with substantial, and sometimes dramatic, contraction of lesion size over time. Solitary tumefactive lesions are usually a herald of MS. In one large series of cases, 70% of patients with tumefactive lesions developed definite MS, but the median interval to a second event was much delayed compared with the overall MS population, suggesting a favorable prognosis.
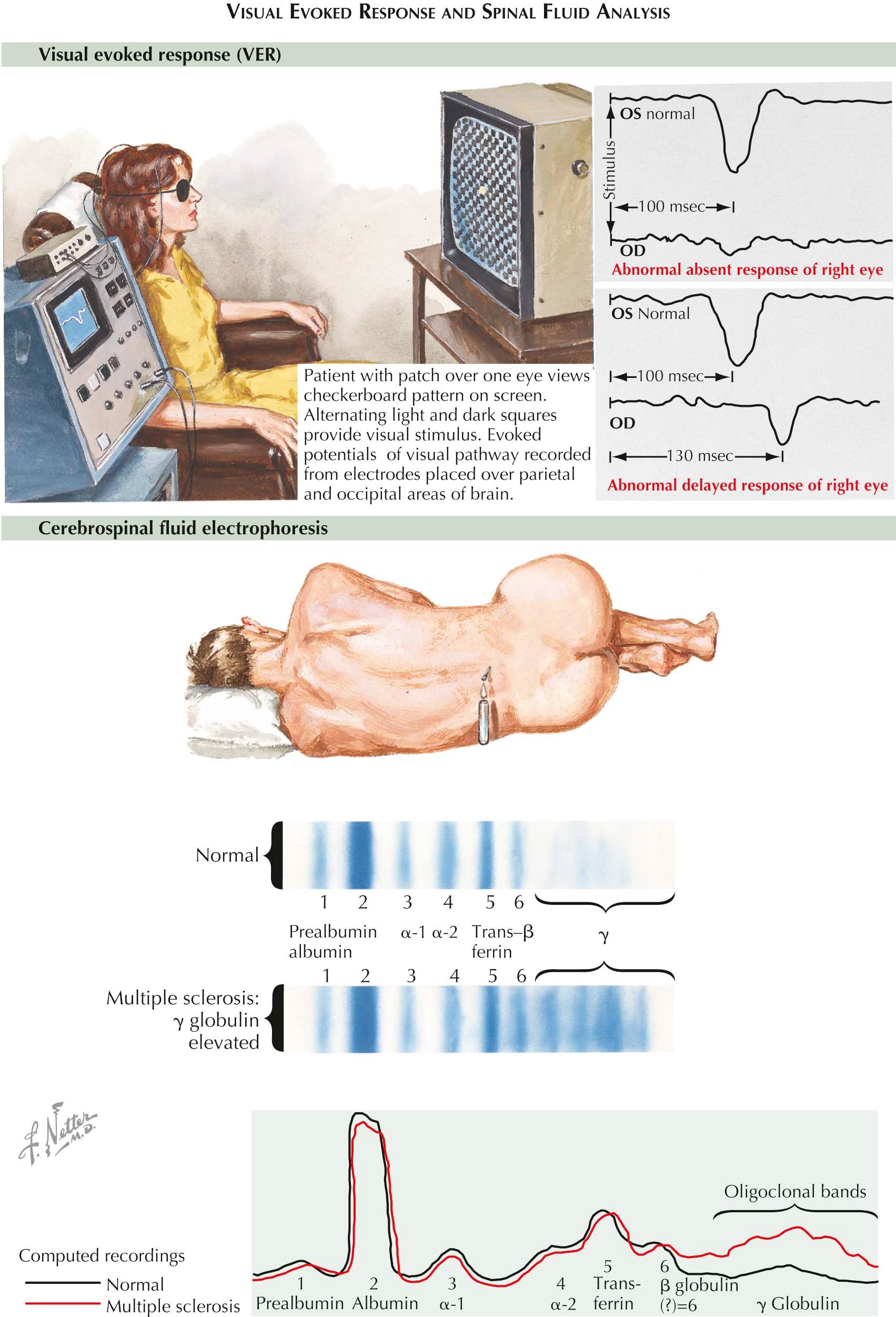
A spinal tap is performed less often today than formerly but may be helpful when the diagnosis is in doubt or to satisfy diagnostic criteria. Total white cell count is elevated in about 25% of MS patients, but rarely above 25 mononuclear cells per mm 3 . The total protein concentration is slightly elevated in 40% of patients but is seldom greater than 75 mg/dL. In 40% to 60%, the γ-globulin (IgG) fraction is elevated above 15% of the total CSF protein, reflecting an increased production of IgG within the CNS. The IgG index provides a more precise estimate of IgG synthesis within the CNS. It is calculated as the ratio of IgG in CSF/IgG in serum divided by albumin in CSF/albumin in serum. A value greater than 0.7 is taken as abnormal and is found in 70% of MS patients. Unfortunately, the IgG index is not entirely specific for MS. Oligoclonal bands in the IgG sector of the protein isoelectric focusing pattern or immunofixation pattern, but not found in blood, are detected in more than 90% of cases and in fewer than 5% of controls once CNS infections are excluded. CSF pressure and glucose content are normal.
These neurophysiologic studies permit an objective analysis of the integrity of neuronal pathways in the CNS. Before the ready availability of MRI, EPs were widely used to identify subclinical CNS disease. Testing is easily performed and requires minimal patient cooperation, particularly when testing the visual pathways by means of visual evoked responses (VERs). In centers where MRI is at hand, brainstem and somatosensory EPs are seldom required today to confirm a tentative diagnosis of MS.
