Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Brushing our teeth seems like a simple voluntary movement. The neural basis for this action is, in fact, richly complex. For example, muscles in the upper limb are used cooperatively with jaw muscles, whereas neck and back muscles provide postural support. Sensory feedback from the teeth and gums is linked to muscle afferents conveying tension and proprioceptive signals from the forearm and hand. This sensory input is an essential feature of efficient motor performance.
Even other less obvious aspects, such as visual system input and memory of prior experience, are involved. The focus of this chapter is on elements of voluntary movement that are regulated by the cerebral cortex.
Control of the voluntary movements just described is a complex, multifaceted process that involves many areas of the brain. Several of the principal control sites are located in the cerebral cortex, specifically the primary motor, premotor, and supplementary motor cortices in the frontal lobe along with portions of the parietal lobe. Although the frontal and parietal lobes have direct projections to the spinal cord, they also work cooperatively through the primary motor cortex ( upper motor neurons ) and the corticospinal and corticonuclear systems to influence the activity of ventral horn and cranial nerve motor neurons ( lower motor neurons ). The latter cells and their axons represent the final common path that links the central nervous system with skeletal muscles. Lesions that damage the descending cortical systems or lower motor neurons produce signs and symptoms of upper or lower motor neuron disease, respectively. These signs are among the most useful in the diagnosis of neurologic deficits related to the control of movement.
Lower motor neurons are those cells whose axons synapse directly on skeletal muscle. When these neurons or their axons are damaged, the innervated muscles will show some combination of the following signs: (1) flaccid paralysis followed eventually by atrophy, (2) fibrillations or fasciculations (involuntary contractions of one motor unit or a group of motor units), (3) hypotonia (decreased muscle tone), and (4) weakening or absence of muscle stretch reflexes ( hyporeflexia, areflexia ).
The term upper motor neuron is commonly used in reference to corticospinal or corticonuclear cell bodies and their axons. Other neurons, such as rubrospinal or reticulospinal neurons, can also be included under the strict definition of this term. Corticospinal neurons are also called pyramidal neurons because their axons pass through the medullary pyramid. Therefore the terms upper motor neuron signs and pyramidal tract signs are often used synonymously. However, as described later in this chapter, these characteristic signs of “pyramidal tract damage” are in fact the result of injury to other descending motor systems in combination with damage to fibers of the pyramidal tract. For example, ischemic lesions of the internal capsule can potentially involve corticostriatal, corticothalamic, and corticoreticular fibers in addition to corticospinal axons because of the close proximity of these tracts to one another within the internal capsule.
Damage to upper motor neurons results in muscles that (1) are initially weak and flaccid but (2) eventually become spastic, (3) exhibit increased muscle tone ( hypertonia ), seen as an increase in resistance to passive movement of an extremity, and (4) show an increase in muscle stretch reflexes ( hyperreflexia ), as may be seen in clonus. Upper motor neuron lesions usually affect groups of muscles, and certain pathologic reflexes and signs often appear. One of the most common is the inverted plantar reflex, also known as the Babinski sign. This involves dorsiflexion of the great toe in response to firm stroking of the lateral aspect of the sole of the foot with a blunt instrument. The response in the normal adult is plantar flexion of the great toe. In contrast, stroking the sole of the foot in a normal neonate may also result in a Babinski sign. This is related to the incomplete myelination of the corticospinal tract and will disappear as this tract matures.
Muscles that are no longer under the influence of upper motor neurons exhibit spasticity. That is, when tested by the physician, the affected muscles offer an increased resistance to passive movement or manipulation. These effects are most pronounced in the antigravity muscles, which in humans include the proximal flexors in the upper extremity and extensors in the lower extremity. Also, the increased resistance to passive movement is velocity dependent: The more rapidly the examiner moves the affected extremity, the greater the resistance. However, after a relatively brief period of applied force, the increased resistance totally collapses; this response is known as the clasp-knife effect (also called clasp-knife rigidity ).
Several hypotheses have been advanced to explain spasticity. One suggests that spasticity, with its associated hypertonia and hyperreflexia, is the result of a release of dynamic gamma motor neurons from descending inhibitory control. This leads to increased gamma motor neuron firing and increased activity transmitted over type Ia muscle spindle afferents, resulting in increased excitatory tonic drive on the associated alpha motor neurons. Another suggestion is that spasticity may represent a generalized failure of the descending cortical activation of spinal cord inhibitory interneurons. For example, supraspinal fibers activate type Ia inhibitory interneurons that contact extensor motor neurons ( Fig. 25.1 A ). If the upper motor neuron input to inhibitory interneurons were to be removed, the extensor motor neurons would be released from inhibitory control, and the result would be hypertonia and spasticity.
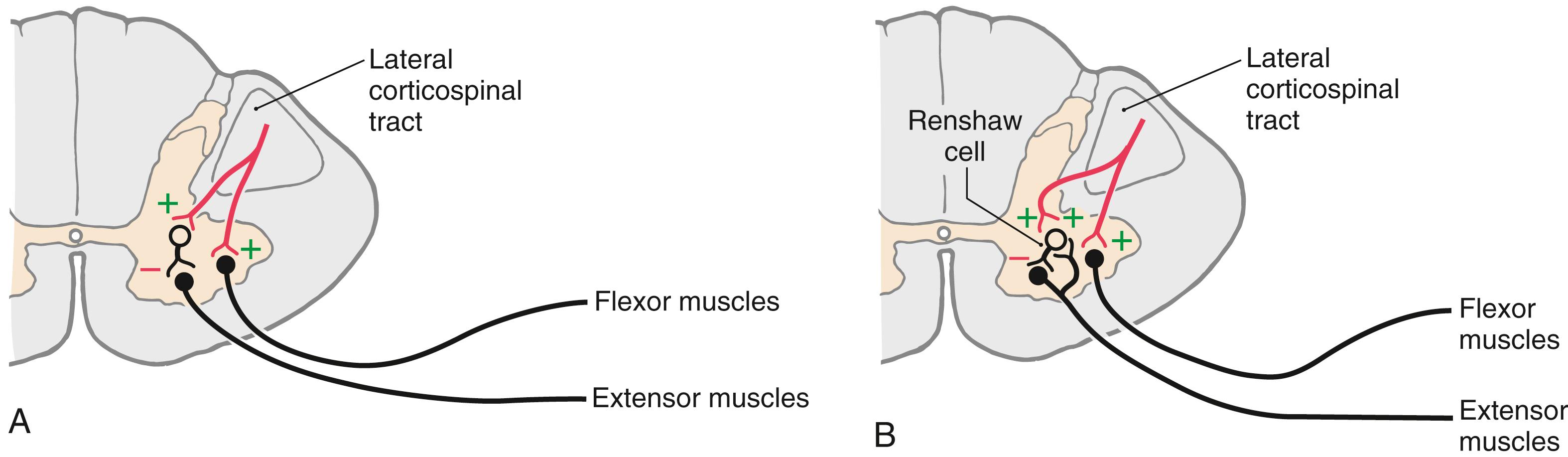
Descending cortical fibers also activate a type of inhibitory (glycinergic) interneuron called a Renshaw cell ( Fig. 25.1 B ). The Renshaw cell receives excitatory input from a lower motor neuron via an axon collateral (and in turn it inhibits the same lower motor neuron) and potentially from adjacent lower motor neurons. It is also known, for example, that cortical fibers activating ankle flexors also contact Renshaw cells (as well as type Ia inhibitory interneurons) that inhibit the antagonistic ankle extensors ( Fig. 25.1 B ). This circuitry serves to prevent reflex stimulation of the extensors when flexors are active. Therefore, when the cortical fibers are lost (upper motor neuron lesion), the inhibition of antagonists is absent. The result is repetitive, sequential contraction of ankle flexors and extensors. Such a phenomenon is referred to as clonus and is often present in combination with spasticity and hyperreflexia.
