Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
These studies were supported in part by a grant from the National Cancer Institute R01 CA135354 , by the M.D. Anderson SPORE in Ovarian Cancer NCI P50 CA83639, the Shared Resources of the M.D. Anderson CCSG NCI P30 CA16672, the Ovarian Cancer Research Fund, the National Foundation for Cancer Research, philanthropic support from the Zarrow Foundation and Stuart and Gaye Lynn Zarrow, Golfers Against Cancer, the Kaye Yow Foundation, and the Mossy Family Foundation.
Ovarian cancer is neither a common nor a rare disease. In 2013 in the United States, 22,240 women were diagnosed with ovarian cancer and 14,030 died from this malignancy. The lifetime risk for a woman to develop ovarian cancer is approximately 1 in 70. A fraction of ovarian cancers can arise from germ cells (3%) or from granulosa-theca cells (7%), but approximately 90% of ovarian cancers arise from epithelial cells. Traditionally, epithelial ovarian cancers have been thought to develop from a single layer of flattened cells that cover the ovary or, more frequently, that line cysts immediately beneath the ovarian surface. Neoplasms with similar morphology and behavior can, however, arise from fallopian tube, endometriosis, endosalpingiosis, and the peritoneum. Recent studies have implicated the fimbria of the fallopian tube as the site of origin for as many as 30% of high-grade serous epithelial ovarian cancers, particularly in women with germline mutations of BRCA1 and BRCA2 .
Advancing age, an increased number of menstrual cycles, and a positive family history are associated with an increased risk for ovarian cancer, whereas oral contraceptives reduce risk in later life by as much as 50%. Approximately 85% to 90% of epithelial ovarian cancers are sporadic and arise in the absence of a family history of the disease, often associated with spontaneous somatic mutations of TP53 . Among the 10% to 15% of familial ovarian cancers, germline mutations of BRCA1 and BRCA2 are found in the majority, associated with a family history of breast, prostate, and pancreatic cancers. In carriers of germline BRCA mutations, TP53 is somatically mutated during malignant transformation and the wild-type BRCA allele is lost, resulting in survival during telomeric crisis, genetic instability, and a homozygous deficiency in homologous DNA repair. Ovarian cancers can also occur in Lynch syndrome families with germline abnormalities in DNA mismatch repair genes, associated with colon and uterine cancers. Rare cases of ovarian cancer are encountered in Li-Fraumeni kindreds with germline mutations of TP53, associated with sarcomas and brain tumors. The lifetime risk of developing ovarian cancer depends on the genetic defect: BRCA1 (30% to 60%), BRCA2 (15% to 30%), HNPCC (12%), and TP53 (<1%). Importantly, modifiers of the effects of BRCA1 and BRCA2 that can improve the ability to predict risk are being rapidly identified and characterized. Other lower penetrance susceptibility genes such as Rad51C and Rad51D likely contribute to familial predisposition to ovarian cancers.
Epithelial ovarian cancers exhibit a distinctive pattern of progression and metastasis ( Figure 37-1 ). Initially, ovarian cancer cells proliferate within the walls of cysts, invade underlying stroma, and enlarge the ovary, forming a pelvic mass. Although epithelial ovarian cancers can spread hematogenously or through lymphatics, the most frequent route of metastasis is over the surface of the peritoneum. In the absence of anatomic barriers, ovarian cancers that arise from the surface of the ovary or the lining of the fallopian tube can spread through the peritoneal cavity before a palpable mass is formed. Ovarian cancer frequently spreads throughout the pelvis and to the right hemidiaphragm, the bowel mesentery, and the omentum. Multiple nodules of metastatic cancer can stud the peritoneal surface and form dense fibrous adhesions that bind adjacent loops of intestine, producing mechanical obstruction ( Figure 37-2 ). Ovarian cancer can also invade the retroperitoneum, affecting the myenteric plexus and producing paralytic ileus. Intestinal obstruction from either mechanism produces nausea, vomiting, and malnutrition. Ovarian cancer patients generally die from inanition, often complicated by intercurrent infection. As control of intra-abdominal metastasis improves, other sites including the lung and brain are becoming more prevalent.