Today the primary value of EP testing occurs when a patient presents with an acute, seemingly monophasic myelopathy plus a normal brain MRI and one wishes to determine whether there might be dissemination of disease in space and time. Some patients may have had an earlier subclinical optic neuritis. Others may have either forgotten a prior episode of visual loss or offer an uninterpretable history of earlier visual disturbance now recovered. In such instances, VER testing may establish or exclude the presence of prior damage to visual pathways. The combination of an abnormal VER and a myelopathy is essentially confined to MS or neuromyelitis optica.
With VERs, a retinal stimulus, typically a reversing high-contrast checkerboard pattern, provides a means to study the integrity of the visual system. Response latencies provide objective data regarding the ability of the nervous system to transmit impulses efficiently from the optic nerve to the occipital cortex. If absence or delay of conduction is unilateral, one can conclude that there is slowed conduction between the retina and the optic chiasm typical for unilateral optic neuritis. VERs are most helpful when patients have fully regained their vision. Remyelination is never perfect so that a VER-documented slowing of conduction velocity provides an indelible marker of prior damage. When delayed latencies, attenuated potentials, or conduction block are bilateral, the lesion cannot be localized precisely because it could be situated anywhere along the visual pathway.
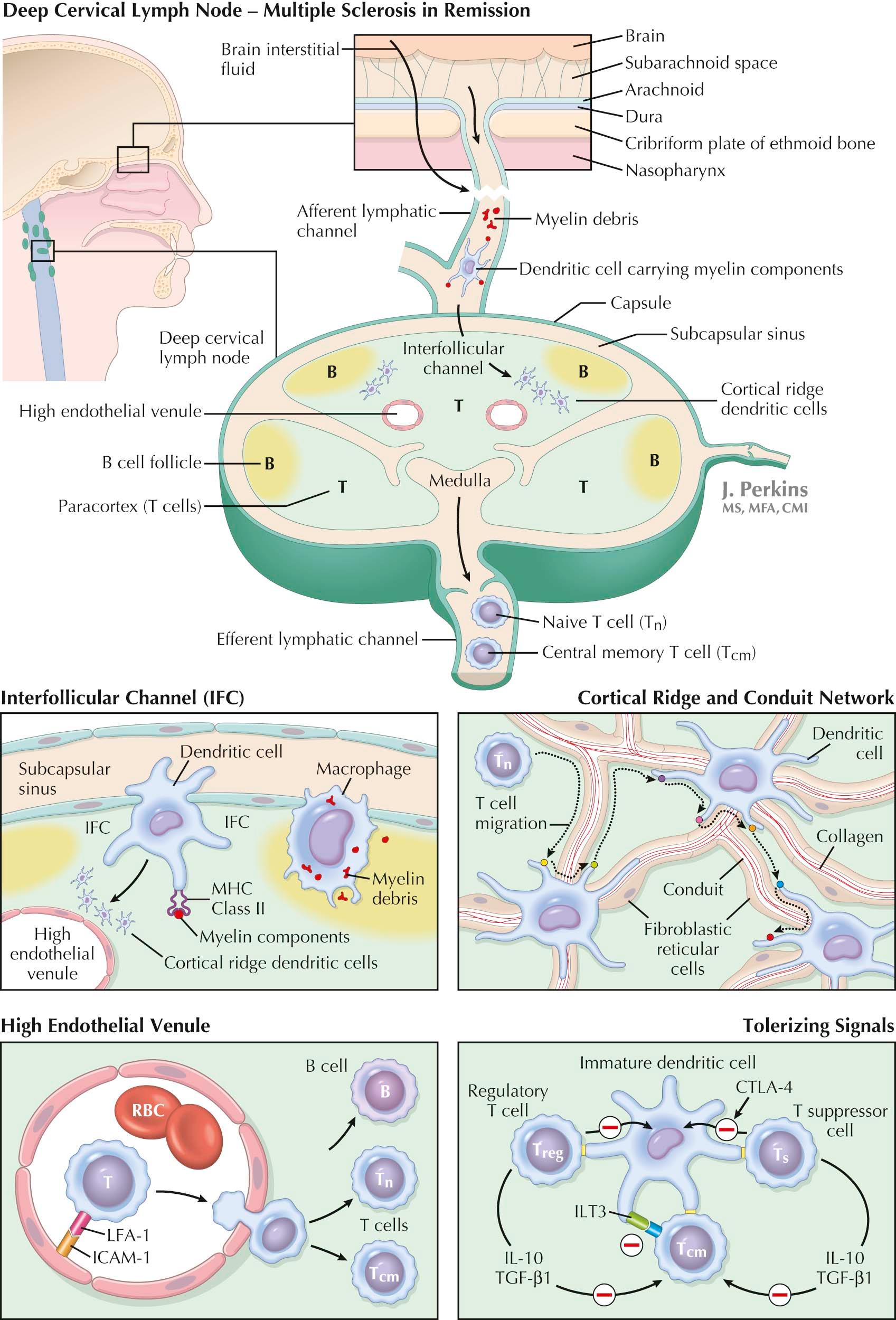
MS relapses evolve subacutely. The accepted environmental antecedent for relapses is an upper respiratory viral illness . Such illnesses activate the immune system, increasing relapse frequency two- to threefold, and leave behind greater deficits than relapses that occur without a history of a viral illness. Immune responses to viruses involve drainage of pathogenic antigens and antigen-presenting cells (APCs) along lymphatic channels to a regional lymph node (LN). Upper respiratory viruses and viral antigens drain via nasal lymphatic channels into the subcapsular sinuses of the deep cervical LNs . Immature dendritic cells (DCs) and monocytes lie beneath the subcapsular sinus and protrude processes into it, permitting them to sample newly arrived lymph and capture viral antigens. Drainage of CSF and of brain parenchymal interstitial fluid occurs through the cribriform plate located below the olfactory bulb into nasal lymphatics and then into these very same deep cervical LNs.
