Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Molecular investigation of the hematopoietic neoplasms has contributed significantly to our understanding of the “genetic basis” of cancer, and progress in this dynamic field continues unabated. Accordingly, new knowledge concerning recurrent genetic abnormalities in myeloid and lymphoid cancers continues to be integrated within the most recent World Health Organization (WHO) classification of the hematopoietic and lymphoid tumors. This chapter summarizes key technical considerations, essential molecular alterations observed in specific tumor associations, and the application of molecular diagnostic approaches to the classification and monitoring of lymphoid and hematologic neoplasms.
Clinical application of laboratory methods to detect chromosomal or genetic aberrations in the hematolymphoid disorders requires a clear understanding of assay sensitivity and specificity, to determine the relative use and limitations of various seemingly complementary techniques in routine practice. Most commonly, reference is made to the concept of analytic sensitivity. This is a very important measurement, defined as the ability of a given assay to detect the lowest amount or concentration of a substance when it is present in a test sample. For molecular (DNA or RNA) analytes, typical analytic sensitivity can attain very low limits of detection (e.g., 10 −3 to 10 −6 ), in contrast to microscopic-based platforms such as fluorescence in situ hybridization (FISH) and conventional karyotyping ( Table 24.1 ). Analytic test sensitivity is further determined in the context of the lowest reproducible level of the intended target, which constitutes the limit of detection (LOD) of the assay. For quantitative assays, the LOD is established along with stringent precision measurements (e.g., mean value with standard deviation and coefficient of variation, 95% confidence intervals); numeric values obtained below this threshold are considered potentially inaccurate despite being detectable owing to stochastic reaction effects. An analytic measurement range (AMR) is also applied to quantitative tests to detail the linearity of the system across the clinically useful biologic range of target concentration or copy number.
| Method | Analytic Sensitivity (%) | Notes |
|---|---|---|
| Cytogenetics | 5–10 |
|
| Fluorescence in situ hybridization | 1–5 |
|
| Chromosome arrays | 20–30 |
|
| Southern blot hybridization | 5–10 |
|
| Polymerase chain reaction (PCR) | 1 × 10 −4 to 10 −6 |
|
| Digital PCR (D-PCR) | 1 × 10 −3 to 10 −6 |
|
| Sanger sequencing | ~20 |
|
| Next-generation sequencing (NGS) | ~5–10 |
|
Good analytic sensitivity is often associated with diagnostic (or clinical) sensitivity, the latter being the ability of an assay to detect a particular clinical condition (signified by the presence of the target analyte) when it is present in a general population of subjects. However, analytic and diagnostic sensitivity can diverge if, for example, the analyte in question is not uniformly represented (e.g., due to cell/tissue distribution effects, temporal or ethnic variations) or if the underlying genetic alteration exhibits significant heterogeneity (e.g., multiple possible mRNA transcripts arising from a single gene rearrangement event, such as the BCR-ABL1 abnormality). This can lead to the situation wherein a clinical test may have exquisite detection sensitivity yet may miss some patients with the condition of interest (i.e., clinical false negativity). Analytic specificity concerns the ability of an assay to identify only the target analyte, even in heterogeneous mixtures with other molecules having potentially overlapping features; evaluating and eliminating potential analytic interferences is also an important component of test validation. For most molecular assays, high analytic specificity can be readily achieved by relying on the highly complementary nature of DNA fragment binding, as well as additional technical maneuvers to enhance high-affinity nucleic acid interactions or amplify positive signals. Clinical or diagnostic specificity refers to the ability of a test to accurately exclude individuals who do not have the disease or condition in question. Once again, these two parameters are linked but can show a significant lack of concordance if, for instance, a truly negative specimen is affected by external contamination with the nucleic acid of interest or if related molecules in the sample are not reliably discriminated by the assay (i.e., analytic false positivity). Amplification-based assays are especially prone to false-positive results, which are an unfortunate potential by-product of high analytic sensitivity; therefore, scrupulous control of contamination is essential in any molecular diagnostic laboratory performing polymerase chain reaction (PCR)-based analyses. Related to the issue of clinical specificity, an understanding of pathologic context is required because a given molecular abnormality may occur in different disease entities. Finally the clinical validity of a biomarker or analyte is an important factor in test development. Validity is determined by several measures, including but not limited to (1) published consensus guidelines from professional bodies; (2) well-established use of an analyte to support a specific disease diagnosis or to monitor a specific therapy; and (3) substantial clinical trial or other peer-reviewed data indicating the required or suggested utility of a marker for diagnostic, prognostic, or predictive purposes. Clinical validity is a major consideration related to obtaining reimbursement for often costly molecular diagnostic tests. All of these facets combine to produce a strong test that must then fit into a framework of clinical practice utility—how and when to use a certain molecular diagnostic assay and, as important, when not to do so. Therefore, when designing molecular genetic diagnostic tests with high analytic and potential clinical utility, the hematopathologist must understand these concepts thoroughly and include sufficient data in validation studies to mitigate potential concerns regarding accuracy, sensitivity, and specificity.
To best understand diagnostic assay design and methodologic advantages and limitations, a basic understanding of the technical aspects of molecular diagnostic methods is required. Although a complete primer on molecular diagnostic techniques is beyond the scope of this chapter, a description of important techniques such as quantitative PCR analysis, digital PCR, next-generation sequencing (NGS), DNA hybridization technologies, microarray, and immune-receptor clonality assays is available as an on-line supplement. Table 24.1 summarizes important sensitivity characteristics of standard methodologies used in hematopathology. The reader is encouraged to review the online supplemental material that provides some technical details on molecular diagnostic methods before proceeding.
The ubiquitous technique of PCR is employed as a stand-alone analytic procedure or as one component of more complex methodologic assays in the majority of molecular diagnostic laboratory operations. PCR using oligonucleotide primers and thermostable DNA polymerases can be performed using genomic DNA or messenger RNA transcripts as templates, in the latter case also requiring a reverse transcription (RT) step to generate complementary DNA (cDNA) prior to amplification (i.e., RT-PCR). A myriad of PCR variations have been developed (e.g., allele-specific, “asymmetric,” use of modified oligonucleotides) to enhance analytic specificity according to particular assay requirements. Detection of PCR-amplified products can be achieved by a number of methods, including agarose or polyacrylamide gel electrophoresis and by capillary electrophoresis; in the latter approach, PCR primers are typically covalently labeled with a fluorescent dye moiety to permit highly accurate fragment length determination following laser scanning of the capillary gel matrix. Routine PCR methods are of great utility in hematopathology, including the determination of B-cell or T-cell monoclonality and the detection of chromosomal translocations characteristic of many leukemias and lymphomas. Translocation events can either produce a novel chimeric gene, which is subsequently transcribed as a unique fusion mRNA species (this is typical of the acute leukemias), or the translocation can result in aberrant overexpression of a highly regulated proto-oncogene owing to its juxtaposition with an unrelated region of DNA containing a strong transcriptional promoter or enhancer (this is most often seen in the non-Hodgkin lymphomas). Other types of genetic rearrangements (e.g., small insertion/deletion events, point mutation changes in genes) can be detected by standard PCR methods, along with additional post-PCR procedures such as fluorescent PCR product sizing, restriction enzyme digestion, oligonucleotide probe hybridization, high-resolution melting curve analysis using DNA intercalating dyes, or automated DNA sequencing. Finally, non-PCR or “isothermal” amplification techniques have been employed in the molecular diagnostic setting, including ligation-mediated amplification, and nucleic acid sequence–based amplification (NASBA), but these alternatives occupy a relatively small niche in relation to the abundance of PCR-based methods.
A variety of tissue or sample sources can be used for the molecular genetic investigation of leukemia and lymphoma, including fresh or frozen tissues and cells, as well as formalin fixed, paraffin-embedded (FFPE) tissue material. Blood and bone marrow specimens should be obtained in anticoagulant (e.g., EDTA, acid citrate dextrose [ACD], or heparin) to ensure that the leukocytic component is adequately available for DNA or RNA extraction. Paraffin-embedded tissue blocks are a common source of material for DNA PCR assays, although fixed tissues are prone to nucleic acid degradation and cross-linking, often reducing the quality of the DNA template. Although technically more difficult, RNA can also be obtained from FFPE tissue; however, the nucleic acid quality is significantly less optimal compared with RNA from fresh or viably cryopreserved cells. Analyzable DNA can also be reliably obtained from air-dried glass slide smears or touch preparations of blood, marrow, or tissue; in this case, unstained slides are preferred, as commonly employed histologic and hematologic dyes may adversely inhibit successful PCR. Regardless of tissue source, the molecular diagnostic laboratory must have adequate procedures to ensure the quality and integrity of each individual sample. Such measures include assessing the amount, purity, and adequacy of amplification of the obtained nucleic acids. RNA samples are particularly labile and subject to rapid degradation by RNases. Given the sensitive nature of PCR technique, isolation of nucleic acid preparations must be done very carefully to avoid sample cross-contamination and rigorous separation of “pre-PCR” (nucleic acid isolation) from “post-PCR” (analysis) areas of the laboratory to prevent pre-analytic sample contamination arising from “floater” or carry-over PCR-amplified products. Standard amplification controls are required in all PCR assays and should include (1) relevant positive and negative nucleic acid controls to ensure specificity; (2) an internal control (i.e., a “housekeeping” gene) of sufficient fragment size to ensure integrity of the RNA or DNA within the expected amplicon size range; and (3) a “no template” control to assess for possible contamination. Assay sensitivity controls (e.g., standard curve or related calibrator reagents for quantitative assays and low-level positive controls near the limit of detection for qualitative assays) are also included to monitor per-run accuracy over the stated analytic range.
Specialized “real-time quantitative” PCR (RQ-PCR) platforms have become invaluable in the molecular diagnostic evaluation of several hematolymphoid neoplasms. RQ-PCR is ideally performed in an automated, multi-sample, closed system format in which the initial amount of a specific nucleic acid target can be accurately quantified with exceptional precision. Commercially available RQ-PCR instruments are based on two major technologies: the 5′ fluorescent nuclease assay, also known as “Taqman” (Applied Biosystems Inc., Foster City, CA) and the dual fluorescent probe hybridization assay based on the concept of fluorescence resonance energy transfer, or FRET (Roche LightCycler, Roche Molecular Diagnostics, Indianapolis, IN). The ability of each platform to deliver highly reproducible quantitative PCR results is very similar, and Taqman chemistry is commonly employed in many molecular diagnostic laboratories. Although standard “end-point” PCR methods can be designed to identify the presence of very small amounts of a target nucleic acid, the plateau phase of PCR product detection (e.g., by gel electrophoresis) often does not reliably reflect the starting concentration of the species of interest, mainly because of late cycle amplification effects on PCR efficiency. In contrast, measurement of the initial quantity of a specific mRNA or DNA species by RQ-PCR is possible by relying on the early detection of PCR product during the exponential phase of PCR. The initial appearance of a fluorescent signal above background at the so-called threshold cycle (Ct) or crossing point (Cp) during this phase of PCR is directly and quantitatively related to the starting amount of the target DNA or RNA in the sample of interest. A standard curve prepared by plotting observed Ct numbers against serial 10-fold dilutions of a given target molecule should produce a linear relationship in which each 3.3-cycle interval represents a change of 1 log of concentration (assuming 100% PCR efficiency). In an ideal PCR assay, the concentration of amplified DNA template doubles with each cycle. In practice, however, PCR efficiency is affected by a variety of issues, such as the composition of the specific target region (GC nucleotide content, or homopolymer repeat region), placement of primers and probes (avoiding known polymorphic nucleotides or pseudogene regions), optimized reagent concentrations and type of polymerase used, among others. Achieving a reliable and precise RQ-PCR assay often requires substantial expertise and some trial-and-error in order to approach the theoretical ideal assay output over the biologically relevant range of detection.
One method of quantifying a nucleic acid of interest in an unknown sample using RQ-PCR is thus to directly read the concentration from a standard curve generated from co-amplified serial dilutions of a positive control template, in essence deriving an “absolute” measurement of the analyte. The standard curve method requires that a quantitative unit value for a particular target be normalized to that of an amplified, unrelated “housekeeping” gene or mRNA transcript, in order to compensate for sample, technical, and PCR condition variability. Alternatively, one can choose to measure the relative quantity of a specific PCR product by comparison to a reference reagent standard or “calibrator,” again after normalizing both the unknown sample and calibrator amplification Ct result to a housekeeping gene or transcript value. This comparative approach also assumes that the sample gene target and calibrator amplify with a PCR efficiency equivalent to the normalizing control gene region, in which case relative changes in the normalized quantities between the unknown sample and calibrator can be reproducibly measured. The comparative method is also known as the 2 -ΔΔCt method, reflecting its mathematical derivation; if carefully validated, the requirement for a formal standard curve with each experiment can be avoided, and the quantitative results are expressed as a-fold or percentage change relative to the calibrator, rather than an absolute numeric measurement. Notably, for RNA analytes, a reverse transcription (RT) step is performed to generate cDNA templates prior to the actual RQ-PCR; the RT reaction is generally assumed to be close to 100% efficient, such that the subsequent RQ-PCR accurately reflects the mRNA target abundance in the sample. Poor RT efficiency can thus adversely affect the reliability of RQ-PCR data. Additional considerations for successful and robust RQ-PCR assays include the appropriate choice of standards (e.g., serial dilutions of positive control cell line DNA, RNA, or cloned template of a particular genetic target), adequate controls (positive, low positive or dilute control, negative, and no template samples), and the careful selection of the normalizing or “housekeeping” gene or transcript. Normalization controls for RNA-based RQ-PCR are typically selected to possess relatively stable gene expression levels in both tumor cells and background cells, to show equivalent PCR efficiency, to have degradation characteristics similar to the target mRNA over time, and to show minimal tendency for significant deviation among clinical subjects. The RQ-PCR assay must also exhibit excellent linearity throughout the testable analytical measurement range (i.e., AMR). The proper evaluation of positive control reagents is also a critical consideration; some cell lines, for example, may have amplification of the target gene or a highly active transcription of an expressed fusion gene relative to clinical samples from individual patients. The choice of positive controls should ideally reflect the biologic range of target values expected in the patient population with the disease when accurate quantitative measurements are required; one approach to assess positive controls is by using the digital PCR (DPCR) technique, as detailed briefly in the ensuing section, to calculate gene or transcript copy number. Finally, the choice of cell line control and housekeeping transcript affects the relative value of normalized target quantity in reverse transcription RQ-PCR assays that are not reported according to a standard consensus (e.g., the International Scale for BCR-ABL1 p210 monitoring); therefore, direct comparisons of measurements from the same patient analyzed in different laboratories may demonstrate some quantitative variability, even though the relative range of target detection (e.g., degree of log-level change) is similar.