Another distinctive feature of ovarian cancer is the formation of ascites fluid that contains leukocytes, mesothelial cells, and a varying fraction of tumor cells. Accumulation of ascites fluid produces abdominal distention, which can be the initial symptom of disease. Fluid generally drains from the peritoneal cavity through diaphragmatic lymphatics (see Figure 37-1 ), which can become occluded by tumor cells, preventing outflow. In addition, tumor angiogenesis produces incompetent vessels that permit greater efflux of proteinaceous fluid from the vascular compartment into the peritoneal cavity. Ovarian cancer cells can produce copious amounts of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) that is both an angiogenic factor and a permeability-enhancing factor. Neutralization of VEGF with monoclonal antibodies can block ascites formation in murine transplant models and in the clinical setting.
Given the location of the ovaries within the pelvic cavity and the difficulty in assessing abnormalities on routine gynecologic examination, the disease is diagnosed only after it has metastasized in approximately 80% of cases. Ovarian cancer is often described as a “silent killer,” but the disease is generally symptomatic, even at early stages in 89% of cases. Symptoms are not, however, specific and are generally attributed to benign gastrointestinal, genitourinary, musculoskeletal, or gynecologic conditions. Detection of a pelvic mass by physical examination or transvaginal sonography generally prompts exploratory surgery to remove the primary tumor and as much of the metastatic disease as possible—so-called cytoreductive surgery. Chemotherapy is generally given for 18 weeks thereafter using a combination of cytotoxic drugs including a taxane (paclitaxel or docetaxel) and a platinum derivative administered intravenously or directly into the peritoneal cavity.
In the 20% of patients with disease that is still localized to the ovaries (stage I), the prognosis is excellent, with up to 90% survival at 5 years using currently available surgery and chemotherapy. As the disease spreads to the other pelvic organs (stage II), to the peritoneal cavity and retroperitoneum (stage III), or to the hepatic parenchyma, pleural cavity, or lymph nodes outside the abdomen (stage IV), the prognosis becomes progressively worse, with a 5-year survival of less than 10% in the last group. Overall, 5-year survival rates have improved significantly ( P < .05) from 37% in the 1970s to 45% in the 2000s, related in large part to improvements in cytoreductive surgery and combination chemotherapy with carboplatin and paclitaxel. Over the past decade, however, median 5-year survival has not improved for patients with newly diagnosed advanced-stage ovarian cancer treated on clinical protocols of the Gynecologic Oncology Group. Moreover, long-term survival for women with advanced disease has not improved dramatically over the past three decades, and 70% of patients eventually succumb to the disease.
As in many other malignancies, epithelial ovarian cancer is a clonal disease that arises from a single cell in more than 90% of cases. Despite a clonal origin, epithelial ovarian cancers exhibit marked heterogeneity at a molecular, cellular, and clinical level.
Among cancers from different patients with invasive cancer , the fraction of cycling cells can vary from 1% to 79% with a mean of 9% to 34% in different series. Cyclin D1, cyclin E, and CDK2 are upregulated in a minority of cancers with their DNA copy numbers or protein levels correlating inversely with survival. Conversely, the p16, p21, and p27 CDK inhibitors are downregulated or mislocalized in a fraction of cancers, associated with a poorer outcome.