Immature DCs in the CNS subarachnoid space ingest myelin elements; after that, they may mature in situ. Mature DCs express the cell surface molecule C-C chemokine receptor 7 (CCR7), whereas endothelial cells in afferent lymphatic channels express chemokine ligand 21 (CCL21) counterligand. Ligation of CCR7 permits mature self-antigen–bearing DCs to migrate via afferent lymphatics to deep cervical LNs. Mature DCs are short lived and have lost their capacity to sample newly presented viral or other antigenic material, whereas long-lived immature DCs or macrophages can do so.
Extracellular debris, including myelin and its peptides, flows from a damaged CNS to deep cervical LNs where subcapsular macrophages and immature DCs capture it. Unlike controls, deep cervical LNs of MS patients with inactive disease contain numerous immature DCs and macrophages with ingested myelin and myelin proteins that can, under appropriate circumstances, be presented to T cells. Immature DCs and macrophages retain the capacity to respond to subsequently encountered viral antigens.
Naïve CD4 + T cells (T N ) move swiftly through LNs, making serial brief contacts with antigen-loaded DCs draped atop the fibroblastic reticular cells (FRC) that enwrap the collagen fiber structural backbone of the LN. Lymph is transferred from the subcapsular sinus to the medulla via the collagen-containing channels of the conduit network. DCs insert processes into the conduit channels to capture peptide elements contained therein, which they can then process and present to T cells. T N cells seek that rare DC expressing the cognate antigen they are programmed to recognize. When a CD4 + T N cell fails in its quest, as usually occurs, it migrates to the LN medulla and exits via efferent lymph. Exit requires expression of the sphingosine-1-phosphate receptor-1 (S1P-1) by migrating CD4 + T N cells. This requirement has been exploited in MS therapy. CD4 + T N cells pass from the efferent lymph into the circulation and move on via the blood to sample another LN. They do not enter the tissues.
Circulating CD4 + T N cells express CCR7 and low levels of the adhesion molecule leukocyte function–associated antigen-1 (LFA-1). CCL21 and intercellular adhesion molecule-1 (ICAM-1), counterligands for CCR7 and LFA-1, respectively, are expressed by the high endothelial cells of venules sited in LN T-cell–dependent areas with a nearby cortical ridge that is enriched in DCs. CCR7 binding to CCL21 and LFA-1 binding to ICAM-1 enables CD4 + T N cells to cross from the blood via these venules into LNs passing either between LN endothelial cells or, intriguingly, as illustrated, through them.
When a CD4 + T N cell finally contacts a cognate antigen–expressing DC, it is either activated or tolerized. Both outcomes are germane to MS. Both require contact between the CD4 + T N cell receptor and an MS-relevant small peptide fragment cleaved from an ingested protein and inserted into a cleft in a human leukocyte antigen (HLA) molecule that then is expressed on the DC surface. Cognate antigen contact lasts longer than contact with DCs expressing irrelevant peptides, and contact is of even longer duration if the CD4 + T N cell is being activated rather than tolerized.
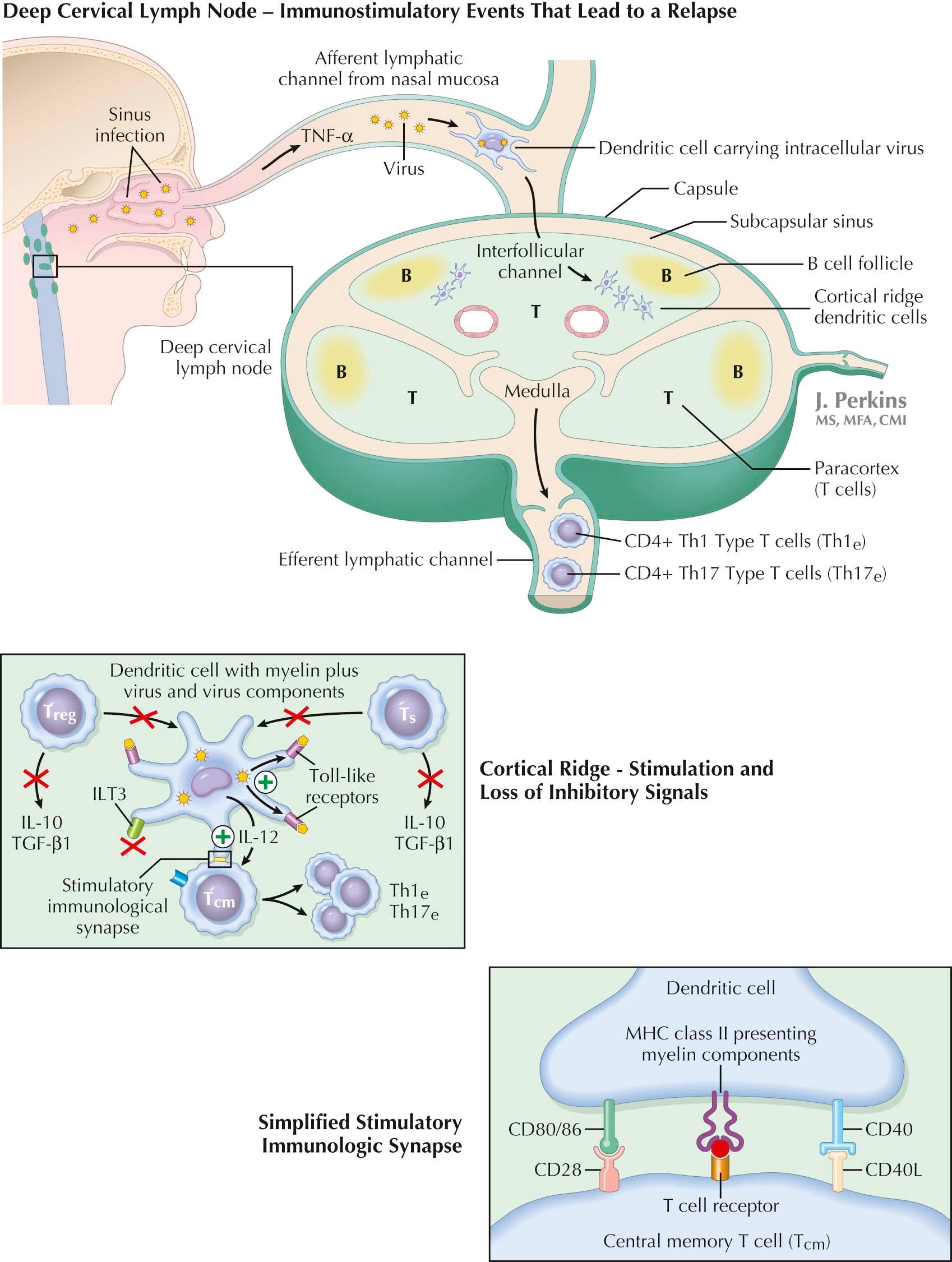