If adequately constructed, RQ-PCR analyses can demonstrate remarkable reproducibility and achieve a high level of analytical sensitivity (i.e., 5–6 log dynamic range). In addition to obvious applications in post-therapy MRD detection, RQ-PCR can also be used to identify and quantify single base changes in nucleic acids for “allelotyping” assays. The sensitive measurement of MRD using RQ-PCR is now an integral component in the management of many hematologic cancers. The rationale of MRD evaluation is to provide more definitive prognostic or predictive information for individual patients in order to monitor treatment efficacy and determine the need for additional intervention. Beyond the concerns inherent in developing high-quality RQ-PCR assays, a variety of other issues are germane to MRD determination, such as the sample source (i.e., blood versus bone marrow), the frequency of monitoring required, and the biologic characteristics of the hematolymphoid tumor being evaluated. In general, serial MRD assessments at key time intervals post treatment are most likely to provide the best predictive data on tumor biologic behavior in a given patient, rather than single measurements at only one or two time points. Although elimination of molecular residual disease (i.e., PCR negativity) is a desirable major goal of treatment (e.g., acute promyelocytic leukemia with PML-RARA ), it is also well known that some long-term leukemia survivors (e.g., patients with BCR-ABL1 chronic myeloid leukemia or RUNX1-RUNX1T1 acute myeloid leukemia) still harbor detectable molecular MRD, albeit at low levels. Since it is not possible to definitively predict which patients have low-level MRD consisting of “dormant” residual tumor cells versus those with minor tumor populations capable of re-constituting overt disease relapse, any low-abundance PCR-positive result must be considered as potentially significant; the ability of RQ-PCR technology to reproducibly assess temporal disease level fluctuations in individual patients is therefore of obvious benefit in this situation.
The detection of “rare events” by highly sensitive RQ-PCR methods can also lead to interpretive difficulties. At the analytic limits of assay detection, one may encounter stochastic effects, such that a sample may test positive in one reaction aliquot but negative in a duplicate one or in a later sample from the same patient, despite the same approximate minimal level of disease being initially present in each case. It is important that laboratories performing molecular MRD detection establish a rigorous definition of the reproducible limit of target detection (LOD) for each quantitative assay. Outlying or spurious low-level results can then be better interpreted in light of these precision standards. Of note, several leukemia-associated fusion gene transcripts (such as the BCR-ABL1 abnormality) have been described at very low levels but with relatively high prevalence in the blood of healthy individuals. These data are consistent with the hypothesis that an abnormal gene fusion is necessary but likely insufficient on its own for the production of overt leukemia. Although these intriguing findings would appear to potentially complicate the interpretation of RQ-PCR MRD analyses in leukemia patients, the detection of such low-abundance fusion transcripts in normal subjects has required the use of modified PCR methods with extended sensitivities typically one or two orders of magnitude greater than the maximal limits obtained by current clinical RQ-PCR assays (i.e., 10 −5 to 10 −6 ). Thus, the chance of identifying a confounding positive PCR result arising from possible rare “bystander” cells should be negligible in a well-designed RQ-PCR assay for clinical use.
Digital PCR (D-PCR) technique has become a recent and powerful addition to the stable of quantitative PCR-based applications and exhibits some important differences compared to RQ-PCR methods. For example, D-PCR quantification relies on end-point product detection in contrast to early exponential phase detection for RQ-PCR. Unlike qualitative end-point PCR assays, however, D-PCR employs a very large partition system of many thousands or millions of small individual “micro-reactors,” each containing nanoliter (or smaller) quantities of a DNA template copy and associated PCR reagents. In theory, adequate partitioning of a sample volume containing the target nucleic acid should create many individual reactions containing either one or no DNA copies in a single compartment. A common mode of sample preparation involves generation of a micro-droplet emulsion suspension from a standard sample volume containing nucleic acid template and PCR reagents. Each tiny reaction is then co-amplified to a cycle maximum, and the individual compartments are analyzed by fluorescence signal detection to identify the presence or absence of PCR product. In this sense, D-PCR is a form of automated, quantitative limiting dilution PCR technique. Accordingly, simple digital counting and scoring of each reaction is a direct reflection of the starting quantity of molecules-of-interest, and if the sum of all compartment volumes is known (i.e., essentially the total input volume dispersed in the digital array), then the starting concentration of the target (copies/sample volume) can be directly computed. Accuracy and precision of D-PCR depend on the target concentration range and at very high levels, non-random errors are introduced to the system (e.g., multiple template copies per partition, or saturation), causing inaccuracies in digital counting and quantitation. At lower target levels, in which fewer PCR-positive events are distributed among a sufficiently large population of negative reaction partitions, the system becomes both highly accurate and sensitive. Furthermore, the probability of a compartment containing some or no copies of a target, along with the degree of measurement uncertainty, can be accurately estimated using the Poisson distribution. The accuracy and sensitivity of D-PCR is optimized when the platform partition space or total number of compartments is very large. D-PCR is gaining popularity because of its advantages in direct determination of target DNA or RNA quantity over useful biologic ranges without the complexities of traditional RQ-PCR methods (e.g., a calibrator reagent for deriving quantitative results is not strictly required). Various commercial platforms employ different strategies to generate micro-reactor compartments (e.g., Raindance Technologies, Billerica, MA, and BioRad, Hercules, CA) and to perform digital counting calculations. The current challenges for D-PCR include the relative inability to process multiple samples at a time, a lack of higher order target multiplexing, and variable lower limit of detection (analytical sensitivity) among different platforms.
Following completion of the first draft sequence of the Human Genome Project in 2000, an intense period of technology advances resulted in the emergence of several “next-generation” NGS platforms. An ideal tool for the genomic characterization of cancer is one that could simultaneously provide information about copy number variations, allelic information, somatic rearrangements, insertion/deletion (indel) events, and nucleotide level mutations in a single experiment. In this regard, the advent of NGS is moving analytic capabilities closer to this goal. Traditional DNA sequencing is performed by Sanger method or alternative approaches like pyrosequencing; however, these are limited in scalability and/or sensitivity. In contrast to Sanger sequencing (which the Human Genome Project was based upon), NGS has a number of distinct advantages (see Table 24.1 ). NGS methodology essentially is composed of four key process elements: (1) choice of sample type (e.g., DNA, RNA, fresh/frozen, or FFPE sources); (2) novel sample preparation techniques to produce sequence-ready libraries; (3) proprietary high-throughout, massively parallel sequencing platforms to detect DNA sequence alterations; and (4) sophisticated bioinformatics analysis pipelines to assess quality parameters, quantitate and classify numerous varied sequence alterations, and graphically display data against a reference genome for localization and visual inspection. A very critical additional element is the careful human assessment of detected variants to determine clinical and biologic significance in the context of disease diagnosis, prognosis, and treatment (i.e., “curation” of the data). NGS technology is truly revolutionary and has quickly found applications in DNA-based sequencing (targeted gene panels to whole exome or genome), RNA sequencing (transcriptomics and expressed mRNA fusions from translocations), mate pair sequencing (detection of larger chromosome or genomic rearrangements), and determination of copy number alterations. Specialized applications including epigenomics (methylation profiling) and structure-function mapping (e.g., CHiP-Seq) have also been developed. NGS has been applied to the detection of antigen receptor gene rearrangements, providing powerful tools for detecting clonal lymphocyte populations and greatly facilitating MRD monitoring in lymphoid neoplasms, as well as deepening the basic understanding of adaptive and cellular immune responses. The depth and breadth of NGS also provides an unprecedented opportunity for unraveling more complex processes, such as genetic mosaicism or tumor genetic heterogeneity and the pathways of neoplastic molecular evolution.
Despite the variety of nucleic acid sources that can be used for NGS analysis, careful considerations are needed for each. High-quality DNA can be readily extracted from fresh (or cryopreserved) tissues, but FFPE samples are prone to degradation and DNA breaks from cross-linking. Nevertheless, FFPE tissues are very commonly used for NGS as long as adequate sample quantity and quality standards are met. RNA must be rapidly extracted and either stored or converted to cDNA to preserve the relative quantities of different transcript species and avoid degradation effects due to RNases. Library preparation methods are numerous, but commonly employed approaches include highly multiplexed PCR amplification of desired target regions, or oligonucleotide probe (“bait”) capture of the regions of interest in a sheared DNA specimen. Each has advantages and disadvantages, such as higher potential sensitivity with PCR-based methods versus more uniform target coverage using capture probe systems. In any library preparation, many steps are involved to incorporate flanking universal “adapter” sequences, sequencing primer sites and unique indexes (or DNA bar codes) to enable downstream sequencing, and analysis of the internal segments of DNA (inserts). The addition of molecular bar codes or indexes enables the simultaneous multiplexing of numerous patient samples, in turn achieving maximum utility of the sequencing capacity of the instrument and providing higher sample throughput with lower cost. Modern NGS platforms use different, innovative approaches to generate large amounts of high-quality data, producing gigabytes to terabytes of data from hundreds of millions of individual sequence reads. These technologies include semiconductor, solid phase support, and “long read” (e.g., nanopore and single molecule) methods. Importantly, NGS instrumentation for most clinical applications requires relatively small fragments of DNA derived from patient samples, referred to as “short read” sequencing; these numerous short sequence reads are subsequently bioinformatically mapped and assembled to produce a consensus final sequence for each target region. Because many hundreds to thousands of individual sequence reads are generated per target region (the depth of coverage), NGS assays routinely reach an analytic sensitivity of approximately 1% to 5%, and even higher sensitivity can be gained with novel chemistry adaptations.
The two most commonly employed commercial platforms include the Ion Torrent (Thermo Fisher, Waltham, MA) and HiSeq or MiSeq (Illumina, San Diego, CA) systems ( Fig. e24.1 ). Ion Torrent sequencing relies on PCR amplification of a prepared library of target DNA fragments attached to millions of microbeads in a droplet emulsion to generate clonal single-stranded DNA sequencing library templates, which are then distributed onto an array of microwells on a semiconductor sequencing chip. Multiple rounds of unlabeled nucleotide additions sequentially elongate the immobilized DNA fragments. Each time a new base is incorporated in an elongating sequence strand, a pyrophosphate molecule and hydrogen ion (H + ) are released, and these tiny changes in pH generate a voltage fluctuation that can be measured across the many thousands of individual microwells. By knowing the order of bases introduced with each cycle, the corresponding nucleotide sequence is deduced by determining which wells “experienced” a pH change. Illumina sequencers use a solid support system in which channels (“lanes”) are coated with adapter molecules that can bind complementary sequences attached to the prepared library fragments. After attachment of sheared, adapter-ligated DNA fragments to the surface of the lanes of the sequencing chip, a limited cycle PCR step is performed to accomplish “bridge amplification” of each spot on the lane, resulting in the formation of millions of individual clonal colonies of fragments. Sequencing is then initiated from consensus sequence primer sites using multiple rounds of fluorescently labeled reversible dye terminator nucleotide addition, digital imaging of the chip, and dye cleavage. If only a single adapter is added during the library step, then sequencing output is termed single-ended . However, Illumina methodology allows for the addition and selection of two adapters for each pair of double-stranded DNA molecules within the library. This permits a second step during sequencing (turn-around phase) in which the initial single-ended sequence product can be re-bridged to the second complementary adapter on the chip surface and another round of sequencing obtained in the opposite direction. So-called paired-end reads are generated, which improves the ability of analytic software algorithms to achieve higher alignment accuracy to the reference genome.
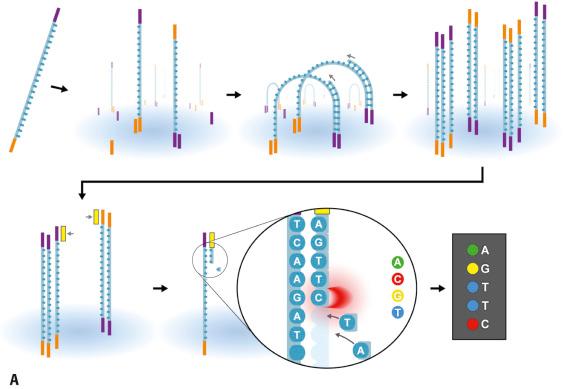
Following sequencing by any NGS method, the raw sequence information is converted to FASTQ file format for initial alignment (mapping) to the genomic reference, base quality scoring, and variant calling and data filtering. The basic process of the bioinformatics “pipeline” involves a variety of software applications that perform sophisticated analyses for accurate alignment of sequenced reads to the reference genome (i.e., genomic and selected coding transcript positions, relative to the specific human genome build used), base substitution detection, and insertion/deletion calling (initial annotation). An extensive quality report is typically generated to detail percent and total sequence reads mapped correctly to the regions of interest and to the genome, base quality scores, sequence error rates, failed regions, off target sequences, and many other parameters. The alignment step and variant calling step of the bioinformatics pipeline create BAM files (binary version of a text readable sequence alignment map) and VCF (variant calling format) files, respectively. Either the BAM or VCF file can be uploaded to genome viewer(s) by the end user for detailed visual review, annotation, and interpretations of genetic variants. Popular viewers include the Broad Institute/MIT Integrated Genome Viewer (IGV) ( www.broadinstitute.org/igv ) and Alamut Visual ( www.interactive-biosoftware.com ) ( Fig. e24.2 ). Notably, additional pipeline algorithms and processes are required for other specialized applications of NGS, such as RNA sequencing or translocation detection. The most labor- and time-intensive aspect of NGS data interpretation concerns the detailed assembly of knowledge to characterize one to often many detected sequence variants in terms of pathogenicity and clinical significance. A large number of publicly available and subscription databases are present to help guide the interpretation of variants; some key examples include those concerned with polymorphic sequence variations (e.g., dbSNP, https://www.ncbi.nlm.nih.gov/projects/SNP ; 1000 genomes, http://browser.1000genomes.org/index.html ; Exome Variant Server [EVS], http://evs.gs.washington.edu/EVS ), cancer genomics (e.g., Catalogue of Somatic Mutations in Cancer [COSMIC], http://cancer.sanger.ac.uk/cosmic ; MyCancerGenome, https://www.mycancergenome.org ), or genetic variants and phenotypes of human heritable diseases (e.g., Human Gene Mutation Database [HGMD], http://www.hgmd.cf.ac.uk/ac/index.php ; ClinVar, https://www.ncbi.nlm.nih.gov/clinvar ). However, the accurate curation of findings requires a thorough understanding of the data quality in databases, knowledge of the specific field of pathology or medicine (often supplemented with extensive and ongoing literature review), and the dedicated efforts of a team of individuals including laboratory technologists, bioinformaticians, genetic counselors and pathologists, or clinical laboratory scientists with relevant molecular genetics expertise. Given that each cancer genome may have thousands of somatic mutations, it is clear that rigorous validation of recurrent candidate alterations in large clinical studies or by detailed biochemical and genetic laboratory research will be required to separate true driver mutations from irrelevant changes (“passenger” mutations). To this end, some studies have advocated the use of matched tumor/normal tissue sequencing to distinguish polymorphic from pathogenic changes and to determine the presence of potential germline conditions. A complete description of NGS applications in diagnostic medicine is beyond the scope of this chapter; although the uses of NGS for currently classifying and determining prognosis in hematologic cancers will be described accordingly, it is important to stress that the information accruing from the explosion in this area will continue to shape hematopathology practice for the foreseeable future.