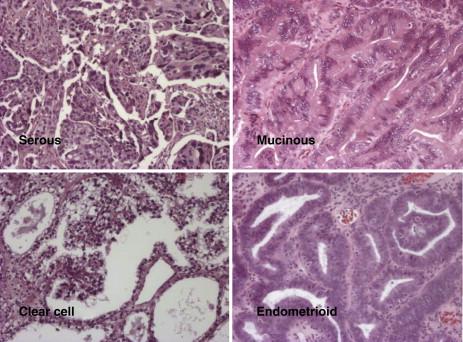
Ovarian cancers exhibit distinct histotypes—serous, endometrioid, clear cell, mucinous—that resemble epithelial components of normal fallopian tube, endometrium, vagina, endocervix, or intestine ( Figure 37-3 ). Histotypes differ with regard to risk factors, genetic abnormalities, expression of tumor markers, and response to chemotherapy. Each histotype exhibits a distinctive pattern of gene expression judged by array analysis, real-time reverse transcriptase–polymerase chain reaction (RT-PCR), and immunohistochemistry. Molecular alterations in ovarian cancers of different histotypes correlate with changes in the normal tissues that they resemble morphologically. The HOX family of homeobox genes plays an important role in determining the histotype of ovarian cancers. During normal development, HOXA9 is solely expressed in the primordia of the fallopian tubes, HOXA10 in the developing uterus, HOXA11 in the lower uterine segment and cervix, and HOXA13 in the future upper vagina. Expression of these HOX genes is retained in adult tissues but is not observed in ovarian surface epithelia. Expression of HOXA9, HOXA10, and HOXA11 is recapitulated in serous, endometrioid, and mucinous epithelial ovarian cancers, and enforced alterations in expression alter cellular histotype, indicating a causal role.
Low- and high-grade ovarian cancers differ not only in histologic differentiation, but also in pathogenesis, genotype, rate of growth, prognosis, and response to therapy, permitting separation into Type I (low-grade) and Type II (high-grade) lesions. Type I cancers include low-grade serous, mucinous, endometrioid, and clear cell histotypes and are often diagnosed in early stage (I or II), grow slowly, and resist conventional chemotherapy. Low-grade tumors frequently express estrogen receptors and may respond to tamoxifen or aromatase inhibitors. The more prevalent Type II cancers include high-grade serous, endometrioid, or undifferentiated histotypes, present at late stage (III or IV), grow aggressively, and respond to conventional chemotherapy, but only occasionally to endocrine therapy. Thus, the distinction between Type I and Type II ovarian cancers can inform choice of treatment.
Whereas Type II serous cancers appear to arise de novo from the walls of ovarian cysts or the surfaces of the ovary or fallopian tube, Type I low-grade serous cancers can grow from noninvasive serous “borderline” tumors of low malignant potential in 60% of cases. High-grade Type II ovarian cancers respond to primary chemotherapy with carboplatin and paclitaxel in approximately 70% of cases. Low-grade serous tumors are resistant, but not refractory, to primary platinum-based therapy. Recurrent low-grade serous ovarian cancer has a very low rate of response. Low-grade mucinous and clear cell histotypes respond to conventional chemotherapy in only 26% and 15% of cases, respectively.
Low-grade serous cancers exhibit a relatively normal karyotype with wild-type TP53 and BRCA1/2 , but exhibit frequent mutations in KRAS genes in 19% to 54% of cases. Low-grade serous cancers express the insulin-like growth factor receptor, and the majority overexpress the IGF-1 growth factor, providing a potential target for therapy. Frequent mutations of KRAS are found in mucinous cancers and in adjacent borderline tumors, consistent with the mutated gene driving malignant progression. A similar pattern of gene expression has been observed in clear cell and low-grade endometrioid carcinomas, consistent with a common cell of origin. Similar mutations have been found in both histotypes. Inactivating mutations of ARID1A , a chromatin remodeling gene, have been reported in 49% of ovarian clear cell carcinomas and 30% of endometrioid ovarian cancers. Mutations of PPP2R1A , the regulatory subunit of a serine-threonine phosphatase required for chromosome segregation, have been found in 7% of clear cell ovarian cancers. Phosphatidylinositol-3-kinase (PI3K) signaling is activated in low-grade endometrioid cancers through inactivating mutations and epigenetic silencing of PTEN and activating mutations of PIK3CA . In nonepithelial granulosa cell tumors, recurrent single base mutations (402C-G) of FOXL2 , a transcription factor implicated in granulosa cell differentiation, have been found in 97% of cases.