DC maturation is critical for activation of a CD4 + T N cell. Maturation involves increased expression of co-stimulatory molecules (e.g., CD80/86 and CD40) and reduced expression of tolerizing molecules (e.g., immunoglobulin-like transcript 3 [ILT3], transforming growth factor-beta 1 [TGF-beta1], and interleukin-10 [IL-10]). Full activation of CD4 + T cells by DCs requires two signals. The first signal is delivered by T cell receptors following contact with their cognate antigen presented to them at an immunologic synapse by a MHC class II molecule. The second set of signals is delivered by a network of interactive costimulatory molecule pairs with CD80/86, expressed initially at low level by DCs, and CD28 expressed constitutively by T cells, providing one prototypic pair and CD40 expressed by DCs in the early stage of their activation and CD40 ligand (CD40L/CD154), induced on T cells in the early hours of their activation, providing a second.
Both cross-communicating pairs upregulate synthesis of their counter ligand, and both pairs, acting in concert, transduce additional activating signals via intracellular second messengers. Each pair synergistically reinforces the actions of the other. CD28 is critical for the initiation of T cell activation, while CD40L has a key role in sustaining it. CD40L also promotes a Th1 cell bias and an even more marked Th17 cell bias. T cells, once activated, proliferate within the LN to generate an up to 10 4 -fold increase of CD4 + effector T cells (T E cells).
Cytotoxic T lymphocyte antigen-4 (CTLA-4), a homologue of CD28, has a critical role in the prevention of autoimmunity. CTLA-4 is expressed by activated T cells and by regulatory T cells. CTLA-4 is focused at the immunologic synapse where it opposes CD28. Both CD28 and CTLA-4 bind CD80/86 but because of its higher affinity CTLA-4 can out-compete CD28 and reverse or blunt the T cell activating actions of CD28. DCs downregulate their expression of CD80/86 in response to the potent negative signal provided by CTLA-4 when it binds to CD80/86. In this way CTLA-4 promotes tolerance.
CTLA-4 is not expressed by resting T cells but comes to be expressed by activated T cells as an immune response evolves. CTLA-4 tempers what would otherwise be an excessive T cell expansion. CTLA-4 is constitutively expressed, and highly so, on the surface of regulatory T cells and, additionally, as a released biologically active soluble splice variant. Regulatory T cells have a major role in autoimmunity prevention. Even when prevention fails, as in MS, regulatory T cells can lessen MS relapse frequency and the severity of those relapses that do occur. Regulatory T cells also participate in the processes that end a relapse (see later). Agents that duplicate actions of CTLA-4 are of interest as MS treatments.
CD4 + T E cells are divided into subtypes known as Th1 cells, Th17 cells, and Th2 cells. Broadly viewed, Th1 cells protect against intracellular organisms, Th17 cells protect against fungi, whereas Th2 cells protect against helminths, certain other extracellular pathogens, and allergens. DCs, over the course of their activation of naïve T cells, can polarize T-cell development along paths that lead to the preferential expansion of a single Th-cell subset. Th-cell subsets interact ; Th2 cells inhibit Th1 cells and vice-versa. Th1 cells and Th17 cells cause damage in MS, while Th2 cells protect because they inhibit Th1 and Th17 cells. Thus any drug (e.g., glatiramer acetate, teriflunomide) or mechanism that shifts polarization from a Th1 to Th2 dominance might be expected to prove beneficial in MS.
The CD4 + T E -cell population in MS contains both interferon-γ–secreting Th1 cells and interleukin-17–secreting Th17 cells . Deep cervical LN-generated MS-relevant CD4 + T E cells migrate to the LN medulla, express S1P-1, move via efferent lymph into the blood and then to the CNS to participate in an MS relapse (see later).