As NGS technology becomes more routinely deployed in clinical diagnostic laboratories, the use of purpose-built targeted gene panels offers a high-yield, cost-effective approach for the molecular profiling of myeloid neoplasms, complementing conventional karyotyping, morphology, and clinical data. A targeted approach has some distinct advantages compared to whole exome sequencing (WES) such as (1) a primary focus on well-documented, recurrently mutated genes enriched in myeloid neoplasms providing analysis of highly curated and clinically relevant (“actionable”) variants, in contrast to the large datasets of WES containing many lower frequency alterations of uncertain clinical significance; (2) improved depth of sequencing coverage compared to WES, thus improving analytic sensitivity to detect subclonal populations or low variant allele fraction (VAF) neoplastic cell populations in a mixed tumor-normal cell background; and (3) higher sample throughput with reduced cost and turn-around time, in terms of post-sequencing analytics. For myeloid neoplasms encompassing myelodysplastic syndromes (MDS), acute myeloid leukemias (AML), myeloproliferative neoplasms (MPN), and myeloproliferative/myelodysplastic neoplasms (MDS/MPN), overlapping genetic mutation patterns are observed. A targeted pan-myeloid neoplasm panel can be effectively designed by selecting genes according to published clinical relevance. By using larger panel designs, smaller subset analyses can be tailored to focus on individual disease types (e.g., “AML-specific” genes) using bioinformatic masking of extraneous gene regions; this ability further extends the flexibility of using NGS panels for hematologic oncology applications. Mutations in specific single genes (e.g., SF3B1 and TP53 ) that are now formally recognized in the 2016 WHO classification can be detected by Sanger sequencing technique, and this may be sufficient in many instances to meet diagnostic criteria; however, the power of NGS in this setting resides in its capacity to interrogate many gene regions and patient samples simultaneously. As additional gene mutation patterns demonstrate clinical utility or therapeutic value, the Sanger approach becomes less tenable with its limited sample throughput and lower sensitivity (15% to 20% vs. 5% for NGS). When deciding on the addition of NGS technology to the molecular diagnostic laboratory, many considerations are required, including the scope of the test (e.g., number of gene regions, overall size of panel), analytic framework (e.g., bioinformatics support, critical software, specialized knowledge, management of potential germline findings), cost and reimbursement potential, sample throughput, and the role of competing methodologies, such as Sanger sequencing or PCR-based techniques. Several position papers and guidelines have been published by organizations such as the Association for Molecular Pathology, College of American Pathologists, and American College of Medical Genetics and Genomics to help meet these challenges.
Southern blot hybridization (SBH) technique occupies a diminishing role in the molecular diagnostic laboratory but continues to have relevance for the analysis of relatively large (kilobase)-scale alterations in genomic DNA. SBH involves digestion of high-molecular-weight DNA with site-specific restriction endonucleases, followed by size separation of the DNA by gel electrophoresis and transfer of the nucleic acid fragments onto a nylon or nitrocellulose membrane. A particular region of immobilized DNA can then be interrogated by stringent hybridization with a specific double-stranded genomic probe, which has been labeled with a radioisotope (e.g., P 32 ) or by a non-isotopic reagent (e.g., for chemiluminescent detection). Exposure of the resultant band pattern on radiographic film reveals the presence of any atypical rearrangement(s) at the genetic locus in question, relative to the expected or “germline” (unaltered) band configuration. SBH has been most widely applied to detect clonotypic rearrangements of the antigen receptor genes in abnormal B- or T-cell lymphoid proliferations, but assays to evaluate specific oncogenes (e.g., KMT2A, BCL2, CCND1 ) have also been described. Visual interpretation of exposed blots is usually straightforward; however, occasional difficulties may arise owing to degraded DNA quality, incomplete enzymatic digestion of DNA, or when novel pathologic bands co-migrate with germline fragments. The multi-step, technically demanding requirements of SBH also increase the possibility of analytic delays, because errors in procedure are seldom identified before the blot is finally exposed. Of note, sample sources for SBH analysis are limited to fresh or frozen cells, owing to the requirement for high-quality, long-strand genomic DNA. For these reasons and with the advent of more comprehensive fluorescence in situ hybridization (FISH), chromosome microarray, or PCR-based methods, SBH is now less frequently performed in molecular hematopathology laboratories.
Conventional cytogenetic analysis remains the standard method to identify numeric and structural chromosomal aberrations in tumors and to assess the visible genome in its entirety for the presence of these large-scale changes. The cytogenetic karyotype in fact retains its prominence in the current WHO disease classification of hematopoietic tumors. However, the limited resolution of chromosome-specific banding obtained by Giemsa techniques (GTG- or G-banding) makes the recognition and interpretation of smaller, masked, or cryptic chromosome aberrations difficult to ascertain and therefore potentially inaccurate. Fluorescence in situ hybridization (FISH) and more recently, chromosome microarray techniques (e.g., array-based comparative genomic hybridization, A-CGH and single-nucleotide polymorphism array, SNP-A) extend the ability to detect smaller scale changes in chromosomal regions and are an integral aspect of molecular cytogenetics practice. The use of these techniques enhances the resolution of both numeric and structural chromosomal aberrations (especially those that are complex or subtle), bridging the gap between conventional chromosomal band analysis and nucleotide base-level molecular genetic analysis.
FISH essentially involves the base-pairing of fluorescently labeled nucleic acid probes to complementary DNA sequences in cell preparations, followed by direct visualization of probe-specific, intranuclear signals utilizing fluorescence microscopy. A large number of different probes designed to identify specific chromosomes or subregions are commercially available for diagnostic purposes, with the choice of probes dependent on the particular application in question. The most commonly used probes in FISH analysis of hematologic malignancies are (1) repetitive sequence probes and (2) locus-specific probes. So-called whole chromosome paints or multiplex probe methods (M-FISH) have been described as well but are technically challenging for standard diagnostic use. Repetitive sequence probes target chromosome-specific satellite sequences of pericentromeric heterochromatin (i.e., centromere-specific probes), or unique DNA sequences at the ends of all chromosomes (i.e., subtelomeric probes). These probes are useful when numeric aberrations carry information of diagnostic or prognostic relevance, as in subgroups of childhood acute lymphoblastic leukemia, or the myelodysplastic syndromes. Locus-specific probes target sequences normally present as only one copy per haploid genome. The main utility of locus-specific probes in hematologic malignancies is the detection of deletions, inversions, and translocations that are often disease specific. Locus-specific FISH technique is highly versatile, and many different probe strategies can be designed to optimize the detection of an abnormality, depending on the clinical application. Dual-color/dual-fusion translocation probe (D-FISH) methods have been developed to increase the sensitivity and specificity of FISH detection. Another strategy of particular utility is the use of so-called break-apart probes (BAP) to target genes with multiple possible translocation partners (e.g., the MLL gene). In this setting, a typical dual-color/dual-fusion FISH assay directed at one particular translocation would possibly miss a variant translocation. A BAP, on the other hand, can detect the presence of a translocation involving a given gene locus independent of the specific partner gene (although the nature of the translocated partner would not be known without additional investigations). Standard karyotype analysis in hematologic malignancies is sometimes hampered by poor chromosome morphology or a low number of requisite tumor metaphases. The ability of FISH technique to use intact, non-dividing (interphase) cells as DNA targets enables the quantitative analysis of a larger number of cells and has considerable added advantages for assessing hematologic malignancies in which the proliferative activity is low, or when the mitotic cells are reactive in nature and do not represent the neoplastic clone. Since culture of mitotic phase cells is not a requirement, FISH is applicable to a variety of specimen types, including fresh or frozen tissue, cytologic preparations (e.g., fine needle aspiration), air-dried unstained slides, and formalin-fixed paraffin embedded tissues (FFPE). In any FISH assay, nuclear DNA is universally stained with a dim fluorescent dye (e.g., 4′,6-diamidino-2-phenylindole, DAPI) to visualize the individual cell nuclei by microscopy. Experimental controls for FISH analyses are based on the false-positive signal rate threshold. Laboratories performing FISH testing must determine the false-positive rate for each assay individually and, in turn, for each type of tissue or specimen used in that assay. Signals from partially overlapping or truncated nuclei (e.g., from thin FFPE tissue sections) can lead to erroneously high positive signal scoring, particularly with fusion probe sets, leading to potentially serious errors in patient diagnosis and clinical management.
Single-nucleotide polymorphisms represent the most frequent type of variation in the human genome, with an average of 1 per 300 bases of genomic DNA. In addition, the discovery of a relatively large number of kilobase- to megabase-sized variations in regional DNA sequences, known as copy number variations (CNVs), is also of significance from the perspective of disease pathogenesis, and these gains or losses can encompass genomic regions harboring many different genes. Although many CNVs represent polymorphic variants, recent studies have begun to identify recurrent alterations that may be critically involved in disease causation, including acquired CNV events in cancer. The advent of array-based automated technologies for assessing global genome architecture has now enabled more widespread application to a variety of disorders, including hematolymphoid neoplasms. Chromosome array platforms differ in design but are similar in the scope of genomic information provided. Array competitive genomic hybridization uses fluorescently labeled patient and control DNA samples to hybridize to a solid substrate or chip embedded with thousands of small individual DNA probes. The ability to densely tile or “spot” the oligonucleotide probes ensures that a very large proportion of total genomic coverage is potentially obtained. SNP array chips similarly consist of spotted oligonucleotides, but the probes instead are designed to include allelic variants of hundreds of thousands to millions of informative SNP loci distributed throughout the genome. SNP-A powerfully combine both CNV analysis and SNP genotyping, the latter based on probe classification of homozygous versus heterozygous status at the given polymorphic loci. In general, signal differences and intensities between sample and control are measured, and bioinformatic software algorithms analyze the hybridization results to denote copy number changes (gains/losses) in relation to a map of the reference genomic sequence and chromosomal position. A-CGH can detect genome-scale alterations at very high resolution (e.g., below 5–10 kb or even lower, far superior to FISH and standard cytogenetics), but this is critically dependent on the probe density and spacing (coverage). A-CGH delimits the boundaries of CNVs and because a paired control DNA is used, true pathologic CNV changes can be more readily distinguished from common polymorphic DNA regions in the patient sample. A-CGH identifies regional gains (gene amplifications), as well as areas of loss of heterozygosity (LOH) or hemizygosity associated with monoallelic deletion of gene loci. However, A-CGH cannot detect copy number neutral LOH (CN-LOH), also called uniparental disomy (UPD). The latter genomic alteration results from duplication of an allelic region of DNA by homologous recombination with the other (maternal or paternal) allele. CN-LOH can be a result of embryonic autozygosity (i.e., congenital UPD) or can be somatically acquired in tumors. CN-LOH is associated with the presence of two identical haplocopies of a gene (or genomic segment), but without a net loss of DNA content within the affected chromosome regions. CN-LOH is thus not visible to cytogenetic or FISH analyses. A-CGH cannot detect CN-LOH because the method is insensitive to balanced DNA changes, and unlike the related SNP-A technology, A-CGH does not have an additional measure of sequence-level variation.
Using SNP-A, hybridization of the sample genomic DNA to both polymorphic probe variants indicates locus heterozygosity, whereas similar signal intensity for only one allele indicates homozygosity. In addition, the relative intensity of the fluorescence signals allows for the analysis of gene region copy number. SNP-A does not require co-hybridization of normal DNA to generate results but instead uses stored data from reference DNA samples to scale sample fluorescence intensities to a normalized copy number value. Bioinformatic algorithms are used to computationally define regions of copy number loss and gain. In addition, genotype analysis identifies SNP loci as either A, B, or AB (based on hybridization results with probes for allele A, allele B, or both, respectively). For each SNP, comparison with reference DNA profiles will reveal if there is heterozygosity, homozygosity, or LOH at the interrogated site. The results of all individual calls along the chromosome are statistically analyzed, delineating genomic segments showing LOH. SNP-A, like A-CGH, provides a high-resolution technique for detecting unbalanced chromosomal defects such as deletions or gains of chromosomal material undetectable by routine metaphase cytogenetic or FISH techniques. As indicated, a major advantage of SNP-A over A-CGH is its ability to detect CN-LOH (UPD). CN-LOH is established when an apparently homozygous constellation of SNP genotyping calls is observed in a genomic region with overall diploid copy number. Recent studies based on SNP-A technology have shown a high rate of acquired UPD in several hematologic malignancies. This process occurs in polycythemia vera, for example, in which a biallelic JAK2 V617F gene mutation is produced by an acquired chromosome 9p CN-LOH event; the recognition of this genomic abnormality enabled the initial discovery of the JAK2 V617F mutation. The non-uniform distribution of SNP markers in human DNA and requirement for sufficient informative heterozygous loci limit the resolution of SNP-A, a drawback when compared to A-CGH, wherein probes can be placed more evenly and can even be targeted to specific regions of interest on individual chromosomes. More recent SNP-A platforms therefore also incorporate additional oligonucleotides to cover SNP-poor regions and increase the sensitivity for detecting CNV, combining the advantages of both approaches. Of note, neither A-CGH, nor SNP-A can resolve balanced chromosomal translocations or low-level mosaicism. The sensitivity of both A-CGH and SNP-A for detecting the presence of genomic abnormalities is variable but typically not better than approximately 20% to 30%, indicating that for somatic changes, normal cells admixed with tumor cells can compromise analysis.