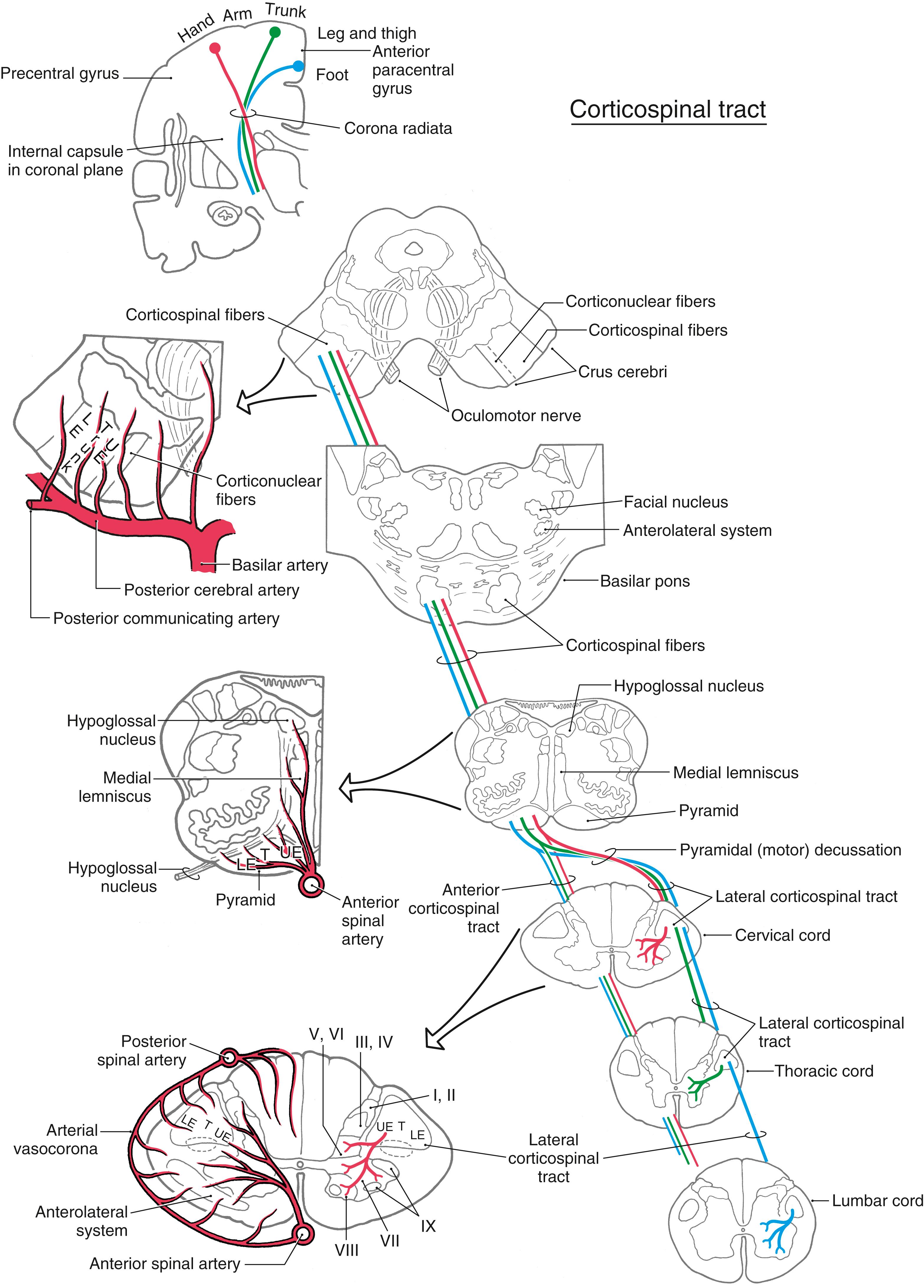
Before the specifics of corticospinal projections are considered, a general point about laterality should be made. The fibers that form the core of the corticospinal system cross the midline in the pyramidal decussation; this structure is commonly called the motor decussation because it is the location where corticospinal fibers cross. Corticospinal fibers arising in the left motor cortex therefore influence muscles on the right side of the body, and vice versa. Consequently, lesions of corticospinal fibers rostral to the motor decussation result in contralateral motor deficits, whereas lesions of the corticospinal tract caudal to the motor decussation (in the spinal cord) result in ipsilateral deficits. An understanding of this concept of laterality is essential in the diagnosis of the neurologically impaired patient.
Neurons that give rise to corticospinal axons are located in deep portions of layer V of the motor cortex ( Fig. 25.2 A ). A small number of these pyramidal neurons are especially large, with somata that may reach 100 μm or more in diameter. These neurons are called Betz cells, and at one time it was believed that they were the sole source of corticospinal axons. It is now known that they account for only 1% to 2% of this fiber bundle.
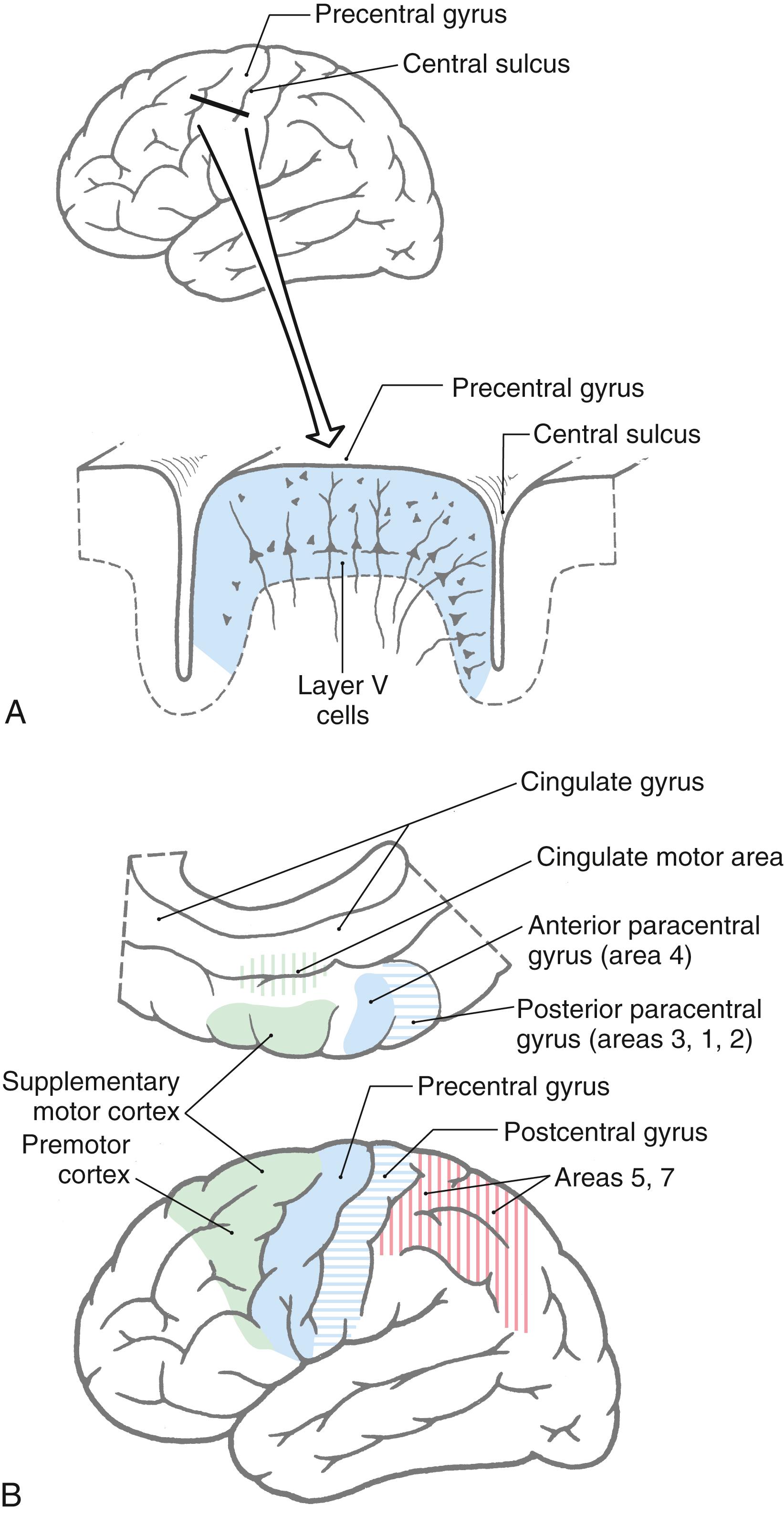
Corticospinal neurons are found primarily in six cortical locations ( Fig. 25.2 B ). The single largest concentration (about 31%) is in Brodmann area 4, which occupies the posterior portion of the precentral gyrus bordering on and extending into the depth of the central sulcus and in the anterior paracentral gyrus. This region is also called MI, the primary motor cortex. The premotor and supplementary motor cortices, which are located in area 6, give rise to about 29% of corticospinal axons. Thus about 60% of all corticospinal axons arise from neurons in the frontal lobe, with more than half of this group coming from the precentral and anterior paracentral gyri ( Fig. 25.2 B ). The remaining approximately 40% arise from the parietal lobe and a few other regions. Included are cells in the postcentral gyrus (areas 3, 1, and 2), the superior parietal lobule (areas 5 and 7), and portions of the cingulate gyrus.