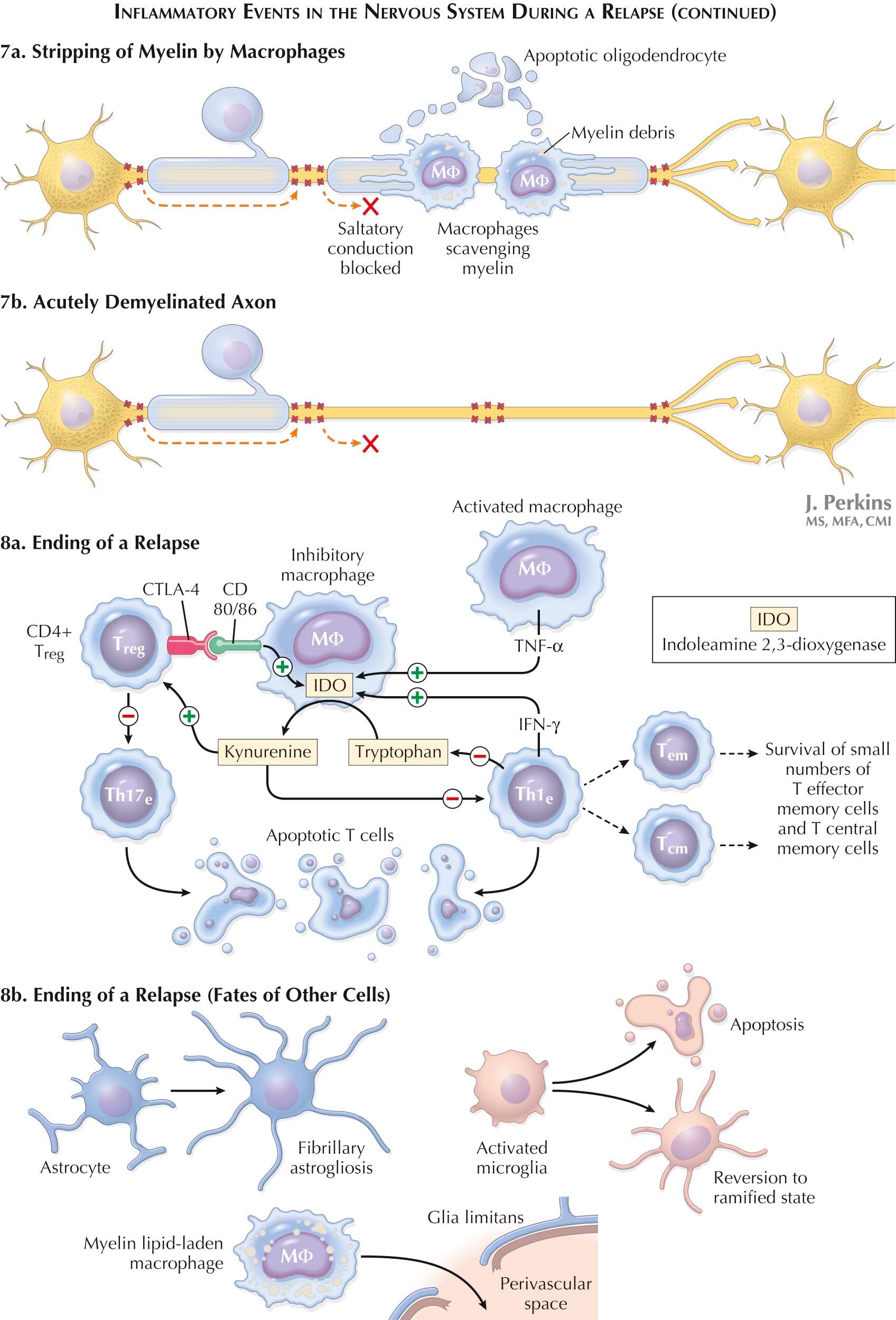
As a relapse ends, most CNS-infiltrating Th1 and Th17 T E cells die in situ by apoptosis, but perhaps 5% survive as T-effector memory (T EM ) cells that remain in the periphery to provide a prompt defense against a subsequent challenge. An additional 5% to 10% remain as CD4 + T central memory (T CM ) cells that express the same adhesion molecules as T N cells so that they too reenter LNs via high endothelial venules, sample DCs, and recirculate via efferent lymph and then the blood to other LNs. Unlike T N cells, MS-relevant T CM cells also survey the tissues and other body compartments, including the CSF, seeking an APC loaded with the MS-relevant peptide they are programmed to recognize. T CM cells outnumber T N cells with the same specificity so that a secondary CD4 + T CM -cell response in a LN is usually more rapid and robust than a CD4 + T N -cell response. Activation of CD4 + T N cells requires peptide presentation by mature DCs, but requirements for CD4 + T CM -cell reactivation are less stringent; they can respond to some extent to antigenic peptide–presenting macrophages and to immature DCs. CD4 + T CM -cell reactivation leads to generation of a new CD4 + T E -cell population that again migrates to the circulation and then to the CNS.
Throughout MS remissions, myelin debris–laden immature DCs and macrophages in the cervical LNs of MS patients are thought to promote tolerance. Deep cervical LNs are favored sites for tolerance induction because they are continuously bathed with products released by the commensal biota of the nasal mucosa. The immune system is programmed to tolerate commensal organisms and to eliminate pathogens. Tolerance is mediated primarily by antigen-specific regulatory (suppressor) T cells that powerfully inhibit T-cell activation. For this reason, when antigen-specific regulatory T cells are deleted, overly robust T E -cell responses ensue. The finding points to a ceaseless positive versus negative competition for control of DC function.
Multiple types of regulatory T cells are described. CD4 + CD25 + T regulatory cells (T REGS ) and CD8 + CD28 − T suppressor cells (T S ) are the most studied in MS. The role of CD4 + CD25 + T REG cells in MS remains unresolved. CD8 + CD28 − T S -cell function is grossly defective during MS relapses . CD8 + CD28 − T S -cell function reverts toward normal as relapses end and is restored, although not always fully, during remissions. Agents that augment CD8 + CD28 − T S -cell function would be expected to be beneficial in MS.
CD8 + , CD28 − T S cells are antigen-specific . The CD8 + , CD28 − T S -cell receptor makes direct contact with its cognate antigen presented by an HLA class I allele expressed on a DC. Contact between them shunts the DC into a tolerizing mode with co-stimulatory molecule (e.g., CD86) expression reduced and tolerance-inducing molecule (e.g., ILT3) expression increased. In the obverse, when DC expression of ILT3 is silenced, proinflammatory cytokine synthesis up-regulates, and generation of disease-provoking T E cells soars. ILT3 expression and CD8 + CD28 − T S -cell function are reduced during MS relapses. Blood levels of type 1 interferons (IFN-α and IFN-β) are low in MS. IFN-β, widely used to treat MS, increases ILT3 expression and augments CD8 + CD28 − T S -cell function.