The concept of “clonality” is central to the cellular definition of cancer and is microscopically represented by characteristic morphologic alterations or by the presence of recurrent or non-random karyotypic abnormalities in a tumor. Lymphoid neoplasms are unique in that the clonal nature of these proliferations can also be readily demonstrated by immunophenotypic or molecular genetic methods. In the primary diagnostic setting, the clonal status of an atypical B-cell lymphoid proliferation can usually be determined by deviations in the ratio of expressed surface or cytoplasmic light chains, as assessed by flow cytometry, immunohistochemistry, or RNA in situ hybridization. However, a number of distinct diagnostic situations require the application of molecular genetic clonality analysis. These include lymphoid processes with indeterminate immunophenotypic features for clonality, many T-cell proliferations, or suspected neoplastic lymphoid expansions that pose difficulty for routine immunophenotypic investigation (e.g., small tissue biopsies).
The presence of unique immunoglobulin or T-cell antigen receptor proteins on the surface of mature lymphocytes is a product of the somatic gene rearrangement process occurring early in the development of B and T cells in the bone marrow and thymus, respectively. The antigen receptor genes consist collectively of many variable (V), diversity (D), joining (J), and constant (C) gene segments, each separated by large intervening regions of non-coding DNA. D-segment regions are integral to the immunoglobulin heavy chain ( IGH ) and T-cell receptor beta ( TRB ) loci but are not present in the immunoglobulin light chain ( IGK , IGL ) or T-cell receptor gamma ( TRG ) genes. In developing lymphoid cells, site-specific rearrangements bring together V, (D), J, and C gene segments across many kilobases of DNA in the antigen receptor gene loci, by a process of coordinated double-strand DNA breakage and recombination mediated by the recombinase activating gene (RAG) system and a variety of DNA repair enzymes. The resultant relatively compact V(D)JC coding “cassettes” consist of rearranged DNA sequences that are distinct in both size and sequence characteristics in each lymphocyte. The remarkable quantity of unique antigen receptor proteins produced by human lymphocytes is thus initially derived from the combinatorial pool of only a few hundreds of individual V, D, J, and C gene segments. The addition of a variable number of non-templated (“n”) nucleotides to the junctional ends of the V and D gene segments (i.e., VnDnJ) by the enzyme terminal deoxynucleotidyl transferase (TdT) during the rearrangement process further dramatically increases sequence diversity ( Fig. e24.3 ). As such, every B or T lymphocyte in a reactive lymphoid population harbors an individual receptor “fingerprint” in both DNA and peptide composition, which in sum comprise a polyclonal population. The expression of heterodimeric antigen receptors on the surface of individual B and T cells (i.e., heavy/light chains on B cells and alpha/beta or gamma/delta chains on T cells) ensures a tremendous range of potential antigen recognition and binding affinities. Although evidently a large spectrum of individual gene rearrangement sequences arise from these recombination events, the coding region segments of the many possible V(D)J recombinations can be reliably detected by relatively simple PCR techniques using “consensus” PCR primers targeted to relatively conserved foci of DNA sequences within related “families” of V and J segments that flank the rearrangement sites. In this manner, a polyclonal B- or T-cell population containing many unique immunoglobulin or T-cell receptor gene rearrangements can be visualized following PCR as a normal (Gaussian) type distribution of amplified fragments within a defined size range bounded by the relative V(D)J lengths and flanking primers. Conversely, a monoclonal lymphoid proliferation, arising from a single clonal cell proliferation, produces only one or possibly two single size fragments, the latter situation reflecting the occurrence of bi-allelic rearrangements of the particular antigen receptor gene analyzed. These concepts are illustrated in Fig. e24.3 .
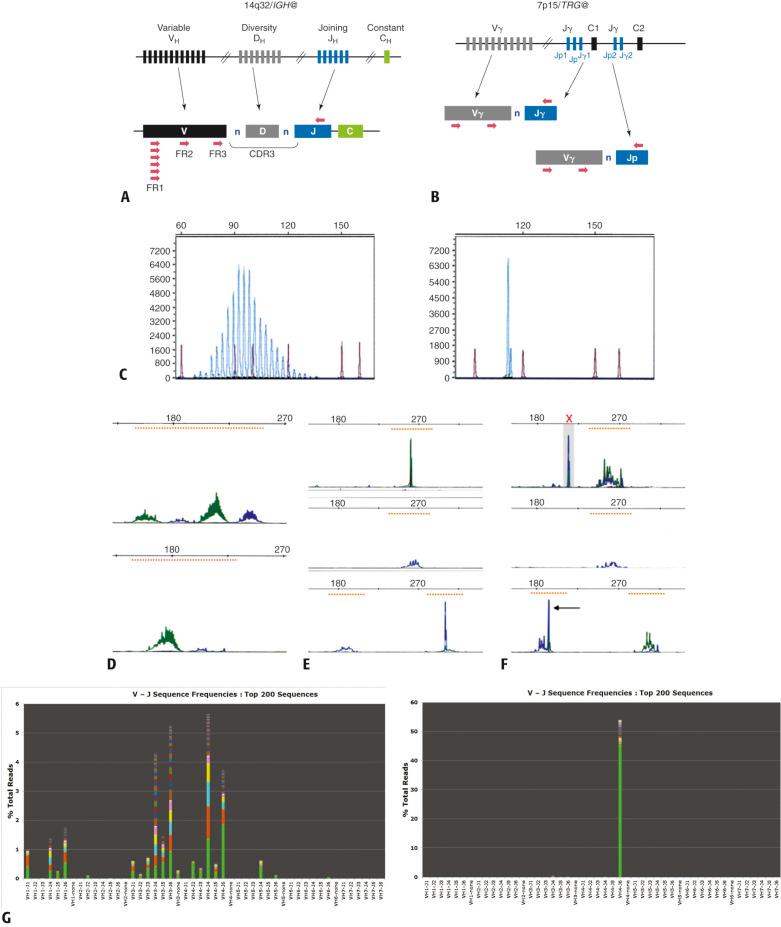
The immunoglobulin heavy chain gene ( IGH ) located on chromosome 14(q32) and the T-cell receptor gamma gene ( TRG ) on chromosome 7(p15) are the most commonly targeted antigen receptor loci for B- and T-cell clonality assessment by PCR methods. The IGH gene consists of 70 to 80 functional V H -region exons, 20 to 30 D H -segments, and 6 J H -segments. Most V H -segments are characterized by relatively conserved nucleotide sequences encoding three framework (FR) or structural peptide regions of the antibody molecule, which in turn are separated by more diverse intervening sequences encoding three complementarity determining regions (CDR) responsible for specific antigen binding (epitope recognition). Similar to V H framework regions, nearly all J H exons also share partial sequence homology. The V H -FR and J H gene segments thus constitute templates for binding by consensus PCR primers (see Fig. e24.3 ). Notably, although these primers are not specific for any particular IGH gene rearrangement, this strategy produces short (100–400 base pair) amplicons that include the hypervariable third CDR (CDR3), which contains the greatest sequence and fragment length diversity and gives rise to the normal distribution of PCR products in a polyclonal B-cell process (see Fig. e24.3 ). The TRG locus is by comparison less complex, containing 11 V-region exons (grouped as 4 families) and 5 J-region segments; nonetheless, sequence and fragment length differences remain significant enough (owing to the segmental recombination process and TdT activity) to create a practically wide distribution of rearrangements in polyclonal T-cell populations. Again, a limited set of consensus and V- and J-specific primers can be utilized to efficiently amplify TRG gene rearrangements by PCR (see Fig. e24.3 ). Following PCR amplification, post-PCR analysis of IGH or TRG products can be achieved by agarose or polyacrylamide gel electrophoresis; however, fluorescent capillary electrophoresis has now become routine in light of its superior resolution and precise fragment-sizing capabilities (see Fig. e24.3 ). One clear advantage of PCR methodology for antigen-receptor gene studies is the ability to use a variety of tissue sources, including fresh or frozen cells, extracted DNA, cells from glass slides, or paraffin-embedded samples.
Although PCR methods represent a powerful means of determining whether a lymphoid proliferation is monoclonal (i.e., neoplastic) or polyclonal (i.e., benign), the technique is associated with potentially serious errors affecting both clinical sensitivity and specificity. For example, consensus primer PCR methods cannot detect all possible V(D)J rearrangements in the antigen receptor genes. A related problem concerns the failed detection of IGH rearrangements in B cells that have experienced a germinal center environment. Germinal center (and post-germinal center) B cells often acquire additional mutations in the immunoglobulin genes resulting from the process of somatic hypermutation (SHM). SHM is mediated by the enzyme activation-induced cytidine deaminase (AID) and its activity increases the affinity of antibody interaction for cognate antigenic epitopes, but during this event, the introduced small genetic mutations may extend into conserved regions (i.e., FR) that are targets for consensus PCR primers. The situations of incomplete gene rearrangement coverage and SHM can lead to false-negative results for true monoclonal IGH rearrangements and a corresponding loss of clinical sensitivity, even if the analytic sensitivity of the method is acceptable. False-negative PCR results can be significantly diminished by the use of multiple primers directed to different conserved V H framework regions or families, in combination with assessment of the IGK locus. Similarly, for analysis of T-cell receptor gene rearrangements, targeting both the TRG and TRB loci can increase the true positive detection rate for monoclonal T-cell populations. Conversely, PCR-based assays can also suffer from false positivity arising from sample contamination (e.g., carryover of tissue fragments or amplified products from an unrelated sample), underscoring the importance of proactively mitigating such events in the molecular diagnostic laboratory. For T-cell receptor PCR studies, another risk of potential false-positive interpretation can occur because of physiologic or iatrogenic circumstances. T-cell repertoire restriction is found in some clinical settings (e.g., autoimmune diseases, post transplantation) and with normal aging. Apparent clonal (“pseudoclonal”) or oligoclonal T-cell populations can be identified by the PCR method in these settings, requiring careful correlation with clinical history and other laboratory or morphologic data in order to avoid over-calling such findings. The use of “peak height” criteria has also been proposed to improve confidence for interpreting clonotypic T-cell PCR results; however, considerable overlap between true monoclonal (neoplastic) and atypical reactive amplification patterns is often encountered in practice. Although PCR is a generally sensitive technical modality, the practical lower detection limit of a monoclonal lymphoid population in a tissue sample is nominally 1% to 5% using standard electrophoretic detection methods. However, this level of sensitivity can vary significantly depending on the particular clonal antigen receptor gene rearrangement and the amount of accompanying polyclonal lymphocytic background in a given case. For specialized indications, such as post-therapy MRD monitoring, higher PCR sensitivity has been achieved by designing patient clone-specific primer and oligonucleotide probe reagents, but this is a labor intensive and technically challenging method.
Although consensus PCR with capillary electrophoresis has been the mainstay of lymphoid clonality assessment for over two decades, the advent of NGS methodology has brought new opportunities and a profound increase in knowledge and diagnostic ability. Using a similar consensus PCR primer strategy, an amplicon library can be prepared with attached NGS adapters and sample-specific bar codes. Deep sequencing of these PCR products provides not only specific sequence identities of thousands of rearrangements within a lymphoid population but also enables quantitation of relative species abundance and direct determination of somatic mutation status (see Fig. e24.3 ). NGS applications for antigen receptor gene rearrangements are providing an unprecedented opportunity to refine epitope mapping, explore lymphocyte subsets, and better understand adaptive or cellular immune responses and repertoires. In major part, this has also been made possible by the development of high-quality antigen receptor gene-specific reference sequence database resources, such as the IMGT immunogenetics site ( http://www.imgt.org ) and IG-BLAST ( https://www.ncbi.nlm.nih.gov/igblast ). In hematopathology practice, NGS is creating a deeper and richer data set to redefine and explore the concept of “clonality” in lymphoid proliferations. The ability to generate millions of sequence reads from a single sample also provides a powerful advantage for NGS in the direct evaluation of MRD; because each gene rearrangement is unique at the DNA sequence level, an individual population can be identified and quantified in a large background of other sequences.
To complete the discussion on lymphoid molecular clonality evaluation, the merits and drawbacks of Southern blot hybridization should be considered. SBH can identify relatively large-scale rearrangements of single-copy genomic DNA at the antigen receptor gene loci, in contrast to the detection of amplified small rearranged V(D)J region nucleotide fragments by PCR technique. The nature of SBH technique and the ability to interrogate substantially larger regions of genomic DNA for pathologic alterations enables the identification of nearly all possible clonal immunoglobulin and T-cell receptor gene rearrangements in lymphoid tumors; this correspondingly lowers the risk of false-negative results, as long as the assay is performed within its analytic sensitivity range. The IGH , IGK , and TRB loci are the most informative antigen receptor genes for SBH clonality assessment. SBH has become less often employed in the routine molecular diagnostic investigation of the lymphomas and lymphoid leukemias for reasons stated previously. In addition, SBH has an analytic sensitivity of optimally 5% to 10% for the detection of monoclonal B- or T-cell populations, typically inferior to that of standard PCR assays. The more refined development of multi-primer and multi-locus antigen receptor gene PCR approaches, as advocated by the BIOMED-2 consortium and related studies, has largely led to a marked decrease in the routine application of SBH, although some diagnostic laboratories retain the capacity to perform this analysis to occasionally complement PCR-based clonality methods.
