Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Microscopic morphologic assessment is a major component of experimental toxicity studies using laboratory animal models. To realize the full value of these studies, toxicologic pathologists should accurately classify all adverse findings and provide a cogent interpretation including a plausible pathogenesis (see Nomenclature and Diagnostic Resources in Anatomic Toxicologic Pathology , Vol 1, Chap 25 ). Cell injury is a common manifestation of chemically induced toxicity. It can occur as a primary event or as sequelae to a variety of predisposing conditions such as inflammation or ischemia, which are also common manifestations of chemically induced toxicity.
Macroscopic morphological changes are evident when large numbers of cells are injured. Standard light microscopic evaluation of formalin-fixed tissue sections, stained by hematoxylin and eosin (H&E), remains the gold standard for classifying cell injury at the cellular level. In chronic studies, sequelae to cell injury include reactive hyperplasia or tissue atrophy and fibrosis that infer earlier cell injury. Unraveling the pathogenesis of such findings may require meticulous evaluations of tissues and cells in short term in vivo studies, or in vitro cell or tissue models that provide the opportunity to investigate initiating events of cell injury in isolation. Utilizing special techniques such as immunohistochemistry, transmission electron microscopy, or digital image analysis may better define molecular mechanisms, identify target organelles, or quantify the extent of injury by classifying a tissue response to cell injury. Ultimately, articulating the sequence of morphologic changes can generate testable hypotheses that define the mechanism of toxicity and potentially refine risk assessment.
This chapter will serve as an overview of the basic histologic and ultrastructural features that reflect cell injury and cell death as well as the sequelae to such processes. Biochemical and molecular changes that underlie these morphologic alterations are discussed in a previous chapter (See Biochemical and Molecular Basis of Toxicity , Vol 1, Chap 2). Injuries that modify the genetic makeup of a cell to produce disturbances in cell cycle and tissue growth are discussed in detail in a succeeding chapter (See Carcinogenesis: Mechanisms and Evaluation , Vol 1, Chap 8). More advanced techniques of analysis, whether for determining adaptive responses, cell injury or cell death, will be covered succeeding sections of this volume (see Vol 1, Part 2, “Methods in Toxicologic Pathology” ).
Living cells are complex structures comprising an interdependent array of many essential components including organelles, fluids, proteins, electrolytes, signaling molecules, and plasma membrane receptors. Although injury to any one of these components can ultimately result in death of the entire cell, some are particularly critical for cell survival: the plasma membrane is the site of osmotic, electrolyte, and water regulation and receptor–ligand signal transduction; the mitochondrion is the site of aerobic respiration and adenosine triphosphate (ATP) generation; the rough endoplasmic reticulum (rER) is the site of most protein synthesis and calcium storage; and the nucleus, where DNA transcription occurs. The biochemical consequences of damage to these structures have been discussed in a previous chapter (see Biochemical and Molecular Basis of Toxicity , Vol 1, Chap 2 ). Other structures, such as lysosomes, peroxisomes, secretory granules, microtubules, microfilaments, and even the extracellular matrix (ECM), may be initially altered in cell injury, depending on the mechanism of action of the injurious agent, but alterations in these structures just as often reflect penultimate secondary changes rather than the proximate cause of cell death.
The onset and progression of cell injury depends on the nature of the noxious insult, including its severity and duration. If injury to the cell is mild and not persistent, recovery is usually rapid and complete; a morphologic manifestation may be absent or imperceptible although a transient functional disruption and/or a brief biochemical alteration may be detectable. For example, transient release of alanine aminotransferase from hepatocytes into plasma after administration of a mild hepatotoxic compound for a short period of time may not correlate to damage microscopically or even ultrastructurally. At the other extreme, a potent hepatotoxic compound may cause such widespread hepatic necrosis after the initial spike in serum levels of the toxicant or toxin. The cause of the massive lysis of hepatocytes cannot be determined as the xenobiotic is no longer detectable in serum due to its relative short half-life at the time of necropsy. However, the lesion is obvious even at the macroscopic level. More information on clinical pathology parameters in toxicity testing can be found in the chapter Clinical Pathology in Nonclinical Toxicity Testing , Vol 1, Chap 10 .
If cells in a tissue are injured, and the compound suspected of causing the damage is still detectable in blood or tissue, a presumptive etiologic diagnosis is possible. But with chronic tissue injury, such as glomerulosclerosis or retinal atrophy, the etiology may remain undetermined.