A single DC can present multiple peptides to CD4 + and CD8 + T cells and can interact with up to 10 T cells, each with a different specificity, at any point in time. Likewise, a CD8 + CD28 − T S cell can interact sequentially with several DCs, provided each expresses the antigenic peptide specific for that CD8 + CD28 − T S cell. It follows that several CD4 + T cells with different specificities can be tolerized in a cascade by a single CD8 + CD28 − T S cell. Further, as relapses recur and additional DCs are driven to maturity, new immune responses to recently captured self-antigens may be generated . This process is known as epitope spreading.
Multiple factors released during viral infections can drive immature tolerance-inducing DCs to maturity and convert them into immune system activators. Reinforcing actions of several factors may be required to ramp up co-stimulatory molecule expression and ramp down tolerogenic molecule expression to extents that permit an override of CD8 + CD28 − T S -cell–mediated tolerance. Included are proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) released at sites of inflammation and swept into LNs plus binding of virus components to Toll-like receptors (TLR) expressed by DCs and macrophages. Viral infections commonly shunt MS-relevant peptide-loaded immature DCs and macrophages into a CD4 + T-cell–activating mode, and in this way, they can provoke MS relapses . This disease-enhancing action of viruses is probably facilitated by an unmasking in MS of a defect in CD8 + CD28 − T S -cell function that is at least in part genetically determined.
Mature DCs present antigen to T cells at the onset of an immune response, but their life span is short (only ≈3 days in mice). During the later stages of an immune response, B cells often assume the dominant APC role . B-cell receptors bind unprocessed antigen rather than the short peptide sequences recognized by T-cell receptors. Nonetheless, once an unprocessed antigen bound to a B-cell receptor has been internalized, B cells can process internalized antigenic material into small peptides, insert them into the clefts of major histocompatibility complex (MHC) class II molecules, and transfer MHC class II–processed peptide complexes to the cell surface for presentation to CD4 + T cells.