Understanding the pathogenesis of the non-Hodgkin lymphomas (NHL) has been greatly aided by the recognition and characterization of recurrent, non-random chromosomal translocation events ( Table 24.2 ). For B-cell NHL, such translocations typically result in the placement of a proto-oncogene adjacent to one of the transcriptionally active immunoglobulin genes, most commonly IGH . In turn, the normal regulation of the oncogene becomes disrupted, leading to aberrant activity resulting in unchecked cellular proliferation or protection from normal cellular apoptosis thresholds. In certain other subtypes of lymphoma (e.g., some extranodal marginal zone lymphomas, anaplastic lymphoma kinase (ALK)-positive anaplastic large-cell lymphoma), translocations produce a chimeric fusion gene, expressed mRNA transcript, and novel oncoprotein. In this situation, pathophysiologic effects arise in part from disruption of key cellular signaling pathways, mediated by aberrant activity of the chimeric protein. In B-cell lymphomas, various mechanisms have been proposed to explain the occurrence of translocation-associated gene rearrangements. These include (1) abnormal targeting of the RAG recombinase machinery during physiologic immunoglobulin gene rearrangements; (2) aberrant resolution of DNA double-strand breaks during germinal center processes (e.g., somatic hypermutation, class switch recombination) mediated in part by the enzyme activation-induced cytidine deaminase; and (3) fragile DNA intermediates prone to breakage and errant recombination.
| Disease Associations | Major Genetic Abnormalities | Molecular Pathogenesis a | Molecular Diagnostic Detection b | Notes |
|---|---|---|---|---|
|
t(14;18)/ BCL2-IGH | Overexpression of antiapoptotic BCL2 protein | FISH (DNA PCR) |
|
| Mantle cell lymphoma (MCL) | t(11;14)/ CCND1-IGH | Overexpression of G1-phase cell cycle protein cyclin D1 | FISH (DNA PCR) |
|
|
BRAF Val600Glu | Constitutive MEK-MAP kinase pathway signaling | Allele-specific PCR (including RQ-PCR), sequencing |
|
|
MYD88 Leu265Pro | Activation of NF-κB pathway signaling | Allele-specific PCR, sequencing |
|
| Extranodal marginal zone lymphomas (ENMZL) | t(11;18)/ BIRC3-MALT1 and t(14;18)/ MALT1-IGH | Upregulation of NF-κB signal transduction pathway activity | FISH (RT-PCR for BIRC3-MALT1 mRNA) |
|
| Chronic lymphocytic leukemia (CLL) | del17p/ TP5 3, del11q, IGH-V region somatic hypermutation status | Deletion of tumor suppressor loci (i.e., TP53 and ATM ); IGH-V mutation status <2% (close to germline) associated with poor prognosis | FISH for chromosome deletion events; PCR and sequencing (Sanger or NGS) for IGH-V mutation status | Gene mutations in SF3B1 , ATM , TP53 , BIRC3 , NOTCH1 may also have prognostic value and can be collectively evaluated by targeted NGS |
|
t(8;14)/ MYC-IGH , MYC-IGK , or MYC-IGL and variant MYC translocations | Overexpression of potent early response mitogenic transcriptional factor MYC | FISH |
|
| ALK+ T-cell anaplastic large-cell lymphoma (ALK+ ALCL) | t(2;5)/ NPM1-ALK and other ALK translocation variants | Constitutive activation and abnormal localization of Alk tyrosine kinase | FISH (RT-PCR for NPM1-ALK mRNA) |
|
| ALK-negative anaplastic large-cell lymphoma | Rearrangements of DUSP22/IRF4 locus on 6p25 or TP63 locus on 3q28 | Current pathogenic effects are uncertain | FISH |
|
| Peripheral T-cell lymphomas (PTCL) with T-follicular helper (TFH) phenotype | ITK-SYK and CTLA4-CD28 gene fusions; recurrent gene mutations (e.g., IDH2 , RHOA , DNMT3A , CD28 ) | Abnormal T-cell receptor and cell signaling activation; epigenetic alterations | FISH (RT-PCR) for gene fusions; targeted NGS panel for gene mutations |
|
| T-cell prolymphocytic leukemia (T-PLL) | inv14 or t(14;14)/ TCL1A gene rearrangement | Overexpression of oncogenic transcription factor | FISH |
|
| T-cell large granular lymphocytic leukemia and chronic NK-cell lymphoproliferative disorders | STAT3 and STAT5B gene mutations | Activation of STAT signaling | Allele-specific PCR, sequencing |
|
a Major pathophysiologic effect resulting from genetic lesion.
b Detection methods in parentheses indicate less clinically sensitive technique.
The identification of characteristic cytogenetic abnormalities is not always essential for establishing a diagnosis or specific subclassification of NHL; most often, a correct diagnosis is arrived at by a combination of morphology and immunophenotypic studies. Furthermore, the detection of expressed oncoproteins by immunohistochemistry provides a simple alternative method of confirming the presence of an underlying specific molecular genetic event in some types of NHLs (e.g., cyclin D1 expression in mantle cell lymphoma and ALK expression in anaplastic large-cell lymphoma). Nonetheless, there are many circumstances in which the presence or absence of these genetic anomalies can be critical for correct disease diagnosis. The detection of lymphoma-associated genetic abnormalities can be achieved by cytogenetics, FISH analysis, or PCR-based methods. Cytogenetics is often limited by the relative unavailability of fresh tissue for sterile culture. PCR assays relying on clustering of genomic breakpoints have been developed to detect some gene fusion abnormalities in NHL, but this approach in general suffers from suboptimal clinical sensitivity because of the large and inconstant breaksite regions in respective chromosome locations precluding the use of standard short-range PCR techniques. FISH methods, in contrast, are very robust in this regard and are most often applied to reveal the presence of translocation events in NHL. The emergence of NGS is also providing new opportunities to potentially evaluate tumor DNA for the presence of chromosome translocations and simultaneously screen for nucleotide base-level mutations. Targeted small NGS panels can be cost-effective solutions to interrogate many disease-related recurrent genetic abnormalities in B-cell neoplasms.
The t(14;18)(q32;q21)/ BCL2-IGH abnormality, which is prototypic of lymphoma translocations, occurs in approximately 90% of follicular lymphomas (FL) and up to 30% of diffuse large B-cell lymphomas (DLBCL) typically arising from preexisting FL. The juxtaposition of BCL2 with transcriptionally active IGH leads to overexpression of BCL2 anti-apopotic protein, which supports neoplastic cell survival in the face of proliferative stress or DNA damage. At the genomic level, breakpoints in BCL2 are most often highly clustered in the distal part of the third non-coding exon, known as the major breakpoint region (MBR), whereas the break-fusion sites in IGH are in the vicinity of the J H exons ( Fig. 24.1 ). Additional BCL2 breakpoint sites have also been identified loosely grouped downstream of the MBR and have been designated as 3′ MBR, intermediate cluster region (icr), minor cluster region (mcr), and 5′ mcr. PCR approaches using extracted tumor DNA can detect nearly 65% of BCL2-IGH translocation rearrangements using a primer situated 5′ to the MBR and a consensus J-region IGH primer (see Fig. 24.1 ). Additional primer sets targeting the mcr and icr can identify these additional breakpoint sites, raising the clinical sensitivity of PCR technique above 80%; however, a significant minority of BCL2 breakpoints remain undetectable even with these more comprehensive protocols. Notably, as is the case for any PCR assay involving fixed, paraffin-embedded material, both the analytic and clinical sensitivity will be adversely affected owing to the effects of DNA degradation. Dual color/dual fusion FISH analysis (D-FISH) using probes spanning the constant through variable regions of the IGH locus on 14(q32) and the entire BCL2 gene on 18(q21) is highly sensitive and specific for detecting this translocation. FISH is also reliable using tissue sections or nuclei extracted from paraffin-embedded samples.
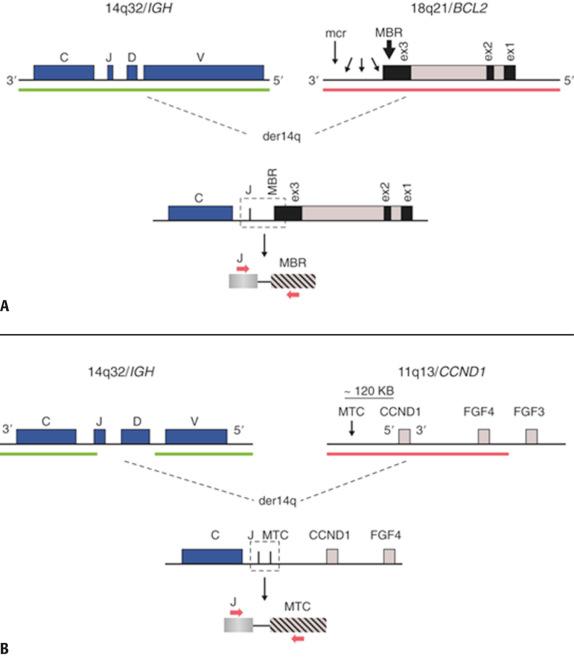
Detection of the BCL2-IGH can be useful to differentiate follicular lymphoma from atypical reactive follicular hyperplasia in challenging biopsies, especially when immunophenotyping and immunoglobulin gene rearrangement studies are not informative. More commonly, the presence of this genetic abnormality is used to establish the diagnosis of follicular lymphoma and exclude other “small B-lymphocyte” neoplasms with potentially overlapping morphologic features. In this regard it is important to keep in mind that BCL2 protein overexpression is commonly observed in many other types of non-Hodgkin lymphomas, resulting from genetic or epigenetic mechanisms distinct from the BCL2-IGH ; the presence of Bcl2 protein overexpression per se is therefore not specific for lymphoma subclassification. Some cases of nodal FL in adults lack the t(14;18)/ BCL2-IGH , and these are usually associated with greater centroblastic (i.e., grade 2 or 3) cytology and a relatively distinct molecular pathogenesis, including BCL6 translocations. BCL2 rearrangements are notably also not observed in the subgroup of pediatric-type FL and in rare patients presenting with high-cytologic-grade FL involving Waldeyer's ring tonsillar tissue with rearrangements of IRF4/MUM1 .
The t(11;14)(q13;q32)/ CCND1-IGH abnormality is observed in mantle cell lymphoma (MCL), as well as a subset of multiple myelomas. Past studies identified the t(11;14) in occasional cases of chronic lymphocytic leukemia (CLL), B-cell prolymphocytic leukemia (B-PLL), and splenic marginal zone lymphoma (SMZL); however, most, if not all such occurrences are now considered to represent clinical and morphologic variations of MCL. Thus, the presence of the t(11;14) can be considered essentially pathognomonic of MCL in the differential diagnosis of mature B-cell NHL. This translocation places the CCND1 locus in proximity to the IGH (see Fig. 24.1 ). CCND1 encodes the G1-phase cell cycle protein cyclin D1, which becomes highly overexpressed as a result of the t(11;14). The three D-type cyclins (D1, D2, and D3) promote early G1-phase entry to the cell cycle by binding to specific cyclin-dependent kinases (cdk4, cdk6), leading to inactivation of retinoblastoma (Rb) protein, transition to S-phase, and subsequent commitment to mitosis. Cyclin D1 (in contrast to its related counterparts) is notable for not being expressed in normal proliferating B cells; thus, overexpression of cyclin D1 via CCND1 translocation is a hallmark of nearly all cases of MCL. Consequently, CCND1 deregulation is thought to result in unchecked cell cycle entry, leading to increased cellular proliferation in MCL. Data from gene expression microarray analysis confirm a prominent proliferation and anti-apoptosis signature that seems to underlie both the pathogenesis of this disease and the observation that patients with MCL have a distinctly adverse outlook compared to other small B-cell neoplasms generally.
From the molecular diagnostic standpoint, approximately 50% of the breakpoints in the 11(q13)/ BCL1 locus aggregate in a region known as the major translocation cluster (MTC) located approximately 120 kilobases (kb) centromeric to CCND1 (see Fig. 24.1 ). The remaining breakpoints can occur broadly on either side of the MTC, including even further centromeric or immediately 5′ of CCND1 . Given the substantial breakpoint heterogeneity in this area of 11(q13) and lack of significant breakpoint clustering outside of the MTC, DNA PCR-based methods to detect the t(11;14) cannot achieve high detection sensitivity. The use of a BCL1 MTC region primer with a consensus J H -region IGH primer detects slightly less than half of locus rearrangements overall, and this is further diminished in paraffin tissue samples. In contrast, D-FISH employing two IGH region probes and a large CCND1/BCL1 region probe can detect virtually all t(11;14) events in MCL (see Fig. 24.1 ). Along with the general advantages of the dual fusion strategy, the 11(q13) probe can simultaneously identify CCND1 gene locus amplification in addition to the presence of the t(11;14). CCND1 amplification has been associated with the more aggressive “blastoid” variant of MCL. Immunohistochemistry using antibodies directed to cyclin D1 has become a widespread ancillary test in establishing a diagnosis of MCL. Regardless, FISH analysis for CCND1 gene rearrangements retains an important function for the specificity of MCL diagnosis, given that aberrant cyclin D1 expression can be seen in other types of B-cell neoplasms (e.g., hairy cell leukemia, rare cases of DLBCL) in the absence of the t(11;14) or CCND1 alteration. Finally, very rare examples of MCL with characteristic morphologic and phenotypic features have been described lacking the CCND1 gene rearrangement. These unusual MCL cases share a similar gene expression profile to CCND1 + MCL, but nearly half demonstrate alternate translocations involving the CCND2 gene, which can be identified by specific FISH probe analysis.