Thus, low-grade Type I cancers appear to be driven by mutations that activate Ras/MAP and PI3K signaling in the context of a relatively normal karyotype with wild-type TP53 and BRCA1/2 . By contrast, Type II high-grade serous cancers exhibit numerous copy number abnormalities with frequent amplifications and deletions, but with mutations in a very limited number of genes including TP53 and BRCA1/2 . The Cancer Genome Atlas Research Network (TCGA) analyzed copy number abnormalities in 489 high-grade serous ovarian cancers, detecting amplification of more than 30 growth stimulatory genes. Amplification and overexpression of genes in the PI3K family occur in more than 50% of Type II cancers, activating the PI3K pathway and conferring “PI3Kness.” In the absence of germline abnormalities of BRCA1/2 , homologous DNA repair can be compromised by somatic mutations of BRCA1 and BRCA2 within the cancer alone, BRCA2 can be silenced, and upstream mutations can downregulate BRCA function combined with mutations in other genes potentially involved in homologous recombination, producing “BRCAness” in up to 50% of patients with Type II cancers. DNA sequencing of exons from 316 cancers detected mutations of TP53 in 96%, most of which appeared to be inactivating. Of the 26,000 genes, only a few were mutated in 2% to 4% of cases, including NF1 , Rb1, BRCA1, BRCA2 , and CDK12 . Unlike Type I cancers, fewer than 1% of Type II cancers had mutations of BRAF , PI3KCA , KRAS , or NRAS . Despite the low prevalence of Rb1 mutations, dysfunction of the Rb pathway was found in 67% of high-grade serous cancers. As in the case of epithelial cancers at other sites, both TP53 and Rb were inactivated in two thirds of Type II ovarian cancers. As indicated earlier, low-grade tumors have a high frequency of mutations in PIK3CA, KRAS, ARID1A , and other putative oncogenes and tumor suppressor genes, whereas high-grade ovarian cancers are characterized by mutations in TP53 and BRCA1/2 and marked alterations in DNA copy number. These distinct molecular characteristics indicate that interconversion from Type I to Type II cancers is either a very rare event or does not occur, and thus Type I and Type II tumors likely represent independent diseases. Embracing this concept and performing independent clinical trials and tailored therapy for Type I and Type II tumors will be necessary to improve patient outcomes.
The pattern of gene expression has been used to identify prognostic subgroups. The Australian Ovarian Cancer Study Group profiled 285 serous and endometrioid tumors to identify six molecular subtypes. Two subtypes were associated with low malignant potential serous tumors and low-grade endometrioid ovarian cancers, whereas the other four transcriptional profiling based subtypes included high-grade serous and endometrioid histotypes including a “mesenchymal” subtype. The immunoreactive subtype expressed T-cell chemokine ligands CXCL11 and CXCL10 and the receptor CXCR3, consistent with a higher level of infiltration by leukocytes. Importantly, lymphocytic tumor infiltration has been associated with an improved outcome. Cases in the proliferative cluster exhibited high expression of the HMGA2 and SOX11 transcription factors, low expression of MUC1 and MUC16 mucins, and high expression of proliferation markers such as MCM2 and PCNA. Differentiated cases have high expression of MUC16, MUC1, and the secretory fallopian tube marker SLPI. Mesenchymal cancers were associated with high expression of HOX genes and markers for stromal components including myofibroblasts (FAP) and microvascular pericytes (ANGPTL2 and ANGPTL1). These subtypes were not associated with changes in overall survival, although a prognostic signature was developed that included 193 genes. Subsequent analyses developed a prognostic “Classification of Ovarian Cancer” (CLOVAR) using gene expression that distinguished groups with markedly different median survival (23 vs. 46 months) and resistance to platinum therapy (63% vs. 23%). Although these classifications can help to stratify future trials, profiles with higher positive and negative predictive value for response to conventional and novel agents will be required in order to affect clinical management.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here