Furthermore, cell injury can be manifested exclusively by functional deficits without perceptible morphologic manifestations if the death of the individual cell occurs very rapidly after exposure or insult (e.g., in a matter of minutes). Such injury could even result in death of the animal. The complete disruption of oxidative phosphorylation in acute cyanide toxicity due to inhibition of cytochromes (CYPs) in the mitochondrial respiratory chain is such an example ( ; ).
The metabolic rate of a cell has a significant effect on lesion development. Cells with high metabolic activity are generally more sensitive to noxious injury than cells with low energy needs. For example, neurons are quite sensitive to hypoxia, while fibroblasts are quite resistant. Metabolically active cells absolutely depend on a continuous supply of oxygen (O 2 ) and functional mitochondria to generate ATP for sustaining cell function. Toxic injuries that break the links between O 2 diffusion from inhaled air across pulmonary alveoli to the O 2 -carrying erythrocytes in blood vessels to the interstitium to the cell and finally to mitochondria, where oxidative phosphorylation and ATP generation occur, can subsequently injure cells with high energy needs. Adequate cellular ATP is necessary to maintain normal structure and function of many vital specialized cell populations, such as neurons. These cells require energy to sustain membrane integrity and polarity as well as neurotransmitter production. Similarly, myocardial cells require energy for myofilament contraction/relaxation and Ca +2 transport, and renal tubular epithelial cells which require energy for transport of fluids, electrolytes, and metabolites. Even small changes in cellular O 2 tension leading to mildly decreased ATP production can cause serious alterations in essential functions of these cell types, with severe consequences for survival. Shifting to anaerobic glycolysis is inefficient for production of adequate ATP and leads to lactic acidosis. By contrast, cells with low metabolic activity, such as fibroblasts and adipocytes, are resistant to low O 2 supply and can tolerate hypoxic conditions. Hence, these connective tissue elements can play prominent roles in regeneration and scarring after tissue damage.
Highly specialized cells are not only sensitive to hypoxia, they are generally more sensitive to toxic insult than connective tissue cells. The latter, such as fibroblasts, possess greater adaptability to a variety of conditions, assuming an assortment of different roles due to their relatively undifferentiated nature and broad functional capabilities. These cells can tolerate anaerobic conditions and many types of toxic insults. In contrast, highly specialized cells, such as retinal rods and cones, must expend abundant energy to maintain their membranes in conformations capable of trapping photons; hence, they are exquisitely sensitive to injury.
The specialized functions of a tissue cell may predispose it to a particular type of noxious insult. Specific receptors on a particular cell population may render it susceptible to insults that would not impact neighboring cell types. For instance, cells expressing the Fas receptor (FasR) may undergo apoptosis when Fas ligand binds to it, whereas cells without this receptor will be unaffected. Some cells may accumulate toxins because they have specific transporters that facilitate their uptake. For example, the nephrotoxicity of gentamicin, and similar aminoglycoside antibiotics, depends on the organic cation transport system that leads to accumulation in lysosomes. Lysosomes eventually rupture, triggering changes that lead to cell injury and then cell death ( ).
Toxicity may be further enhanced if the target cell is incapable of metabolic detoxification or metabolizes the toxin to a more reactive chemical species. Phase I metabolism, especially by certain members of the CYP450 family, is particularly important in bioactivation of xenobiotics to toxic intermediates. For example, the presence in the hepatocyte of CYP 2E1 makes it uniquely susceptible to damage by acetaminophen, which is bioactivated to a highly reactive quinone imine by this CYP isozyme ( ).
The “innate” ability of a cell to counter an injurious stimulus can be critical for resisting a toxic insult. For example, cells with high levels of antioxidants are typically resistant to oxidative injury. The liver, which receives 60% of its blood supply directly from the gastrointestinal tract through the portal vein, can generally tolerate a remarkably high level of potential noxious agents originating from the gut. This tolerability is attributed to high concentrations of antioxidants such as reduced glutathione and the vitamins C and E, and a broad array of phase I and phase II detoxification enzymes within hepatocytes. Of course, metabolizing enzymes are a “double-edged sword” since otherwise innocuous compounds may be metabolized highly bioactive, toxic intermediates which deplete glutathione and then bind to innate proteins or nucleic acids. Cells lacking high concentrations of endogenous antioxidants and/or antioxidant enzymes or missing the appropriate quantities or combinations of phase I and phase II enzymes can be especially prone to toxic injury. This vulnerability is heightened if cells tasked with detoxification, like the hepatocyte or the bronchiolar exocrine (club) cell in the lung, fail to detoxify harmful substances before they reach the general circulation.