Classic presentations of hairy cell leukemia (HCL) are associated with well-recognized clinical and pathologic features. Nevertheless, the usual presentation of splenomegaly and bone marrow involvement and the cellular immunophenotype associated with HCL can overlap with other low-grade B-cell chronic neoplasms, notably splenic marginal zone lymphoma. In this regard, the discovery of a recurrent Val600Glu mutation (V600E) in the BRAF gene now provides an additional molecular marker to support the diagnosis of HCL, since this abnormality is present in more than 90% of HCL but very rarely seen or not observed in other low-grade B-cell tumors. The same BRAF mutation is also present in approximately 50% to 60% of Langerhans cell histiocytosis and is similarly found in the rare presentations of Erdheim-Chester disease. In a significant subset (16%) of HCL with BRAF V600E, cooperating mutations in the CDKN1B gene encoding the p27 cell cycle regulator are also present. Detection of the BRAF V600E is not strictly necessary for the diagnosis of HCL but can be a highly useful adjunct in situations where morphologic and immunophenotypic features are conflicting or unclear. In relapsed or refractory disease, the presence of the mutation provides a rationale for targeted therapy with B-Raf inhibitors. The BRAF V600E mutation can be detected using PCR and Sanger sequencing or more sensitively by allele-specific or quantitative PCR methods. Bone marrow aspirate preparations from patients with HCL are usually hemodilute secondary to associated reticulin fibrosis, and most individuals have significant pancytopenia at presentation; thus, a relatively sensitive molecular diagnostic approach is preferred. However, highly sensitive assays for BRAF V600E must also be constructed carefully to ensure accurate mutant allele discrimination and optimal specificity. Notably, the BRAF V600E mutation is not identified in a small proportion of classic HCL associated with IGH VH4-34 rearrangements or in cases of hairy cell leukemia–variant (HCL-V); however, mutations in the downstream MAP2K1 gene have been described in these tumors, again highlighting the importance of deregulation of the mitogen activated protein (MAP) kinase pathway in this B-cell tumor and underscoring the potential for drugable therapeutic targets using Raf and MEK1 inhibitors. Neither BRAF nor MAP2K1 mutations have been described recurrently in SMZL, providing useful molecular markers in this differential diagnostic situation.
Low-grade B-cell lymphomas with plasmacytic differentiation include lymphoplasmacytic lymphoma (LPL) and marginal zone lymphoma. Differentiation of these entities based on morphologic and immunophenotypic features can be difficult, particularly in the bone marrow, at extranodal sites, or in small tissue biopsies. Even though both types of lymphomas exhibit indolent disease biology, the clinical manifestations of LPL (Waldenström's macroglobulinemia) can create distinct challenges for patient treatment, underlying the importance of accurate diagnostic classification. LPL is now much better defined by the presence of a recurrent somatic mutation in the MYD88 gene, causing a leucine-to-proline substitution at amino acid 265 (i.e., p.Leu265Pro, or L265P), which is present in approximately 90% of cases. In the setting of characteristic morphologic features and IgM paraproteinemia, the finding of MYD88 L265P is therefore a highly specific molecular marker for LPL. This mutation is also present in approximately 50% of IgM monoclonal gammopathy of uncertain significance (MGUS) but absent from true cases of IgM-positive multiple myeloma, suggesting that patients with IgM MGUS share a common pathogenesis more akin to LPL than plasma cell myeloma. Notably, IgG or IgA expressing lymphoplasmacytic lymphomas may harbor the MYD88 L265P mutation in approximately 40% of cases. Although MYD88 L265P is highly associated with LPL, it has also been noted to occur in approximately 10% of splenic marginal zone lymphomas, requiring careful attention to clinical and pathologic features to establish a correct diagnosis. The same mutation is also identified in nearly one third of diffuse large B-cell lymphomas (typically of non-germinal center or activated B-cell origin) and up to 90% of large B-cell lymphomas occurring in “immune privilege” sites such as testis, brain, or vitreoretinal tissue.
The MYD88 L265P mutation can be detected by several techniques, including Sanger sequencing of the exon 5 region or more sensitive allele-specific or quantitative PCR approaches. Relatively high sensitivity (e.g., at least to 1% mutation level detection) is required because of the patchy nature of LPL infiltrates in bone marrow aspirate samples and the lack of significant peripheral lymphocytosis in many cases. Design of a short amplicon PCR method is also important to achieve equivalent clinical and analytic sensitivity from fixed paraffin-embedded bone marrow clot or tissue samples. In nearly 30% of LPL cases with MYD88 L265P, a concurrent mutation is also present in the CXCR4 gene. CXCR4 is the receptor for the chemokine CXCL12/SDF-1, acting to increase intracellular calcium levels and promote cellular signaling through MAPK1/3. Germline mutations of CXCR4 are also found in WHIM (warts, hypogammaglobulinemia, infections, myelokathexis) syndrome and unusually, somatic CXCR4 mutations in LPL show a similar pattern of C-terminal truncation (e.g., p.Ser338Ter) or frameshift alterations; these mutations can be detected by PCR and Sanger sequencing of the involved region of CXCR4 exon 2. The co-occurrence of CXCR4 mutations and MYD88 L265P in LPL is associated with decreased blood counts, higher disease burden, and poorer response to targeted ibrutinib therapy, although overall survival is not significantly different from patients with LPL without CXCR4 alterations. Nonetheless, the identification of CXCR4 mutations can provide additional prognostic information to the clinician and enhance the diagnostic specificity for LPL.
The extranodal marginal zone (B-cell) lymphomas (ENMZL) or mucosa-associated lymphoid tissue lymphomas (“MALTomas”) are a group of indolent B-cell tumors encompassing a variety of anatomic sites and several tumor genetic abnormalities. Although numeric chromosome imbalances (e.g., trisomies of 3, 18) are often observed in ENMZL, several recurrent chromosomal translocations have been identified in these lymphomas. The t(11;18)(q21;q21) is most frequently observed in gastric and pulmonary ENMZL, with a much lower incidence in orbital and salivary gland sites. This translocation results in the formation of the BIRC3-MALT1 gene fusion. BIRC3 , also called API2 (or IAP2 ) encodes an apoptosis inhibitor protein, whereas the MALT1 (or MLT1 ) gene produces a “paracaspase” protein. The chimeric BIRC3-MALT1 is transcribed and creates a novel oncogenic protein. A second, less frequent translocation in ENMZL is the t(14;18)(q32;q21) generating the MALT1-IGH abnormality, with resultant deregulation of MALT1 gene expression. The MALT1-IGH has been associated mainly with orbital, salivary gland, lung, and cutaneous ENMZL but is very infrequently seen in gastrointestinal primary sites. The genomic breakpoints on chromosome 18q21 are only slightly centromeric to the BCL2 locus, such that classic karyotyping studies cannot differentiate between the BCL2 translocation of follicular lymphoma and the MALT1 rearrangement in ENMZL; FISH technique can, however, accurately distinguish these different rearrangements. A third rare t(1;14)(p22;q32) event has been found in gastric and pulmonary ENMZL and is characterized by the BCL10-IGH genetic fusion with overexpression of BCL10 protein. Remarkably, although substantial diversity is present in the anatomic location and genetic features of ENMZL, a common molecular pathogenesis has emerged regarding these translocation events. Both BCL10 and MALT1 are cytosolic adaptor proteins involved in a ternary complex with the CARD11 protein, which together mediate antigen receptor-induced activation of the nuclear factor kappa B (NF-κB) signal transduction pathway in normal B cells. In ENMZL, the respective translocations effectively deregulate either MALT1 or BCL10 expression, inducing constitutive activation of the NF-κB pathway. In contrast, the BIRC3-MALT1 fusion protein directly stimulates NF-κB activation and concurrently mediates an anti-apoptotic effect. Altered NF-κB effects on cellular proliferation and survival are considered critical to promoting tumor cell growth. The FOXP1-IGH abnormality arises from the t(3;14)(p14.1;q32) and has been identified in a small number of ENMZL and rare large B-cell lymphomas often in extranodal (e.g., gastric) sites. FOXP1 is a forkhead transcription factor that associates with nuclear corepressor proteins or modifiers to silence transcription at target-specific genes and is an important regulator of normal B-cell development.
Overall, FISH methods are best suited to identify the common translocations in ENMZL. MALT1 gene abnormalities derived from the t(11;18) or t(14;18) abnormalities can be ascertained by D-FISH strategies. The chimeric BIRC3-MALT1 mRNA is expressed and can also be detected by RT-PCR technique; however, owing to substantial breakpoint heterogeneity in both of these genes, one of several fusion transcript isoforms could be present in any given case of ENMZL, requiring the use of multiple PCR primers. Molecular diagnostic evaluation is not routinely sought in ENMZL, although some situations may require specific investigations. For gastric ENMZL, identification of the t(11;18)/ BIRC3-MALT1 abnormality can be of clinical importance because translocation-positive tumors do not respond to antibiotic-mediated eradication of Helicobacter pylori infection, a therapeutic option which is otherwise often successful in a significant proportion of translocation-negative cases. Small extranodal tissue biopsies involved by low-grade B-cell NHL (B-NHL) can sometimes pose a difficult differential diagnosis, and FISH studies in this setting can be helpful for subclassification. Given that the FISH probes for BCL2 and MALT1 rearrangements are non-overlapping, this approach can differentiate between these t(14;18)-related abnormalities, providing an important clinical and pathologic distinction between follicular lymphoma and ENMZL with colonization of germinal centers. Extranodal tissue specimens with suspicious lymphoid populations are often also assessed for monoclonal immunoglobulin gene rearrangements by PCR to support the diagnosis of B-cell lymphoma or to check for minimal persistence of lymphoma following treatment. Positive molecular clonality studies in these scenarios require careful clinical and morphologic correlation in light of reports describing “pseudoclonal” B-cell populations in some reactive lymphoid hyperplasias or in cases with histologically negative post-therapy biopsies showing delayed clearance of true molecular disease.
Several molecular genetic assays are becoming more frequently incorporated in the initial assessment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). FISH analysis is commonly performed to detect changes in specific chromosomes: -13 or 13q-, 11q-, +12, and 17p-. Of these, 11q- and 17p- are associated with adverse outcomes, characterized by risk of early disease progression or therapeutic failure. Trisomy 12 is considered an intermediate-risk prognostic marker, and sole abnormalities of chromosome 13 appear to connote a more indolent disease course. The significance of 11q- and 17p- is related in part to monoallelic loss of the tumor suppressor genes ATM and TP53 , respectively, in the regions of chromosomal deletion. For 17p-, which occurs in approximately 5% of untreated CLL/SLL patients, a deleterious point mutation of the remaining TP53 allele is almost always present, indicating biallelic loss of p53 function. Patients undergoing therapy or who experience a transition to more aggressive disease have an even higher prevalence of 17p-/ TP53 mutations. Patients with 17p- disease tend to progress earlier, with poor responses to purine nucleoside analog drugs, and thus require alternative treatment options. Detection of the 17p- abnormality and TP53 mutations in CLL/SLL is of critical importance clinically. Complementing FISH analysis for the 17p- abnormality (often performed as part of a CLL FISH prognostic panel), TP53 mutation testing can be accomplished using tumor DNA by PCR and Sanger sequencing of exons 4 to 9, which encompasses the DNA-binding domain.
Approximately half of patients with CLL demonstrate nucleotide alterations relative to reference germline IGH sequences, which are indicative of somatic hypermutation (SHM) following B-cell transit through the germinal center environment and antigen exposure. Extensive studies have shown that an IGH V H mutation load greater than 2% from germline is associated with significantly better outcome (designated “mutated” CLL) versus those cases with SHM 2% or less (“unmutated” CLL). Of note, a subset of CLL cases with V H 3-21 rearrangements is associated with poor outcome regardless of SHM status. Determination of SHM requires an optimized PCR assay spanning the entire leader (or 5′ FR1) through J H region of the clonal IGH rearrangement, followed by Sanger sequencing and database comparison to a reference human IGH germline sequence library (e.g., http://www.imgt.org ; https://www.ncbi.nlm.nih.gov/igblast ). Either DNA or reverse-transcribed mRNA can be used as the starting material, and the PCR strategy can employ leader region consensus primers, FR1 family-specific primers, and J H primers in a multiplex or multiple single reaction approach. Recommendations for CLL IGH V H SHM analysis have been put forth by the European Initiative in CLL (ERIC). Database analysis to determine clonal identity and SHM percentage from assay-generated sequencing data is now very robust and rapid. Newer developments utilize NGS methods for directly analyzing PCR products and identifying the exact V H DNA sequence, presence of SHM, percent mutation from germline, and the translational status of the predicted rearrangement (e.g., in-frame versus unproductive) (see Fig. e24.3 ). NGS techniques also demonstrate very high analytic sensitivity and specificity because of the ability to identify many thousands of individual B-cell rearrangements at great sequencing depth, in turn also providing a basis for ongoing MRD monitoring. Clonal identity evaluation using PCR and Sanger sequencing or possibly NGS has been recognized as a prognostically important factor in patients with CLL who experience transformation to large B-cell lymphoma (Richter transformation). Patients exhibiting the same clonal immunoglobulin gene rearrangement sequence in both the original CLL and large B-cell lymphoma (i.e., true-transformation) appear to have a significantly worse outcome compared to individuals with distinct CLL and large B-cell clones (i.e., de novo large B-cell lymphoma). Broader application of NGS methods in the study of CLL genomics has identified additional recurrent gene mutations of interest, most commonly including somatic alterations in SF3B1 , NOTCH1 , ATM , and BIRC3 . Mutations in these genes have been associated with adverse clinical behavior, although currently the optimal use of this information relative to established prognostic factors is not entirely clear in the era of new immunotherapy agents and tyrosine kinase inhibitor use (e.g., Bruton's tyrosine kinase [BTK] targeting by ibrutinib). With the increased use of small-molecule signaling pathway inhibition, acquired resistance mutations are now known to occur in target genes, including BTK and phospholipase C-gamma 2 ( PLCG2 ). The mutation hotspots are recurrent, allowing for detection by allele-specific (AS)-PCR, melting curve, or sequencing analyses in patients who become refractory to these treatments.