Within MI, corticospinal neurons are somatotopically organized in patterns that reflect their influence over specific muscle groups. The caricature thus created is called the motor homunculus ( Fig. 25.3 A ). Neurons in medial MI, the anterior paracentral gyrus, project to lumbosacral cord levels to influence motor neurons that innervate muscles of the foot, leg, and thigh. Thoracic and cervical cord levels, which contain motor neurons innervating the trunk and upper extremity, receive input from neurons in the medial two thirds of the precentral gyrus. The musculature of the head, face, and oral cavity is influenced by neurons in the lateral third of the precentral gyrus ( Fig. 25.3 A ). These cells contribute to the corticonuclear tract that projects to cranial nerve motor nuclei. The disproportion in body part size in the homunculus reflects the density and distribution of corticospinal neurons devoted to the control of musculature in each particular region of the body ( Fig. 25.3 A ). Complete but less precise body representations are also found in other motor cortical regions. Thus a single muscle or muscle group can be influenced from multiple locations in the cerebral cortex.
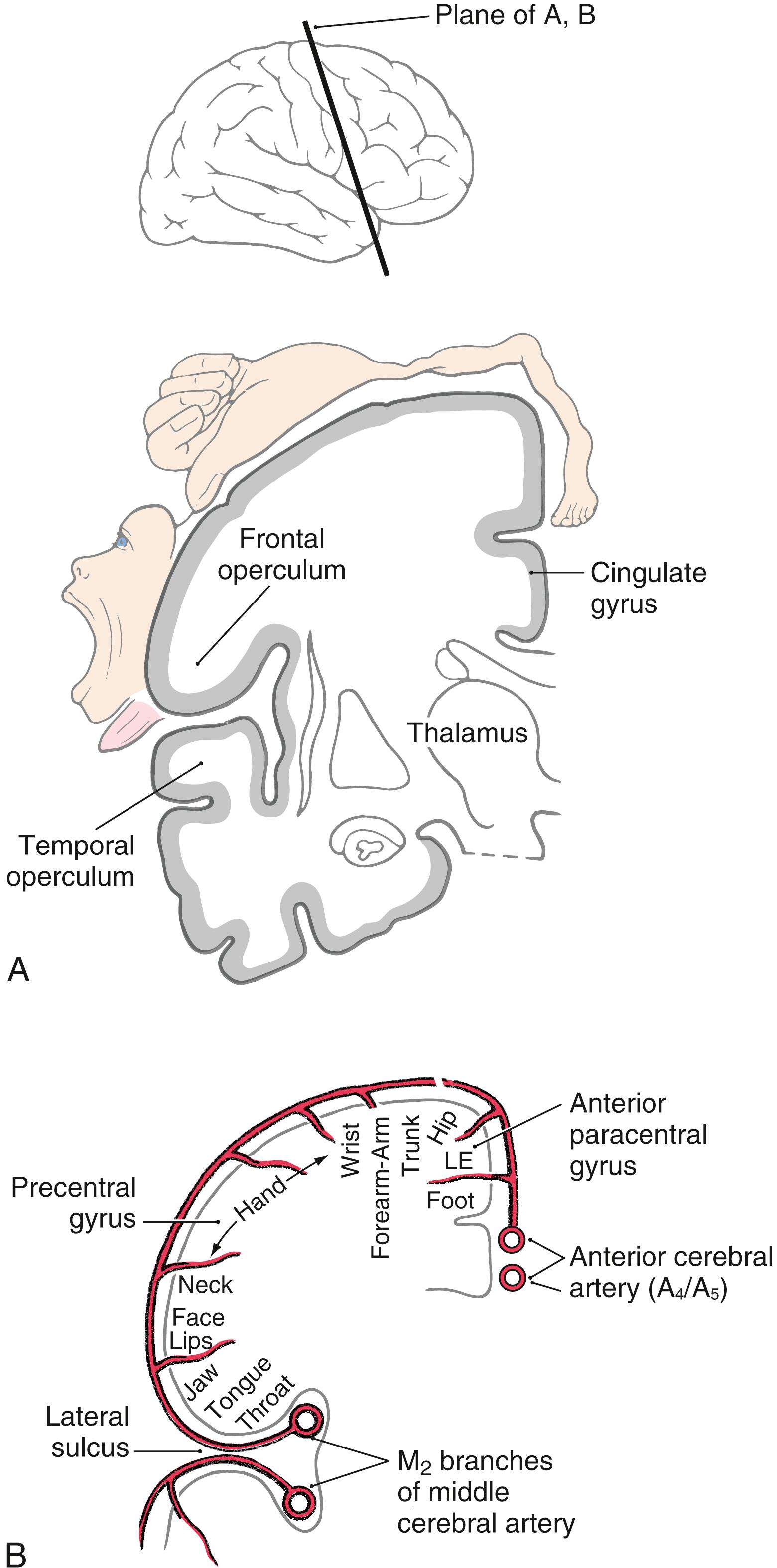
The blood supply to MI arises from branches of the anterior and middle cerebral arteries ( Fig. 25.3 B ). The lower extremity area of MI is served by terminal branches of A 4 and A 5 segments of the anterior cerebral artery. Specifically, these branches arise from the callosomarginal artery. The trunk, upper extremity, and head areas of the motor cortex are supplied by branches of the M 4 segment of the middle cerebral artery, mainly its rolandic and prerolandic branches.