Host reaction to injured or dead cells often plays a key role in the morphologic manifestation of toxic cell injury. The inflammatory reaction to injured cells can amplify the original toxic injury to a more substantial lesion or even a life-threatening condition. Thus, what may be a relatively mild and recoverable injury can progress to a severe, nonresolving lesion. For example, acute necrotizing pancreatitis induced by acinar cell toxins is exacerbated by activated neutrophils attracted by released zymogen granules. Neutrophils release a variety of preformed and de novo generated chemicals that indiscriminately destroy even previously unaffected cells in the vicinity of the original injury.
It is important to consider that the inflammatory reaction to “programmed” cell death such as apoptosis or other related forms of death is muted or even absent altogether. With necrosis and other forms of mechanistically related cell death, the inflammatory reaction is provoked by leakage of normally sequestered intracellular contents [e.g., cytochrome c from mitochondria, partially degraded cell components (e.g., cross-linked fragments of damage membranes), cytoplasmic enzymes, and signaling proteins from disintegrating cells]. This process especially attracts neutrophils, the “first responders” of the acute inflammatory response. In contrast, apoptosis and related forms of cell death proceed in an orderly sequence of cell senescence preserves membrane integrity. The sequestration of intracellular materials within the debris (“apoptotic bodies”) permits macrophages to scavenge potential proinflammatory products before they can be released.
All the aforementioned factors and more contribute to the varied susceptibility of different cells and tissues to injury. Regardless of the type of injury or the factors present that mitigate or exacerbate that injury, a given cell population has only a limited number of responses available for survival and repair.
A cell exists within a narrow range of physiochemical conditions necessary to maintain a viable state. Thus, a cell, even a highly specialized one, dedicates much of its resources toward maintaining this internal environment. Ion gradients, intracellular pH, and cytosolic osmolarity are vigorously controlled by the cell, even at the cost of its own specialized functions. A cell threatened with a loss of these basic conditions will often jettison its specialized structures and cut back on its specialized functions, regardless of whether these functions are critical