The diffuse large B-cell lymphomas share similar morphologic features but exhibit substantial tumor genetic heterogeneity underlying the variability in clinical outcomes observed among patients. Gene expression profiling (GEP) of DLBCL has revealed a general cell of origin (COO) dichotomy between lymphomas with “germinal center” (GCB) and non-germinal center or “activated” B-cell (ABC) signatures. GCB DLBCL has been shown to have significantly better overall survival and treatment response compared to ABC DLBCL in the era of routine chemo-immunotherapy protocols. A standard gene expression-based clinical molecular diagnostic method using a limited gene set has been difficult to reproducibly establish. Most lymphoma diagnostic practices therefore rely on immunohistochemical algorithms as a surrogate for GEP. Although immunohistochemistry provides fairly good correlations when applied rigorously, a substantial subset (nearly 20%) of cases is difficult to categorize confidently as GCB or ABC. Recent commercial developments for GEP, such as the Nanostring nCounter technology (Nanostring Technologies, Seattle, WA), appear promising for widespread diagnostic adoption to support COO classification for DLBCL and also enable GEP analysis from paraffin-embedded fixed tissues and small biopsy specimens. This platform employs hybrid capture and fluorescent reporter probes to bind to many specific target mRNA molecules directly, allowing the resolution, digital counting, and precise quantification of transcripts that define either GCB or ABC profiles. Along with improvements to COO classification methods, the genomics of DLBCL continue to be defined using NGS. Important examples include the identification of deregulated toll-like receptor (TLR) and NF-κB pathways, or aberrant (“chronic active”) B-cell receptor signaling in many ABC-type DLBCL related to mutations in MYD88 , CARD11 , or CD79A among others. Some gene mutations are also typically observed almost exclusively in GCB-type lymphomas (e.g., gain of function mutations in the epigenetic modifier EZH2 ). Somatic TP53 gene alterations are also seen at low frequency in DLBCL regardless of subtype, and this finding has been associated with poorer prognosis. Although NGS testing is currently not in widespread use for DLBCL evaluation, COO classification and comprehensive sequencing analysis of gene mutations represent complementary aspects of integrated genomic profiling for DLBCL; the COO information is directly used for assessing patient prognosis, whereas the detection of specific tumor-associated genetic abnormalities can potentially identify targets of cellular pathway deregulation for novel therapeutic interventions.
Several recurrent cytogenetic abnormalities have been identified in DLBCL, including the t(14;18)/ BCL2-IGH (20% to 30% of cases with GCB phenotype) and rearrangements of the BCL6 , MYC , and ALK genes. BCL6 is located on chromosome 3(q27) and can become deregulated by translocation to the IGH gene in a t(3;14)(q27;q32) event. However, BCL6 also becomes aberrantly activated by promoter substitutions arising from translocations with many other non-immunoglobulin genes. Overall, approximately one third of DLBCL show the presence of a BCL6 rearrangement; an even higher proportion reveal somatic mutations involving the 5′ regulatory region of BCL6 , sometimes concurrent with gene translocation. The BCL6 gene product is a critical transcription factor responsible for normal secondary follicle formation during the germinal center reaction in lymphoid tissues and for proper T-cell–dependent antibody responses on exposure to antigen. Accordingly, Bcl6 is strongly expressed by normal germinal center (GC) B cells but is not present in naive and post-germinal center B cells. BCL6 is thought to protect normal GC B cells during the affinity maturation process (i.e., somatic hypermutation) in part by downregulating pro-apoptotic stimuli elicited by physiologic DNA double-strand breakage. BCL6 is overexpressed in a subgroup of B-cell lymphomas, most notably those tumors that derive from normal GC counterparts (e.g., FL, many DLBCL, Burkitt lymphoma). BCL6 rearrangements in DLBCL can be detected using break-apart probe (BAP) FISH technique. MYC gene rearrangements occur in approximately 5% to 10% of typical centroblastic DLBCL via translocations to the IGH or light chain ( IGK , IGL ) genes, or occasionally to non-immunoglobulin genes. The presence of an MYC translocation in general confers a significantly worse outcome, although some data have suggested a less adverse prognosis for non-immunoglobulin partners in this context. Because the morphologic and immunophenotypic features of MYC -rearranged DLBCL are not otherwise distinctive, routine FISH evaluation of the MYC locus in de novo cases has been advocated, although this remains controversial. Importantly, because some classic DLBCL cases and a subset of B-cell lymphomas with high-grade (e.g., blastoid, “Burkitt-like”) morphologic features and GCB phenotype also have gene rearrangements of MYC concurrent with BCL2 and/or BCL6 (i.e., “double- or triple-hit” lymphomas), many practices combine COO classification, Myc protein expression, and a reflex FISH strategy to recognize these highly aggressive tumors at diagnosis. One such approach could involve initial screening for MYC abnormalities by BAP FISH and, if positive, D-FISH to identify the immunoglobulin partner, followed by BCL2 and/or BCL6 BAP FISH.
Burkitt lymphoma (BL) is an uncommon, yet distinctive and highly aggressive, B-cell NHL. BL is classically associated with the t(8;14)(q24;q32) cytogenetic anomaly resulting in the fusion of the MYC and IGH genes, but less commonly, MYC can be involved with translocations to the IGK and IGL immunoglobulin light chain genes on 2(p12) or 22(q11), respectively. The normal MYC gene product is a highly regulated early response transcription factor that coordinates mitogenic stimuli with nuclear responses for DNA synthesis and replication. MYC gene translocation thereby leads to marked overexpression of Myc protein and upregulation of many genes driving cell proliferation. The break-sites in MYC are variable and occur over a large genomic span. The so-called endemic (or African) type of BL is characterized by a strong association with chronic Epstein-Barr virus (EBV) infection and demonstrates a molecular pattern of MYC breakpoints far upstream of the gene and corresponding IGH disruption within the J H -region. Sporadic BL cases show genomic breaks involving the immediate 5′ region of MYC and the heavy chain switch region of IGH ; sporadic BL is less frequently EBV-positive. The observed variation in IGH locus breakpoints suggests that the translocation occurs in B cells at different maturational stages in the endemic versus sporadic types, although there is no apparent clinical relevance to this phenomenon. Recent data implicate activation-induced cytidine deaminase in the pathogenesis of a significant subset of MYC-IGH translocations. Mutations in the transcription factor gene TCF3 (a regulator of CCND3/cyclin D3) or its negative regulator ID3 are present in a substantial fraction of both sporadic and endemic variants. The definitive diagnosis of BL requires the demonstration of a solitary MYC gene rearrangement and, more specifically, the presence of a translocation to the IGH , IGK , or IGL loci in the correct morphologic and immunophenotypic context. The breakpoints occurring in MYC and IGH are not amenable to standard DNA PCR methods, because of the large intervening DNA regions and lack of significant break-site clustering, but are readily detected by FISH technique. BAP FISH technique can be used to rapidly identify MYC gene disruption, in conjunction with a D-FISH approach to specifically identify the MYC-IGH or light chain gene fusion ( Fig. 24.2 ). Expedient detection of MYC gene rearrangement is critical in BL because the tumor doubling time is rapid and chemotherapy can be curative in many younger patients. As already noted, MYC gene rearrangements are also found in several other examples of aggressive B-NHL, including a minority of de novo diffuse large B-cell lymphomas, many “double-hit” high-grade B-cell lymphomas (in conjunction with BCL2 and/or BCL6 rearrangements), some large-cell or blastoid transformations of indolent B-cell lymphomas, HIV/AIDS-associated lymphomas, and in some monomorphic posttransplant lymphoproliferative disorders (PTLD).
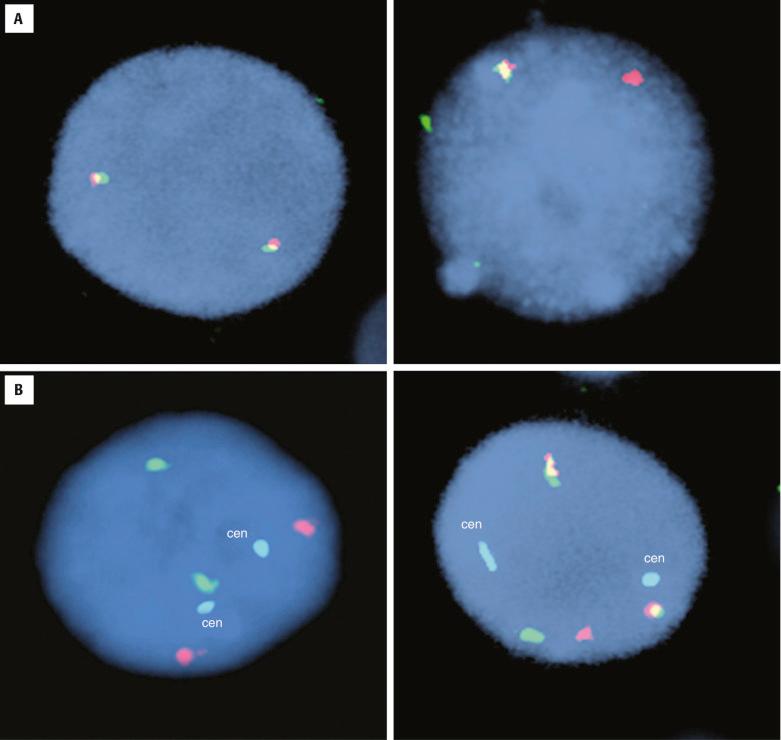
Among the peripheral T-cell lymphomas, anaplastic large-cell lymphoma (ALCL) is a distinctive pathologic entity. Recognized primarily from its distinctive morphology and uniform expression of CD30 antigen, a substantial subset of systemic ALCL is also genetically defined by the presence of anaplastic lymphoma kinase ( ALK ) gene rearrangements. The most commonly characterized abnormality is the t(2;5)(p23;q35), which couples the nucleophosmin gene ( NPM1 ) to ALK . NPM1 is a multifunctional protein involved in nuclear-cytoplasmic shuttling of ribosomal units, cell cycle regulation through stabilization of p53 protein, and maintaining proper chromosome alignment during mitosis. ALK is a tyrosine kinase protein that is not expressed in normal lymphocytes. The resultant NPM1-ALK fusion gene produces a chimeric protein causing constitutive deregulation of ALK and consequent aberrant phosphorylation of downstream signaling target proteins. Other translocations involving ALK also occur in this group of ALCL, including the t(1;2)(q25;p23)/ TPM3-ALK , t(2;3)(p23;q35)/ TFG-ALK , and inv(2)(p23q35)/ ATIC-ALK abnormalities. A key feature of ALK gene fusion products in ALCL is that the partner gene in each case dictates the intracellular distribution and activity of the aberrantly expressed ALK kinase. Determination of ALK gene rearrangement status in a case of morphologic and phenotypic ALCL is critical, since ALK -positive neoplasms are characterized by relatively favorable treatment responses and outcome. ALK gene rearrangements are best identified using a BAP FISH strategy, one advantage of which is the applicability to paraffin-embedded fixed tissue biopsies. Identification of the specific involved partner gene or chromosome is not necessary, as no particular prognostic significance has been ascribed to the different translocation variants. The technically simple application of ALK immunohistochemistry largely obviates the need for molecular cytogenetic evaluation in many cases, and the cellular pattern of ALK distribution can provide some information concerning the nature of the ALK translocation.
The group of ALK rearrangement-negative ALCL has also become better defined and now includes two subsets characterized by chromosomal rearrangements of 6p25.3 involving the locus encompassing the dual specificity phosphatase ( DUSP22 ) and interferon regulatory factor-4 ( IRF4 ) genes, and a small group of tumors with rearrangements of the p53 family TP63 gene locus on chromosome 3q28. ALK -negative ALCL with DUSP22 rearrangements are associated with a relatively better prognosis compared with CD30-positive peripheral T-cell lymphomas generally, whereas TP63 rearranged cases have a very aggressive course. Both of these genetic abnormalities are best identified by interphase FISH strategies. A subset of primary cutaneous ALCL also has rearrangements of the DUSP22/IRF4 locus. Of note, rare cases of CD30-negative large B-cell lymphoma with an unusual immunoblastic (plasmablastic) morphology and cytoplasmic IgA positivity are described with rearrangements of the ALK gene arising from t(2;17)(p23;q23)/ CLTC-ALK or, occasionally, t(2;5)(p23;q35)/ NPM1-ALK abnormalities. The pattern of ALK protein expression in CLTC-ALK –positive B-cell tumors shows a distinctive punctate membranous distribution reflecting localization in clathrin-coated pits.
Although the genomic pathology of peripheral T-cell lymphoma (PTCL) remains incompletely understood, some entities, in addition to ALCL, have become more distinctly defined. The group of nodal T-cell lymphomas derived from T-follicular helper (TFH) cells is one category, which include angioimmunoblastic T-cell lymphoma (AITL). Certain translocations, such as the ITK-SYK and CTLA4-CD28 gene fusions, have been associated with this group, although the latter abnormality has also been reported in other PTCL subtypes and extranodal NK/T-cell lymphomas. Detection of the ITK-SYK fusion is accomplished by specific D-FISH analysis, and both FISH and RT-PCR techniques can detect the CTLA4-CD28 anomaly. These gene fusions may represent therapeutic targets with the goal of blocking aberrant intracellular and T-cell receptor signaling, respectively. More extensive analysis by NGS methodology has disclosed recurrent gene mutations involving IDH2 , TET2 , RHOA , DNMT3A , and CD28 , among others, in the TFH category. IDH2 R172 mutations, for example, appear to define a unique subset of AITL. Molecular diagnostic evaluation of TFH lymphomas typically involves the supportive assessment of clonal T-cell receptor gene rearrangements when indicated; however, with the recognition of recurrent gene mutations involving metabolic, cell division, and epigenetic and immune processes, focused NGS panels may represent an attractive approach to identify alterations that may be amenable to rational small-molecule inhibitor or immunologic therapies. Despite advances in ALCL and TFH categories, the large group of PTCL unspecified remains genetically complex, and further insights from gene expression profiling and NGS mutation profiling will be central to improving the classification and outcome for these patients.
T-cell prolymphocytic leukemia (T-PLL) is characterized by rearrangements of the TCL1A gene. In 80% of T-PLL, TCL1A is altered and abnormally activated by an inv14(q11q32) or t(14;14)(q11;q32) event. In either case, the TCL1A proto-oncogene is brought in proximity to the T-cell receptor alpha locus ( TRA ) on 14(q11). This pathogenic process leads to overexpression of TCL1A, a transcription factor that is not normally present in mature T cells. TCL1A gene rearrangement is also not observed in other types of peripheral T-cell lymphomas, constituting a relatively specific genetic marker for T-PLL. FISH analysis can detect rearrangements of this gene arising from either the inversion or translocation anomalies. Immunohistochemistry can also be used to identify overexpression of the protein. Of note, a small group of T-PLL demonstrates intact TCL1A alleles but instead harbor the alternate translocation events t(X;14)(q28;q11) or t(X;7)(q28;q35), which involve fusion of a TCL1A -related gene MTCP1 , to either the TRA or T-cell receptor beta ( TRB ) loci, respectively.