Lesions that involve only areas of motor cortex outside the MI usually do not result in paralysis, and the effects may dissipate over time. For example, vascular infarcts of the premotor or supplementary cortex may produce an apraxia. This disorder involves difficulty in using the affected part of the body to perform voluntary actions, such as grasping a pencil, even though there is no obvious spasticity, paralysis, or altered tone in the muscles. For example, a premotor lesion may result in the inability to perform voluntary actions with the contralateral hand, although the strength and tone of the hand muscles are normal. Similarly, unilateral lesions in the supplementary motor cortex affect the ability to coordinate actions on the two sides of the body. The muscles, again, are normal. In contrast, lesions that affect the primary motor cortex in combination with another motor cortical region usually result in spastic paralysis and hyperreflexia, signs characteristic of upper motor neuron lesions.
The largest axons in the corticospinal tract are myelinated, range from 12 to 15 μm in diameter, and have conduction velocities up to 70 m/s, but they make up less than 10% of the total corticospinal population. The remainder are less than 5 μm in diameter, and many are lightly myelinated or unmyelinated with proportionally slower conduction velocities.
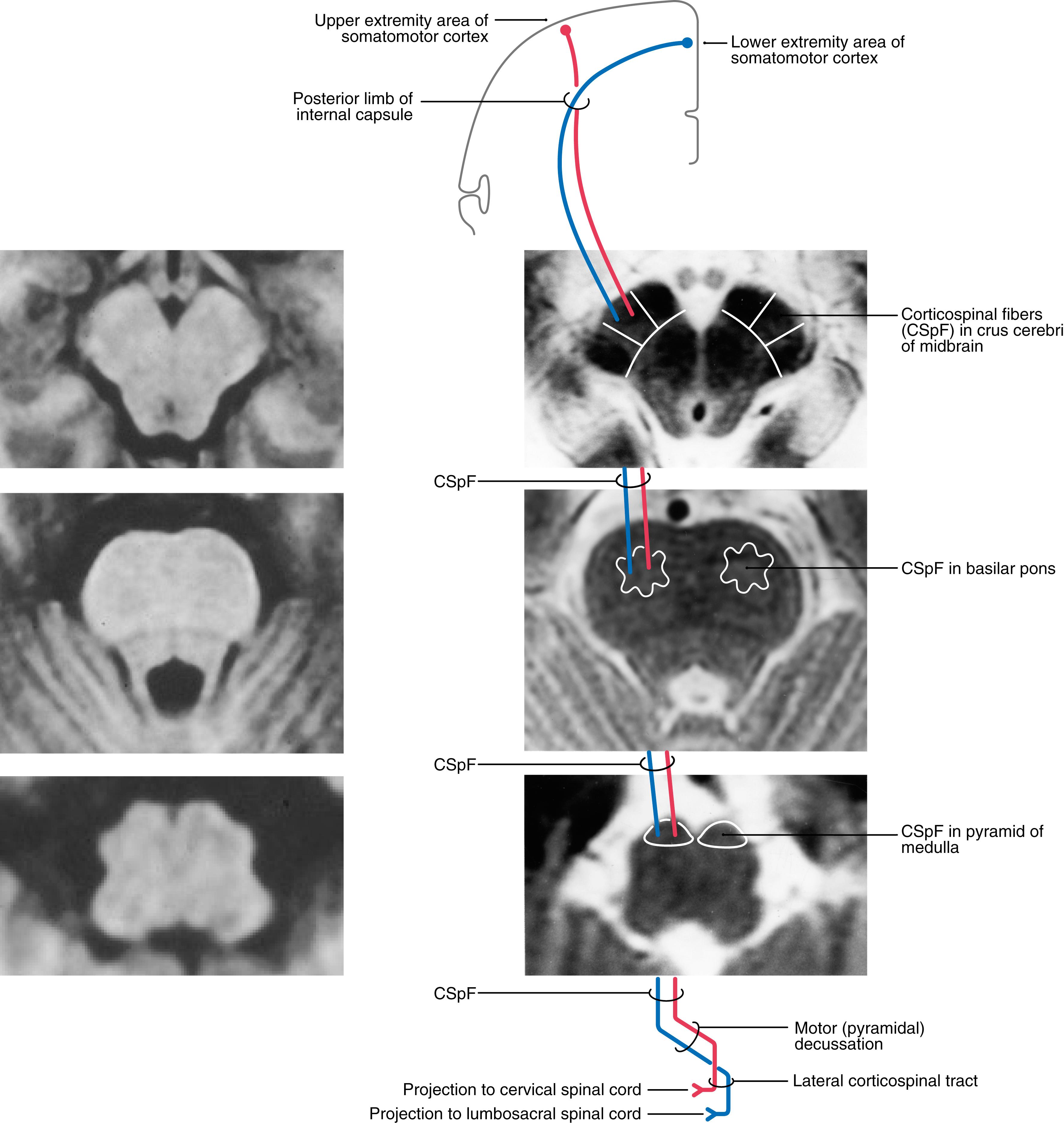
Corticospinal fibers pass through the corona radiata and converge to enter the posterior limb of the internal capsule ( Fig. 25.4 A ). Here the fibers are somatotopically organized in about the caudal half of the posterior limb such that the axons that terminate at the highest cord levels are located most rostrally, and the axons that terminate at progressively lower levels are located more caudally ( Fig. 25.4 B ).