to the survival or the host at large. Substantial deviations from homeostasis lead to death of the cell. Less substantial deviations may lead to a new, usually reduced, level of function or metabolic activity in an attempt compromise between overall cell survival and specialized cell function. The response of a cell to disrupted homeostasis while maintaining some degree of function and avoiding death is called adaptation.
Atrophy is an adaptive change characterized as a generally visible reduction in the size (volume) of a cell, tissue, or organ. Cell atrophy can simply result from “lack of use” (i.e., reduced demand for the specialized function or product of the affected cell type), such as occurs in skeletal muscle fibers after denervation or with immobilization. The lack of muscle contraction leads to a reduction in sarcoplasmic contractile proteins which is evident microscopically as a decrease in myofiber diameter ( Fig. 6.1 ).
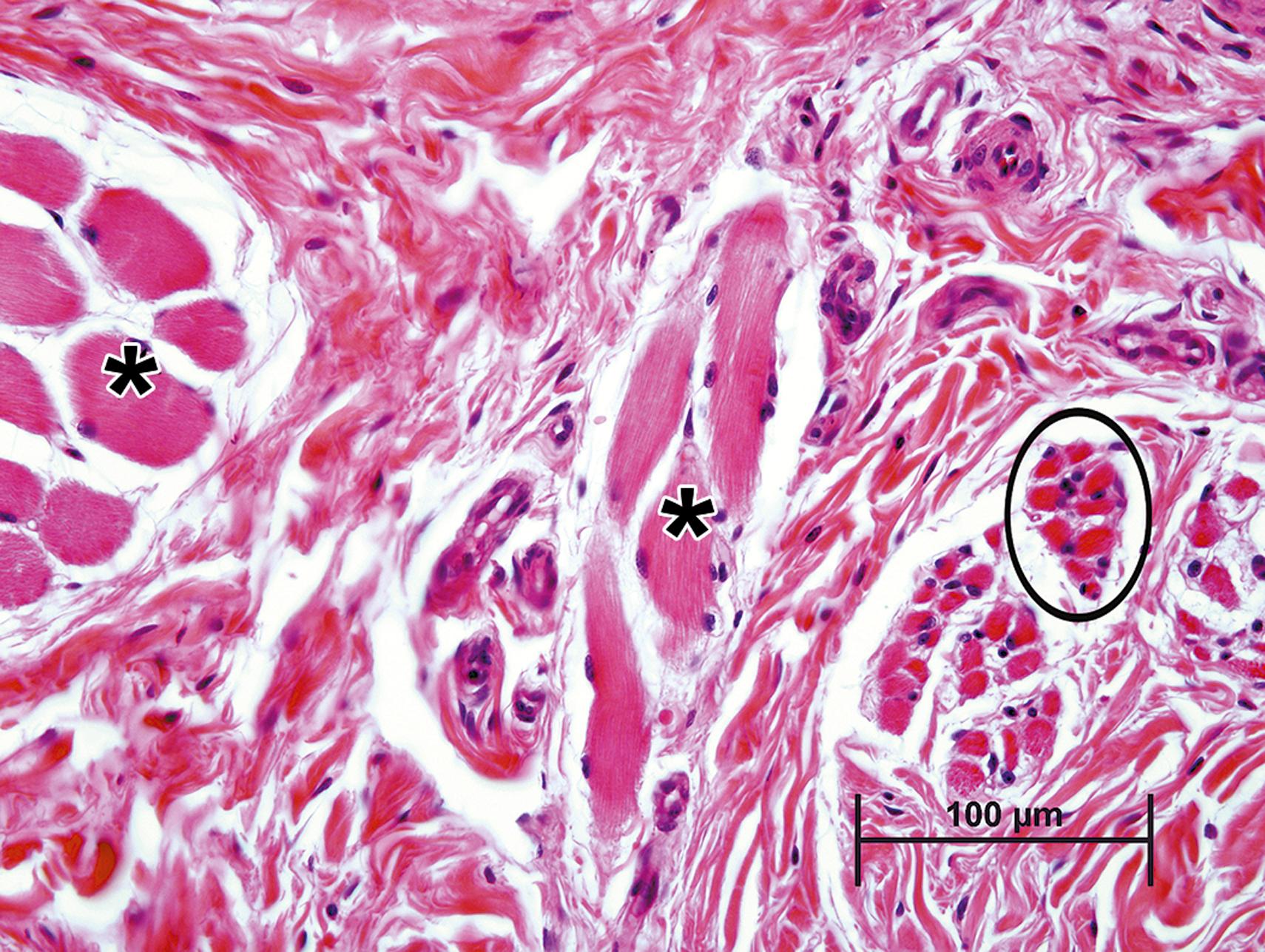
Atrophy can also result from a lack of hormone stimulation. Deficiencies in pituitary trophic hormones, such as thyroid stimulating hormone (TSH) or adrenocorticotropic hormone, lead to atrophy of thyroid follicular and adrenal cortical epithelial cells, respectively, resulting in atrophy of that portion of each gland where unstimulated cells reside. This type of atrophy, in which cells simply disappear from a tissue, is sometimes termed numerical atrophy. This process occurs by a programmed process of cell death termed apoptosis (see below). The severity of atrophic changes is dependent on both the degree and duration of stimulus withdrawal. Profound or complete loss of stimulation for an extended period of time can lead to large scale autophagy and even cellular senescence.
Cell atrophy can also result from an insufficient supply of energy or substrates required to maintain structure and function even with no change in demand for its products or function. For example, pressure from an adjacent tumor can disrupt normal circulation leading to hypoxia or insufficient delivery of glucose for ATP generation or amino acids to maintain structural proteins. Cells that are capable of surviving in such an adverse environment will generally undergo atrophy. Cells in the immediate vicinity of the tumor may be compressed and distorted by physical displacement.
At the subcellular level, an atrophied cell is not only reduced in size but also may lack organellar features typical for a fully functional cell of that particular phenotype ( Fig. 6.2 ).
