Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Monoclonal antibodies have emerged as mainstays of cancer therapy and have had a significant impact on the morbidity and mortality of several cancers. This chapter will discuss the attributes that make antibodies powerful cancer therapeutics, how some antibodies are used clinically, and the development of new antibodies with clinical promise.
Antibodies are heterodimeric proteins composed of two heavy chains and two light chains connected by disulfide bridges. The two light chains each contain one variable region (V L ) and one constant region (C L ), whereas each heavy chain contains one variable region (V H ) and up to three distinct constant regions (C H 1 to -3) ( Figure 50-1 ). Differential utilization of C H domains can be used to classify antibodies into five main groups, or isotypes: IgD, IgA, IgG, IgM, and IgE. For example, IgM uses heavy-chain constant domains from the C H μ gene, whereas IgG uses the C H γ gene. IgG is the isotype most commonly used in cancer immunotherapy and is the focus of this chapter.
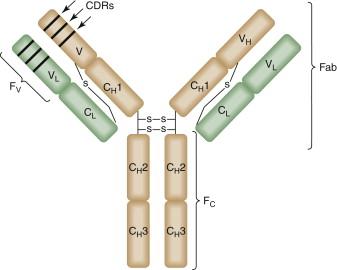
Structurally, antibodies can be divided into two functional modalities: the fragment of antigen binding (Fab) and the fragment of crystallization (Fc).
The Fab is responsible for antigen binding and is composed of the full-length light chain (V L + C L ) and the V H and C H 1 domains of the heavy chain. The V L and V H domains, collectively referred to as the variable fragment (Fv), contain six hypervariable regions called complementary determining regions (CDRs). The antigen binding pocket, also referred to as the paratope , is formed by opposition of three CDRs located on the V L domain (CDR-L1, -L2, -L3) with three CDRs on the V H domain (CDR-H1, -H2, -H3). The paratope of the antibody binds to a small region of approximately five to eight amino acids on the antigen called the epitope . A complex system of genetic recombinations and rearrangements is responsible for generating diversity among CDR sequences, resulting in a human antibody repertoire with between 10 10 and 10 11 distinct paratopes.
The Fc portion of IgG antibodies is composed of C H 2 and C H 3 domains and is required to initiate effector immune responses such as complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC). IgGs engage immune effector cells via Fc-γ receptors (FcγRs), which are expressed on a diverse range of hematopoietic cells. In humans, there are three activating FcγRs: FcγRI (CD64), FcγRIIA (CD32A), and FcγRIIIA (CD16). When engaged, these receptors transduce activating signals via immunoreceptor tyrosine-based activation motifs (ITAMs), resulting in initiation of ADCC or phagocytosis. Similarly, FcγRs can transduce inhibitory signals via immunoreceptor tyrosine-based inhibitory motifs (ITIMs). The primary inhibitory receptor in humans is FcγRIIB (CD32) which is expressed on a wide range of effector cells, with the notable exception of natural killer (NK) cells. In addition, neutrophils express FcγRIIIB, which is a GPI-linked isoform that serves as a decoy receptor and a sink for immune complexes. IgGs can also bind to neonatal Fc receptors (FcRn), which mediates transplacental transfer of maternal antibodies to the fetus. FcRns are also expressed in the vascular endothelium, where they bind IgGs and return them to the circulation, thereby prolonging their serum half-life.
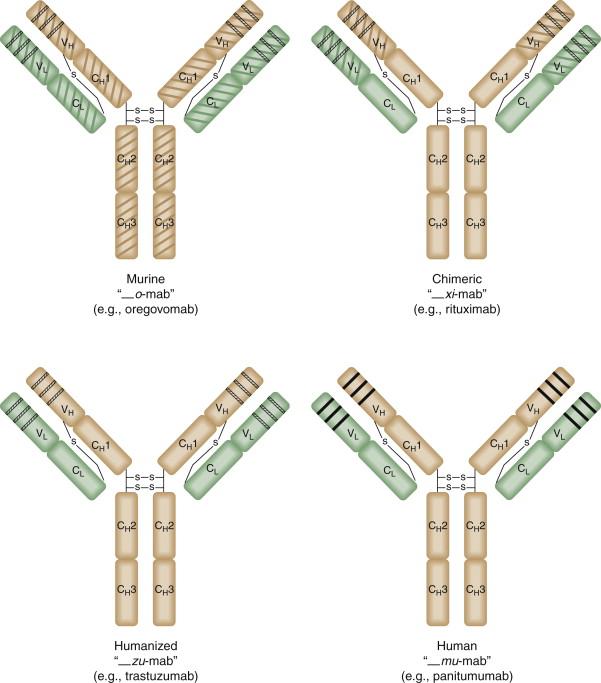
The introduction of hybridoma technology in 1975 by Kohler and Milstein allowed for the mass production of murine monoclonal antibodies. This technology used murine B cells from immunized mice fused together with an immortalized murine plasma cell line. Through a series of selections and limiting dilutions, a clonal population could be isolated that produced antibodies specific for a single epitope. Eventually, antibodies to tumor antigens were developed and tested in clinical trials. The results of early trials demonstrated limited therapeutic benefit, primarily due to the production of human anti-murine antibodies (HAMAs), which resulted in rapid clearance of the drug and occasionally significant immune-mediated adverse events such as rash and renal failure. In addition, the lower affinity of murine Fc to human FcRn may contribute to shorter serum half-life. In the early 1980s, IgG genes were cloned and expressed in mammalian cells, paving the way for the development of chimeric antibodies, which contain murine variable regions and human constant regions. Chimeric antibodies have reduced immunogenicity compared to their murine counterpart, but some patients still develop an immune response to the residual murine component ( Figure 50-2 ). Humanized antibodies, which contain human heavy and light chains and murine CDRs, were developed in the late 1980s in an effort to further reduce immunogenicity.
Currently, fully human antibodies are being produced using two common approaches: screening recombinant antibody libraries and engineering transgenic animals to express human immunoglobulin genes. A library of antibody fragments can be generated from human B cells, or by using cloning techniques, and can be used to construct phage or microbial display libraries. These libraries are subjected to multiple rounds of screening against the antigen of interest, and eventually high-affinity antibody fragments can be isolated. These fragments can be used to generate full-length human antibodies.
More recently, transgenic mice expressing various human antibody gene sequences have been used to develop high-affinity, highly specific, fully human antibodies. Panitumumab, an antibody targeting the epidermal growth factor receptor (EGFR), is an example of an antibody generated in transgenic animals and is currently approved for the treatment of refractory metastatic colon cancer.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here