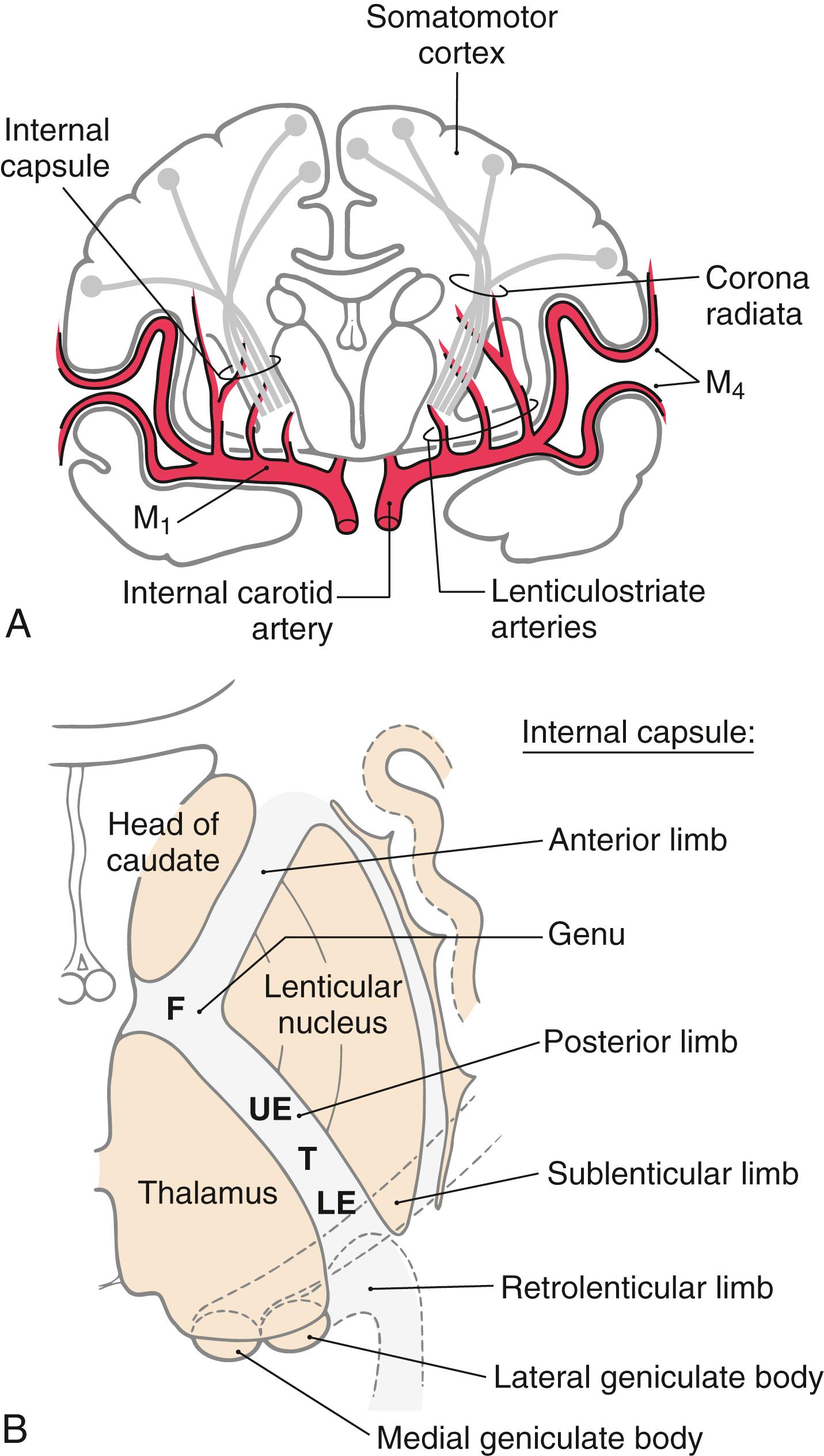
Unlike with lesions of the cortical gray matter, interruption of axons in the posterior limb of the internal capsule often results in catastrophic motor deficits. A common cause of lesions in this area is hemorrhage from lenticulostriate branches of the M 1 segment of the middle cerebral artery ( Fig. 25.4 A ). Motor symptoms of capsular infarcts appear in the contralateral upper and lower extremities and consist of weakness and transient flaccid paralysis of variable duration, which is followed by spastic paralysis (upper motor neuron signs) that typically never resolves. Apparently these symptoms appear because not only corticospinal fibers but also many other types of cortical axons are interrupted. Included are axons projecting to the neostriatum, thalamus, and brainstem as well as thalamocortical axons involved in somatic sensation and vision. Damage to thalamocortical axons explains why hemisensory loss or homonymous hemianopia (in the case of an anterior choroidal artery syndrome) may accompany the motor deficits. Deficits such as spasticity, hypertonia, and hyperreflexia, although commonly associated with pyramidal tract lesions, are in fact due to damage of other descending systems in combination with damage to corticospinal fibers.
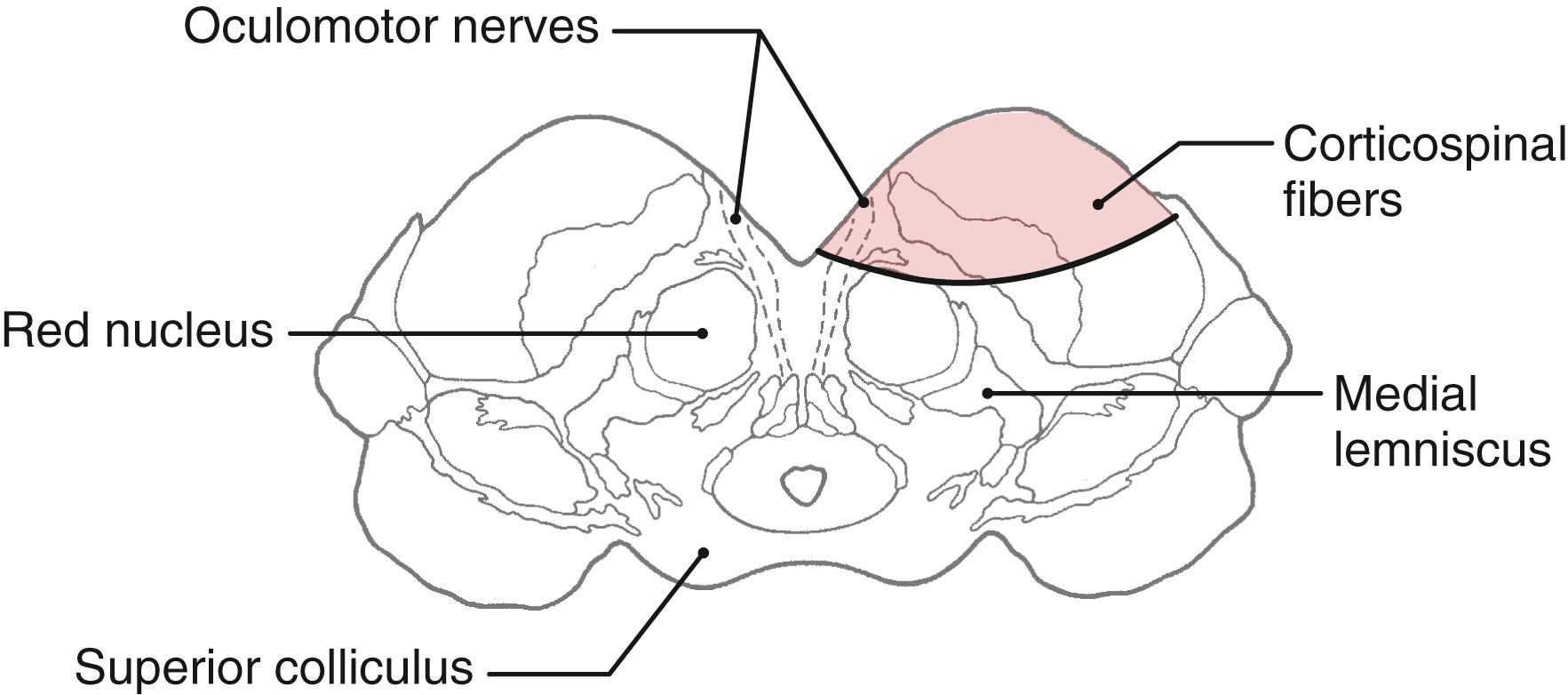
As they pass caudally from the internal capsule, corticospinal fibers traverse the various divisions of the brainstem. In the midbrain they coalesce to form the middle third of the crus cerebri ( Figs. 25.5 A , 25.6, and 25.7 ). Within this part of the crus, fibers from forearm-hand (upper extremity) areas of MI are located medially, whereas those from leg-foot (lower extremity) areas are located laterally.