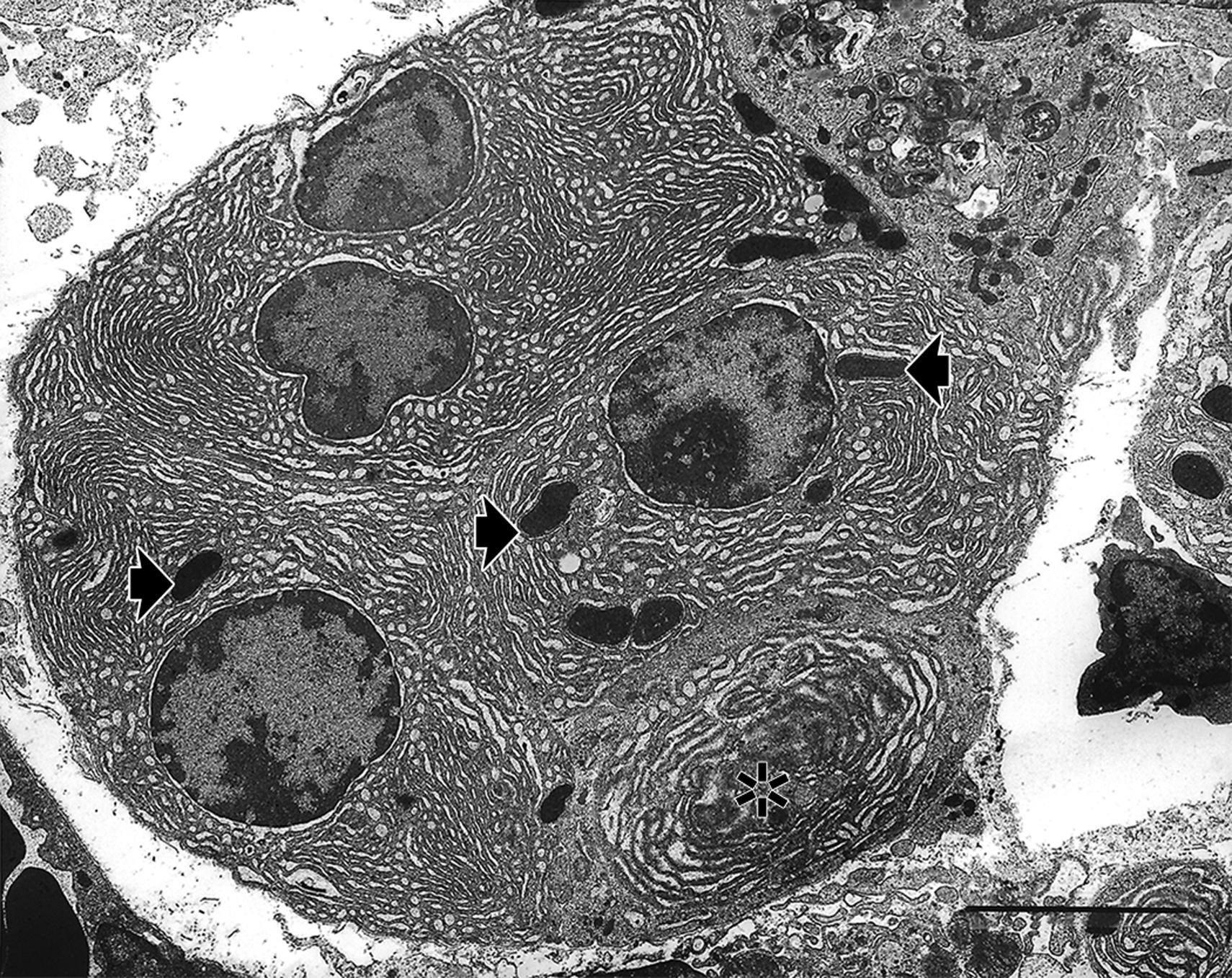
Cellular atrophy is typically accomplished by a process termed autophagy. Autophagy has two major forms that can be observed morphologically—microautophagy and macroautophagy ( ; ). Although these autophagocytic processes may be observable by light microscopy and transmission electron microscopy, the changes observed are dependent upon which adaptive process occurs and at what stage the particular autophagocytic process is in when the cell is assessed morphologically.
The term “microautophagy” represents a cellular process utilized mainly for getting rid of excess soluble protein product either free within the cytosol, sequestered within ER or within secretory granules (e.g., zymogen in pancreatic acinar cells). The process is apparently mediated by direct uptake by the lysosome of the targeted protein using an endocytosis-like process or by fusion of a protein-laden endosome with the lysosome, leading to activation of lysosomal proteases and degradation of the discharged protein ( ). Given the relatively small size of a lysosome, autophagolysosomes may be difficult to visualize histologically, at least in early stages. They have been characterized as being small, indistinct basophilic, sometimes eosinophilic 1–2 micron bodies usually without a well-defined vacuole or halo around them. These bodies may eventually coalesce and condense into dark intensely hyperbasophilic or hypereosinophilic residual bodies (see below).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here