B cells enter LNs via high endothelial venules in T-cell–dependent areas. Most B cells move quickly from the T-cell–rich paracortex into B-cell zones called follicles . Follicle entry occurs because follicle-destined B cells express the chemokine CXCR5 that binds to its CXCL13 counterligand expressed by follicular dendritic cells (FDC), a distinct population of follicle-confined stromal cells. Follicle-infringing subcapsular macrophages transport particulate material into the follicle and pass it along to FDCs that can then present it to B cells. In addition, soluble antigens percolate into the follicle, are captured by FDCs, and presented to B cells. Antigen-pulsed B cells next leave the follicle to form monogamous immunologic synapses with DC-primed CD4 + T cells at the T-cell–B-cell boundary. Each B cell drags its T-cell partner along the border for a time, but the B cell then separates from its T-cell partner with the B cell moving back into the follicle, only to be followed much of the time by its prior partner T cell that has now come to express the CXCR5 chemokine, a marker for so-called follicular helper T cells (T FH ).
Bidirectional interactions between CD4 + T FH cells and B cells are important for (1) germinal center formation, (2) expansion of both populations, (3) hypermutation of B cells that diversifies their antigen receptors and permits affinity-driven clonal selection, (4) B-cell differentiation into long-lived plasma cells that secrete high-affinity antibody and confer long-lasting protection from secondary challenge and, (5) development of memory B cells.
Of importance, T cells can shift lineages . Antigen-presenting B cells may shift CD4 + T FH cells into CD4 + T CM cells, CD4 + IFN-γ–secreting Th1 T E cells and CD4 + Th17 T E cells in response to low doses of autoantigens. Thus B cells can contribute in a major way to MS relapse severity.
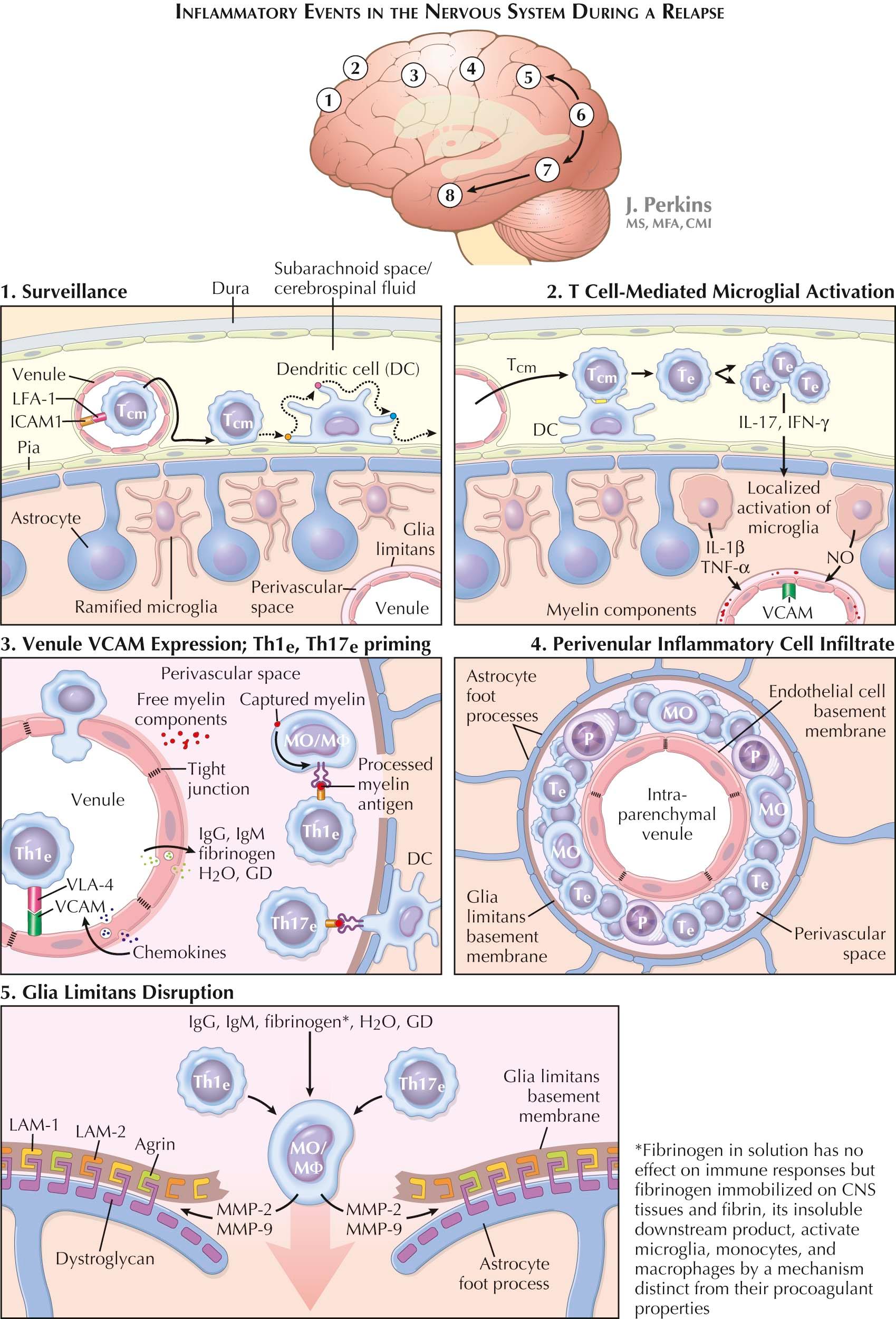
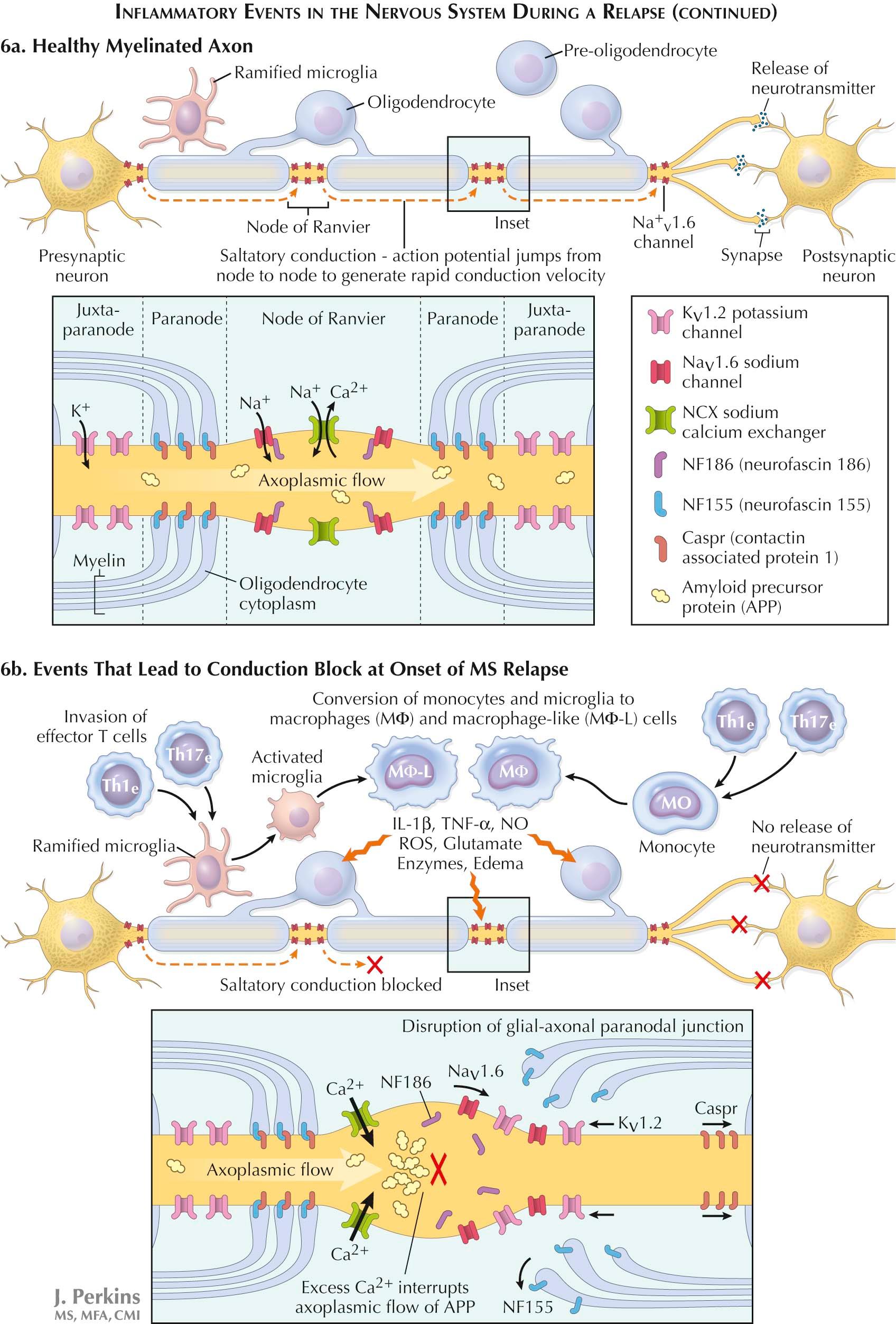
The initiation of MS relapses is thought to involve five sequential CNS-restricted steps. Much evidence to support this concept derives from study of experimental autoimmune encephalomyelitis (EAE), an inducible rather than a spontaneous animal model for MS. This raises concerns as to the validity of extrapolating findings from EAE to human disease. Even so, fundamental mechanisms, such as antigen recognition, are likely to be similar if not identical.