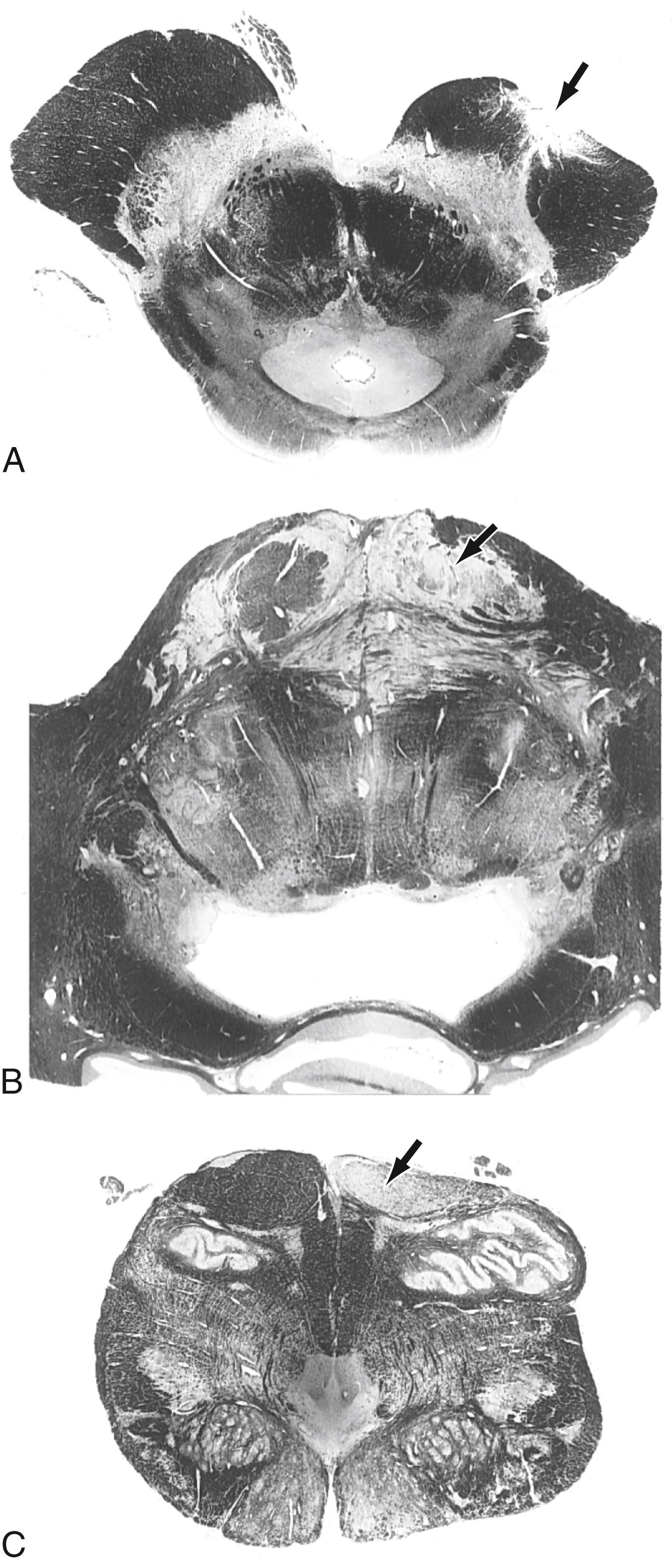
Fibers in the medial two thirds of the crus cerebri (frontopontine, corticonuclear [corticobulbar], and corticospinal) and the exiting rootlets of the oculomotor nerve fibers are served by paramedian branches of P 1 and branches from the adjacent posterior communicating artery ( Fig. 25.6 ). Hemorrhage of these vessels will damage these groups of fibers, resulting in (1) contralateral hemiparesis of the arm and leg with spasticity and (2) deviation of the ipsilateral eye down and laterally because of damage to the oculomotor nerve resulting in unopposed action of the superior oblique and lateral rectus muscles. Direct and consensual light reflexes and accommodation may also be lost in the eye on the side of the oculomotor nerve lesion. This condition is known as superior alternating hemiplegia because cranial nerve signs are seen on one side and corticospinal signs on the “alternate” side; it is also called a crossed deficit. In clinical parlance, this combination of deficits is known as a Weber syndrome ( Fig. 25.8 ; Table 25.1 ).
| Syndrome | Structures Involved | Corresponding Deficit |
|---|---|---|
| Benedikt syndrome (Weber and Claude) | Corticospinal fibers in crus | Contralateral hemiplegia |
| Oculomotor nerve fibers | Ipsilateral oculomotor palsy, dilated pupil, diplopia | |
| Red nucleus | Contralateral tremor, hyperkinesias | |
| Cerebellothalamic fibers | Contralateral ataxia | |
| (Medial lemniscus) | (Contralateral loss of vibratory sense, position sense, discriminative touch) | |
| Claude syndrome † | Oculomotor nerve fibers | Ipsilateral oculomotor palsy, dilated pupil, diplopia |
| Red nucleus | Contralateral tremor, hyperkinesias | |
| Cerebellothalamic fibers | Contralateral ataxia | |
| (Trochlear nucleus) | (Weakness of contralateral superior oblique muscle) | |
| Dejerine syndrome (medial medullary) | Corticospinal fibers in pyramid | Contralateral hemiplegia |
| Hypoglossal nerve fibers or nucleus | Ipsilateral deviation of tongue on protrusion | |
| Medial lemniscus | Contralateral loss of vibratory sense, position sense, discriminative touch | |
| Foville syndrome ‡ | Corticospinal fibers in basilar pons | Contralateral hemiplegia |
| Abducens nerve fibers | Ipsilateral abducens (lateral rectus) palsy, diplopia | |
| Middle cerebellar peduncle | Ataxia | |
| Gubler or Millard-Gubler syndrome § | Corticospinal fibers in basilar pons | Contralateral hemiplegia |
| Facial nerve fibers or nucleus | Ipsilateral weakness of facial muscles | |
| (Anterolateral system) | (Impaired pain and thermal sense on contralateral side of body) | |
| (Trigeminal nerve fibers) | (Impaired pain and thermal sense on ipsilateral side of face) | |
| Midpontine base syndrome | Corticospinal fibers in basilar pons | Contralateral hemiplegia |
| Trigeminal nerve fibers | Ipsilateral paralysis of masticatory muscles; ipsilateral loss of pain and thermal sensations on face | |
| Middle cerebellar peduncle | Ataxia | |
| Raymond syndrome | Corticospinal fibers in basilar pons | Contralateral hemiplegia |
| Abducens fibers in basilar pons | Ipsilateral abducens (lateral rectus) palsy, diplopia | |
| Wallenberg syndrome (lateral medullary, posterior inferior cerebellar artery) | Spinal trigeminal tract | Ipsilateral loss of pain and thermal sense on face |
| Anterolateral system | Contralateral loss of pain and thermal sense on the body | |
| Vestibular nuclei | Vertigo, nystagmus, nausea, vomiting | |
| Nucleus ambiguus | Hoarseness, dysphagia, deviation of the uvula to opposite side on phonation | |
| Restiform body | Ataxia | |
| Weber syndrome | Corticospinal fibers in crus | Contralateral hemiplegia |
| Oculomotor nerve fibers | Ipsilateral oculomotor palsy, dilated pupil, diplopia | |
| Corticonuclear fibers in crus | Contralateral weakness of facial muscles on lower half of face; deviation of the tongue to contralateral side on protrusion; ipsilateral weakness of trapezius and sternocleidomastoid muscles | |
| Substantia nigra | Contralateral Parkinson-like tremor, akinesia |
∗ Syndromes are listed in alphabetical order.
† Although this syndrome does not include corticospinal fibers, it is included here for completeness.
‡ This syndrome is described, in some sources, as including the facial nerve or nucleus, anterolateral system fibers, paramedian pontine reticular formation (lateral gaze center), and medial lemniscus, each with their corresponding deficits.
§ Some sources also include the fibers of the abducens nerve as being involved in this syndrome.
From the midbrain, corticospinal fibers continue into the basilar pons, where they make their way longitudinally between the masses of neurons forming the basilar pontine nuclei ( Figs. 25.5 B , 25.6, and 25.7 ). As corticospinal axons pass through the pontine gray, they give rise to collaterals that synapse on these neurons.
Corticospinal fibers in the basilar pons and the exiting fibers of the abducens nerve in the caudal pons are within the domain of the paramedian branches of the basilar artery. Occlusion or rupture of these vessels results in hemiplegia and upper motor neuron signs in the contralateral extremities. The lesion may also involve intraaxial abducens fibers, resulting in lower motor neuron paralysis of the ipsilateral lateral rectus muscle ( Fig. 25.9 ). This combination of deficits (ipsilateral abducens paralysis and contralateral hemiplegia) is (1) a characteristic of brainstem lesions, that is, a crossed deficit, (2) called a middle alternating hemiplegia, and (3) one of the variations of the Foville syndrome ( Table 25.1 ). The paramedian branches of the basilar artery may penetrate deep into the pons and also serve the medial lemniscus ( Fig. 25.9 ). In such cases, damage to these vessels can produce not only the motor deficits described earlier but also contralateral loss of vibratory sense and two-point tactile discrimination.
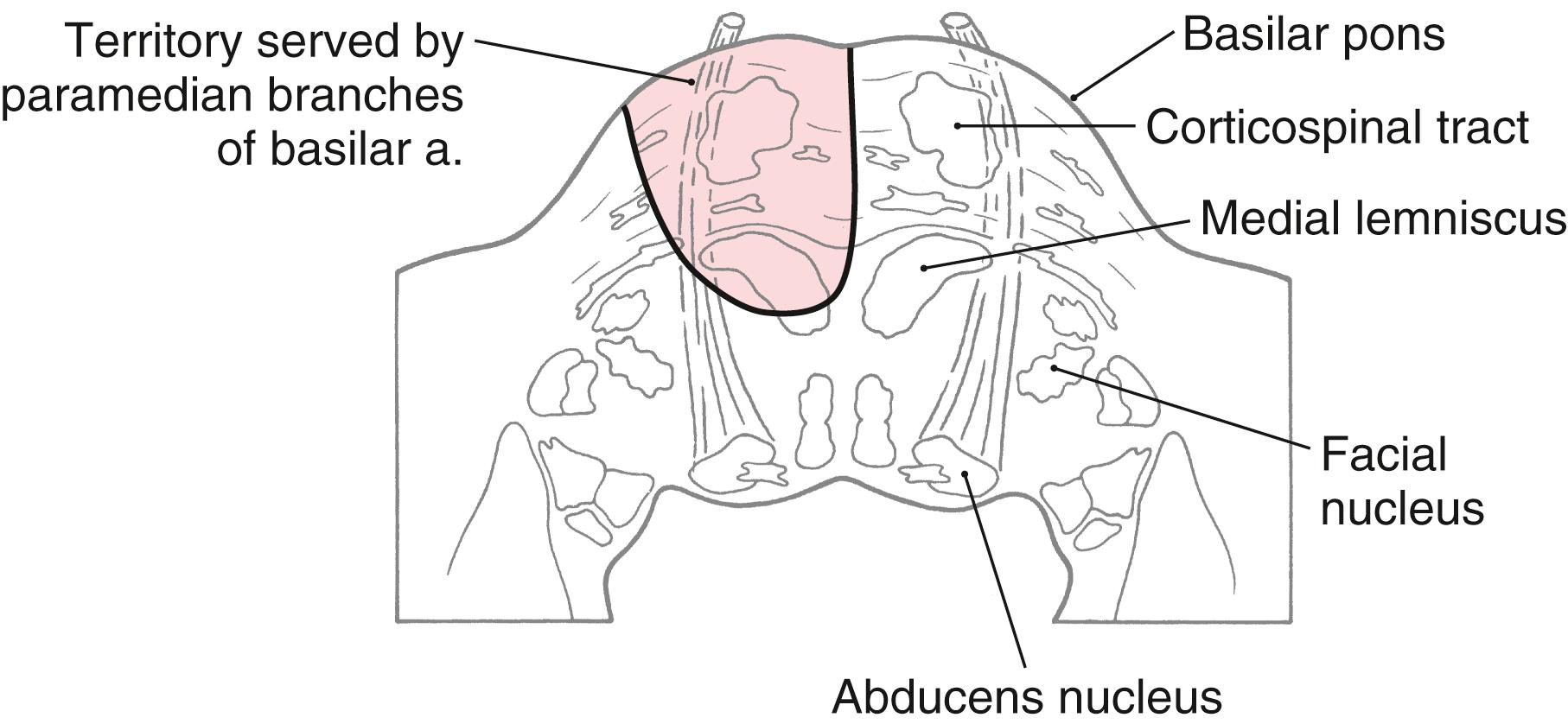
In the medulla, corticospinal fibers aggregate on the anterior surface of the brainstem, where they course within the medullary pyramids ( Figs. 25.5 C , 25.6, and 25.7 ). Within the pyramid, fibers that terminate at cervical levels tend to be located medially, whereas those projecting to lumbar and sacral levels are progressively more lateral. Collaterals of these axons innervate the inferior olivary complex, posterior column nuclei, and various medullary reticular nuclei.
The pyramid, the laterally adjacent exiting fibers of the hypoglossal nerve, and the medial lemniscus receive their blood supply through penetrating branches of the anterior spinal artery ( Fig. 25.6 ). Occlusion of these branches results in a contralateral hemiparesis of the extremities (with spasticity, corticospinal) and an ipsilateral flaccid paralysis of the tongue ( hypoglossal nerve ). When it is protruded, the tongue deviates toward the side of the lesion (the weak or flaccid side). This combination of symptoms is called inferior alternating hemiplegia. Because branches of the anterior spinal artery also serve the medial lemniscus, an inferior alternating hemiplegia is typically accompanied by a contralateral loss of two-point discrimination and vibration sense. Lesions of the medial medulla characterized by crossed (or alternating) deficits, as described earlier for other brainstem levels, are also known as the Dejerine syndrome ( Table 25.1 ). Additional brainstem syndromes that may involve cranial nerves and corticospinal fibers in various combinations are summarized in Table 25.1 .
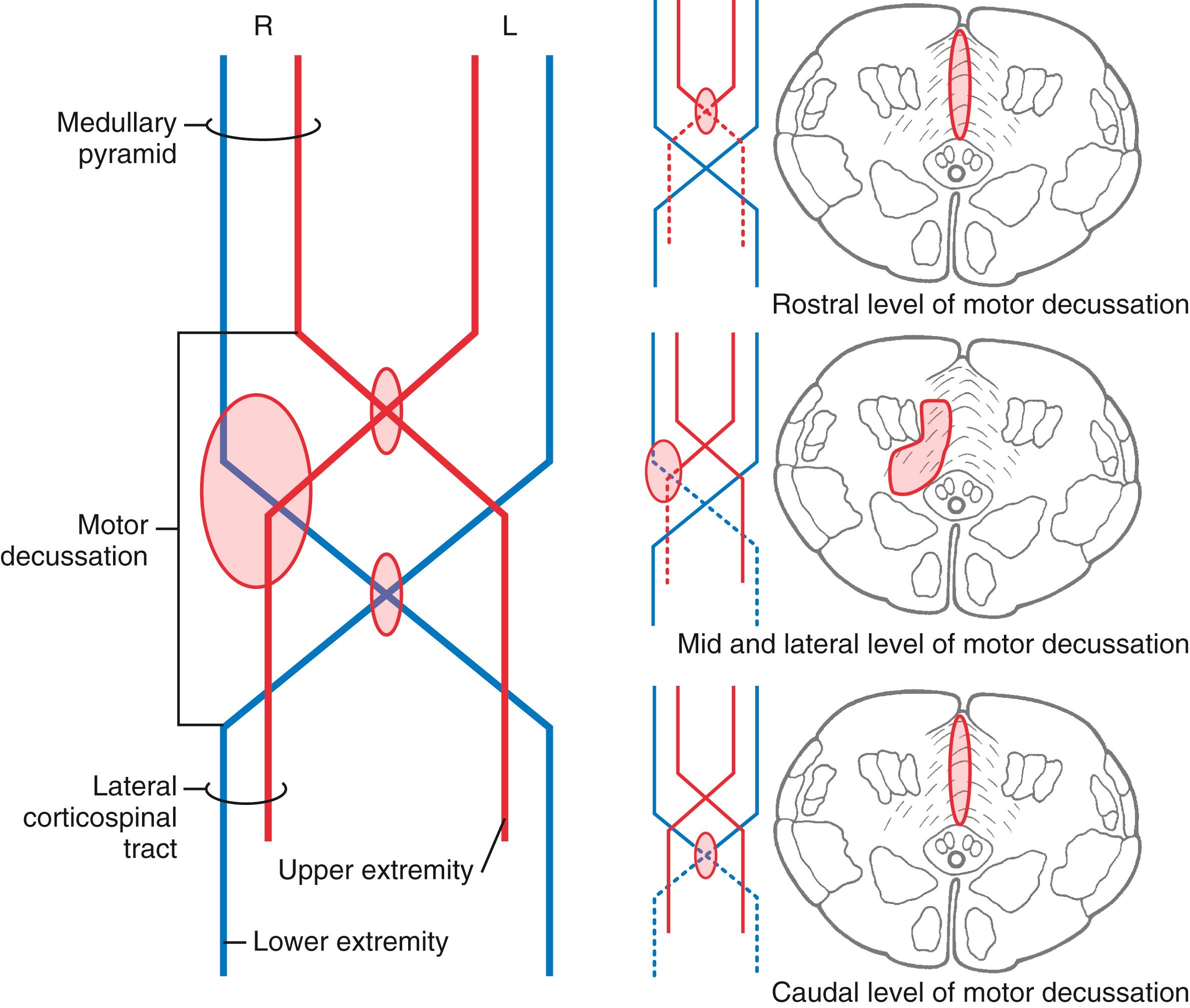
At the medullospinal junction, 85% to 90% of the corticospinal fibers cross the midline as the motor decussation (sometimes called decussation of the pyramids ) ( Fig. 25.6 ). The corticospinal fibers within the motor decussation are somatotopically arranged ( Fig. 25.10 ). Fibers that originate in the upper extremity portion of the MI cortex cross in rostral portions of the decussation and preferentially terminate in cervical cord levels. In similar manner, fibers that arise in the lower extremity portion of MI cross in the caudal parts