This is clinically silent and takes place in the subarachnoid space (SAS) and within the cerebral ventricles. Between 10 5 and 5 × 10 5 lymphoid cells are present in the noninflamed CSF of humans at any point in time. Eighty percent of these are CD4 + T CM cells in search of their cognate antigen, with lesser representations of CD8 + T CM cells and of CD4 + T E cells, these last probably derived from CD4 + T CM cells that have found their cognate antigen (see later).
Postcapillary venules on the pial surface and within the choroid plexuses express CCL21 and ICAM-1, whereas CD4 + T CM cells express CCR7, the counterligand for CCL21, and richly express LFA-1, the counterligand for ICAM-1. CCR7-CCL21 and LFA-1–ICAM-1 interactions permit CD4 + T CM cells to pass from postcapillary venules into the SAS and from choroid plexus venules into the ventricles. CD4 + T CM cells, once in the SAS, move first along the outer surface of meningeal vessels and then along the pial surface, searching for their cognate antigen. Should they encounter their cognate antigen, offered to them in processed form by an APC, a local immune response is initiated. Should surveillance of the SAS prove fruitless, CD4 + T CM cells return to the blood.
In humans, major histocompatibility complex (MHC) class II–expressing DCs with up-regulated co-stimulatory molecule expression are present in the CSF compartment. They are situated on the surface of meningeal microvessels and on pial and ependymal surfaces. Their numbers appear to be enriched in MS. Abundant myelin debris with contained protein antigens, residua of prior MS relapses, follows the established drainage paths of interstitial fluid through the ependyma into the ventricular CSF and via Virchow-Robin spaces into the SAS. Residual myelin particles are extracellular for the better part but are also detected within DCs and macrophages. Myelin debris is not found in controls.
CD4 + T CM cells prepare the terrain for the subsequent entry of T E cells into the CNS parenchyma and the onset of the clinically evident component of a relapse. Adhesion molecules are not expressed by resting brain parenchymal endothelial cells, nor do meningeal vessels ordinarily express vascular cell adhesion molecule-1 (VCAM-1), the adhesion molecule required for CD4 + T E -cell exit from the blood. CD4 + T CM cells, having made synaptic contact with a DC, may undergo several cycles of division, still within the meninges or the ventricles to generate a small locally restricted CD4 + T E -cell cohort. Some members of this cohort cross the pia or ependyma to enter the subjacent CNS parenchyma. These pioneer CD4 + T E cells secrete interferon-γ (IFN-γ), as does a subpopulation of still SAS-confined CD4 + T CM cells that are perhaps transitioning into T E cells.
Released IFN-γ diffuses from the pial and ventricular ependymal surfaces and from the immediately subjacent CNS parenchyma for a considerable depth into the CNS parenchyma, oozing preferentially along fiber tracts. In addition, the released IFN-γ activates intraparenchymal microglia as evidenced morphologically by retraction and thickening of their processes. Microglial activation is essential for the subsequent invasion of the CNS parenchyma by T E cells and by blood monocytes destined to become macrophages. Activated microglia secrete cytokines, and notably tumor necrosis factor-α (TNF-α), which activates nearby parenchymal postcapillary venules. These venules quickly come to resemble the high endothelial venules of LNs and, most important, now begin to express the adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and VCAM-1.
Expression of microglial-driven ICAM-1 and VCAM-1 by parenchymal venular endothelial cells permits very late antigen-4 (VLA-4)–expressing CCR7 − CD4 + Th1-type T E cells and CCR7 − CD4 + Th17-type T E cells to traverse CNS parenchymal venules and form perivascular inflammatory cuffs. Monocytes also express VLA-4 and interactions between VLA-4 and VCAM-1 are critical for their co-transmigration into perivascular cuffs and their subsequent disease-promoting activities. Cell passage across CNS venular endothelium is transcellular rather than intercellular. The tight junctions that join CNS endothelial cells to create the blood-brain barrier (BBB) are not disrupted during MS relapses. The perivascular space is bounded by an inner endothelial cell, laminin-containing, basement membrane in which pericytes and vascular smooth muscle cells are embedded. This endothelial cell basement membrane is permissive for T-cell and monocyte transmigration into the perivascular space and the formation of perivascular inflammatory cell cuffs. Of note, these perivascular cuffs are still silent clinically.
Perivascular cuff monocytes function as APCs. They capture local antigenic material, process it, and present processed antigen in the context of surface-expressed MHC class II molecules so as to prime abutting CD4 + T E cells for full effector function. In addition, microglial-derived DCs, resident in the juxtavascular CNS parenchyma, extend processes between astrocyte foot processes and through the basement membrane of the glia limitans (see later), express MHC class II alleles, and likewise present antigen to perivascular T E cells, further priming them in anticipation of their movement into the CNS parenchyma.
Plasma proteins leak into the CNS parenchyma at sites of acute MS disease activity . Plasma proteins, including fibrinogen and high-molecular-weight IgM, are carried across endothelial cells by an energy-requiring intracytoplasmic vesicular transport mechanism. Gadolinium (GD) is also transported into the CNS parenchyma in this same fashion, at least in guinea pigs with EAE. Transport is maximal across capillaries rather than across the postcapillary venules that CNS-invading immune system cells traverse. Most gadolinium positive lesions are silent clinically so that transendothelial cell transport of blood elements into the perivascular space need not, even when extensive, engender symptoms of an MS relapse.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here