of the decussation and terminate primarily in lumbosacral cord levels. This arrangement explains why small vascular lesions in the motor decussation (which is also served by branches of the anterior spinal artery) may result in selective bilateral weakness or paralysis of only the upper extremities or only the lower extremities ( Fig. 25.10 ). The pattern of crossing fibers in the motor decussation also explains the somewhat unusual picture of weakness of the upper extremity on one side and of the lower extremity on the opposite side. Lesions in the rostral half of the decussation on one side, and not on the midline, damage upper extremity fibers that have already crossed (ipsilateral upper extremity weakness) and lower extremity fibers that have not crossed (contralateral lower extremity weakness) ( Fig. 25.10 ). If a lesion producing this alternating upper extremity–lower extremity pattern of deficits extends laterally, it may damage the accessory nucleus and the anterolateral system with corresponding deficits. The decussating fibers extend into the lateral funiculus to form the lateral corticospinal tract. The corticospinal axons that do not cross in the decussation continue into the ipsilateral anterior funiculus of the spinal cord as the anterior corticospinal tract ( Figs. 25.6 and 25.7 ). Damage to this tract is of little clinical significance. This is because most of the fibers in this tract cross in the spinal cord before termination.
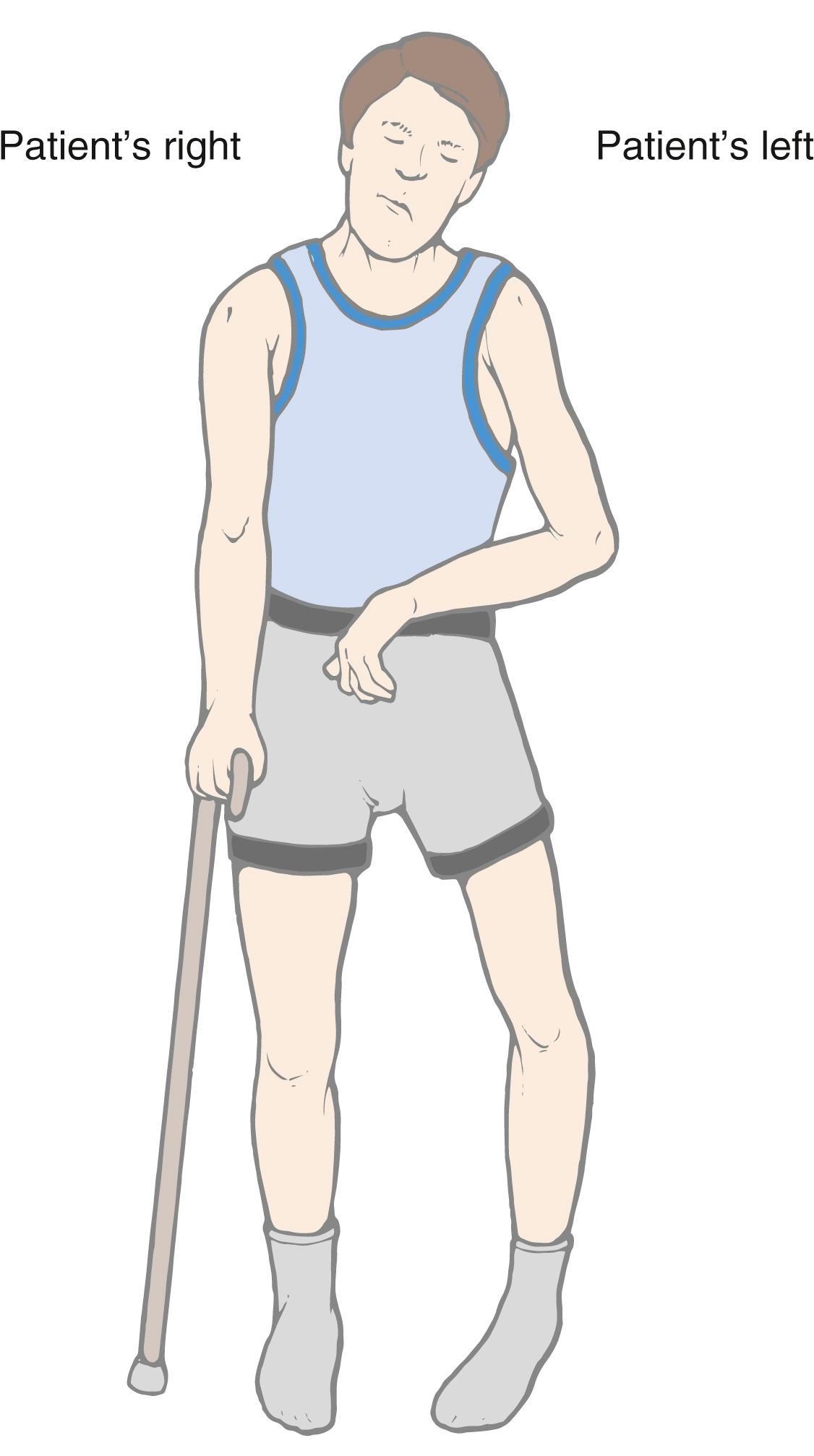
The crossing of corticospinal fibers at the motor decussation is the anatomic basis for the contralateral deficits seen in a patient with a lesion in which these fibers are rostral to (above) this decussation. For example, a patient with a capsular lesion on the right side will have hemiparesis of the upper and lower extremities on the left ( Fig. 25.11 ). This patient may also exhibit additional deficits related to damage of corticonuclear fibers in the genu of the internal capsule, such as drooping of the face and weakness of the sternocleidomastoid muscle; these deficits are discussed later in the section on the corticonuclear system.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here