Recent data have identified deregulation of the JAK/STAT pathway, with the finding of mutations involving STAT3 (in approximately one third of cases) and less frequently STAT5B (<5% of cases) in T-LGL disease. STAT3 gene mutations are also observed in a subset of chronic lymphopoliferative disorders of natural killer (NK) cells. Mutations in these genes are considered activating and can be detected by PCR and sequencing or by target allele-specific PCR methods. Given the difficulty in resolving a reactive subset-restricted T-LGL expansion from true chronic leukemia in many patients, identification of these gene mutations can be a valuable molecular diagnostic adjunct to support the latter diagnosis.
The molecular genetics of B-cell lymphoblastic leukemia (precursor B-cell acute lymphoblastic leukemia, B-LBL) represent a paradigm for linking tumor genetics with clinical outcomes, especially in the pediatric population. In childhood B-LBL, a wide spectrum of genomic alterations has been identified, including numeric and structural chromosome alterations, activating chimeric kinase gene fusions, gene amplifications, and pathogenic gene mutations or deletions. These events have distinct prognostic value and help to classify patients according to relapse risk (e.g., standard, low, or high risk) and in turn provide the basis of current risk-adjusted therapy ( Table 24.3 ). Some of the same genetic changes are also found in a subset of adult patients with B-LBL, whereas others are exclusive to childhood presentations. This section is primarily focused on the utility of cytogenetic and molecular diagnostic assessment of B-LBL. Genetic abnormalities in T-cell lymphoblastic leukemia/lymphoma (T-LBL) continue to become better defined and include translocations of HOX genes with the T-cell receptor gene loci, mutations of NOTCH1 , PHF6 , or JAK1 genes, TAL1 oncogene activation, and gene fusions involving ABL1 . Cases of early T-cell precursor (ETP) acute lymphoblastic leukemia have been characterized by gene mutations involving cell signaling (e.g., RAS , FLT3 , JAK1 / 3 ), transcription factors ( RUNX1 , ETV6 , etc.), and epigenetic modification (e.g., EZH2 , SUZ12 ) that surprisingly resemble abnormalities observed in myeloid malignancies. Application of molecular diagnostic testing is currently less frequently applied to T-LBL beyond FISH analysis for specific genomic rearrangements and is therefore not further detailed in this section.
| FAVORABLE PROGNOSIS ABNORMALITIES | ||
|---|---|---|
| Genetic Abnormality | Molecular Diagnostic Detection | Notes |
| t(12;21)/ ETV6-RUNX1 | FISH, RT-PCR | 20% of childhood B-LBL, rare in adult patients |
| Hyperdiploid chromosome content (>52) with trisomies of 4, 10, and 17 | Cytogenetics, DNA index (flow cytometry), and FISH | 25% to 30% childhood B-LBL |
| UNFAVORABLE PROGNOSIS ABNORMALITIES | ||
| Genetic Abnormality | Molecular Diagnostic Detection | Notes |
| Hypodiploid chromosome content (<44) | Cytogenetics, DNA index (flow cytometry), and FISH | Rare (<5%) childhood B-LBL |
| t(9;22)/ BCR-ABL1 | Cytogenetics, FISH (RT-PCR) |
|
| iAMP21 | FISH chromosome microarray (A-CGH, SNP-A) |
|
| KMT2A ( MLL ) rearrangements | Cytogenetics, FISH (RT-PCR) | Numerous KMT2A partner genes preclude efficient assessment by PCR methods |
| “Ph-like” genetic abnormalities | Multimodal approach including next-generation sequencing (NGS), FISH, gene expression profiling, chromosome microarray, RT-PCR |
|
| Minimal residual disease (MRD) positivity | Flow cytometry, leukemia-specific immunoglobulin and T-cell receptor gene rearrangements detected by clone-specific PCR or NGS |
|
Numeric chromosome aneuploidies and a set of recurrent translocations are identified in approximately half of pediatric patients with B-LBL. A hyperdiploid chromosome complement is present in nearly one third of pediatric B-LBL at diagnosis; this is defined as a tumor chromosome number greater than 52 associated with trisomies of chromosomes 4, 10, and 17, and these patients have a “good” risk profile with a favorable long-term outlook. Hyperdiploid cases can be identified by cytogenetics or a combination of flow cytometric assessment of relative DNA content analysis of lymphoblast cell nuclei (DNA index >1.16) and targeted FISH probe analysis. Conversely, the presence of a hypodiploid chromosome number (<44) defines a rare group of individuals with aggressive disease and early treatment failure. These patients may be considered as early candidates for allogeneic stem cell transplantation. Tumor hypodiploidy can similarly be ascertained by standard cytogenetics and FISH analysis, or it can be inferred from flow cytometric DNA index. An intrachromosomal amplification of chromosome 21 (“iAMP21”) has also recently been associated with poor outcome, arising from complex break-fusion events (chromothripsis) mainly involving the long (q) arm and leading to amplification of transcription factor genes such as RUNX1 and ERG . Cases of iAMP21 can be identified by FISH analysis of specific loci (e.g., RUNX1 ) or chromosome array (e.g., SNP-A or A-CGH) to detect the variable copy number alterations.
In approximately 25% of pediatric B-LBL, common recurrent chromosome translocations are identified, including the t(12;21)(p13;q22)/ ETV6-RUNX1 , t(9;22)(p34;q11)/ BCR-ABL1 , t(1;19)(q23;p13)/ TCF3 - PBX1 , and rearrangements of the KMT2A ( MLL ) gene. The t(12;21)/ ETV6-RUNX1 fusion is the most common recurrent gene fusion in pediatric B-LBL (20% of cases), although it is very rarely identified in adult patients. ETV6-RUNX1 –positive patients have an excellent clinical outcome following chemotherapy, similar to the group with chromosome hyperdiploidy and associated “good prognosis” trisomies. The occurrence of late relapses in ETV6-RUNX1 B-LBL is a concern for a subset of these individuals, although secondary treatment responses are often successful. The ETV6 gene, which encodes an Ets family transcription factor, is joined with RUNX1 to form the chimeric fusion gene on chromosome 12p. The hybrid ETV6-RUNX1 protein disrupts the normal function of core binding factor (CBF) transcriptional activity and, similar to the RUNX1-RUNX1T1 in acute myeloid leukemia (AML), this leads to profound alterations affecting progenitor cell proliferation and normal differentiation. In addition, a large proportion of ETV6-RUNX1 lymphoblastic leukemias also have deletions of the remaining ETV6 allele on 12p, indicating that complete inactivation of ETV6 function may also be pathogenically important. In the majority of t(12;21)/ ETV6-RUNX1 –positive B-LBL, the translocation is cytogenetically cryptic, requiring either RT-PCR or FISH molecular methods for detection. Breakpoints in the ETV6 gene are generally invariant within intron 5; however, the RUNX1 gene can show heterogeneity both in genomic break-site location, as well as by alternative splicing of RUNX1 exons in the chimeric mRNA. This situation leads to as few as one and potentially as many as four ETV6-RUNX1 fusion transcripts that can be amplified and detected by RT-PCR technique in any given patient; the most frequent types, present in nearly all cases, involve fusion of ETV6 exon 5 with exons 2 or 3 of RUNX1. FISH technique also readily detects the ETV6-RUNX1 translocation in pediatric B-LBL, and this approach further enables the simultaneous identification of an accompanying deletion of the non-translocated ETV6 allele, if present.
The t(9;22)(q34;q11)/ BCR-ABL1 abnormality (Philadelphia, or Ph, chromosome) is present in 3% of B-LBL in children and 20% to 25% of adult patients and is associated with a very poor prognosis typified by primary chemoresistance and high relapse risk. Approximately 80% to 90% of pediatric and two thirds of adult Ph-positive B-LBL produce a BCR-ABL1 fusion mRNA transcript termed e1-a2, resulting in a 190-kD oncoprotein. Remaining cases are associated with a p210 protein and corresponding e13-a2 or e14-a2 (formerly b2-a2 or b3-a2) BCR-ABL1 transcripts. The chimeric BCR-ABL1 protein alters the normal cellular localization and regulation of the ABL1 tyrosine kinase and renders it constitutively active, leading to increased cellular proliferation. This oncoprotein is readily transforming in in vivo transduction models of leukemia, but its phenotypic outcomes as related to acute versus chronic disease are dependent on the hematopoietic cell type context (i.e., stem cell versus committed lymphoid progenitor). Molecular details of the BCR-ABL1 fusion gene are discussed in more detail in the section on chronic myeloid leukemia (CML) and illustrated in Fig. 24.3 ; the BCR-ABL1 abnormality can be readily identified by cytogenetics, FISH, or RT-PCR.
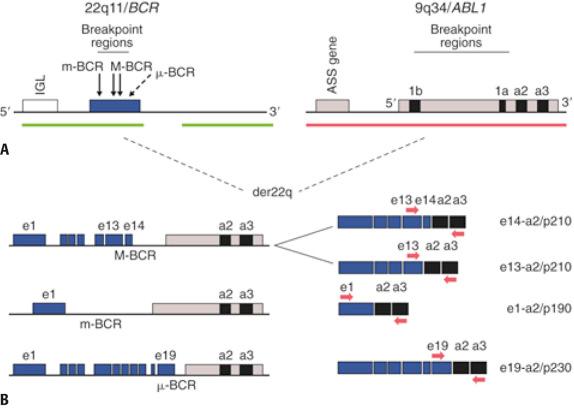
A small proportion of pediatric B-lineage B-LBL, most commonly with a “pre-B” cell (cytoplasmic IgM positive) immunophenotype, harbor an unbalanced t(1;19)(q23;p13) with the resultant formation of a chimeric TCF3-PBX1 oncogene (the TCF3 gene is also known as E2A ). This abnormality joins elements of two disparate transcription factors to create a novel leukemogenic TCF3-PBX1 protein. TCF3 is an important mediator of normal lymphoid cell and myocyte development, whereas PBX1 is a DNA binding protein that is not expressed in normal lymphocytes. Leukemia patients with this translocation fusion gene have been linked to more aggressive disease biology, although optimized therapeutic management has now improved the clinical outcome in most cases. A single chimeric TCF3-PBX1 mRNA is produced in nearly all cases, and this transcript can be readily detected by RT-PCR analysis; FISH technique can also be employed to detect the genomic abnormality. Rare occurrences of a balanced t(1;19)(q23;p13) also occur in B-LBL and do not involve the TCF3 and PBX1 genes but rather create novel DAZAP1-MEF2D and reciprocal MEF2D-DAZAP1 gene fusions associated with poor outcome.
The M ixed L ineage L eukemia ( MLL ) gene, now designated KMT2A , is located on chromosome 11(q23) and is abnormally rearranged in approximately 5% of childhood leukemias, including the majority of cases occurring in infants less than 1 year of age. The KMT2A gene product encodes histone-lysine N -methyltransferase 2A (MLL protein), which differentially regulates a set of homeobox, or HOX , genes that are variably responsible for coordinated skeletal development during embryogenesis and, significantly, normal post-fetal hematopoietic progenitor cell development. In B-LBL, KMT2A becomes juxtaposed with a different gene locus via chromosome translocation, resulting in the formation of a chimeric gene product with concomitant disruption of normal MLL protein function. Infantile acute leukemias with KMT2A gene rearrangements present in a clinically aggressive fashion with a dismal prognosis, particularly when associated with a poor therapeutic steroid response. Abnormal MLL fusion proteins can promote leukemogenesis by aberrantly deregulating certain HOX gene subsets in hematopoietic stem cells. The central role of KMT2A in this neoplastic process is underscored by the numerous (>50) described reciprocal pairings at other “partner” genetic loci. Of these, the t(4;11)(q21;q23) is most commonly encountered in infantile B-LBL, resulting in the formation of the KMT2A-AFF1 fusion. In this event, the KMT2A gene exhibits substantial genomic breakpoint heterogeneity encompassing a large region containing exons 5 to 11. Likewise, the AFF1 gene (also known as AF4 ) also shows variability of breakpoint location among B-LBL cases. A second translocation, the t(11;19)/ MLLT1 (or ENL )- KMT2A abnormality, is also encountered in infant B-LBL, as well as lymphoblastic and acute myeloid leukemias in older children and adults. Although one fusion transcript type is associated with any given leukemic tumor, the propensity for many possible chimeric transcripts arising from KMT2A gene break-fusion events coupled with the large number of potential rearranging partner genes in MLL translocations creates significant challenges for detection. Multiplex RT-PCR approaches have been designed to identify many different KMT2A gene fusions using a single platform; however, comprehensive molecular evaluation of this gene in childhood leukemias by PCR methods remains problematic owing to both biologic and technical complexity, thus limiting clinical sensitivity. FISH based on a BAP probe strategy (with probes spanning either side of the breakpoint cluster region in the KMT2A gene) is very powerful for the rapid identification of translocations. This strategy is diagnostically sensitive for identifying KMT2A rearrangements regardless of the translocation partner; D-FISH probe strategies can then be employed to specifically define the more common translocation variants. FISH analysis also discriminates between true 11(q23)/ KMT2A locus rearrangements and other genetic recombinations not involving the gene but clustering within the 11(q22;q25) region; this distinction may be difficult to discern by routine cytogenetic analysis. On occasion, distal deletions of the KMT2A locus may accompany a true gene translocation event with a resulting “deletion only” signal pattern suggested by BAP FISH technique; in these situations, confirmation of an abnormal KMT2A gene fusion can be obtained by targeted D-FISH or possibly RT-PCR analysis.
Further underscoring the importance of MLL dysregulation in leukemogenesis is the finding of KMT2A translocations in the subgroup of therapy-related, or “secondary” AML following treatment with DNA topoisomerase II inhibiting agents (e.g., etoposide, daunorubicin). Therapy-related AML is associated with a very poor prognosis, and remarkably, the gene breakage sites situated in the 3′ region of the KMT2A breakpoint cluster overlap with those of de novo infant KMT2A -rearranged B-LBL. Several studies have examined the underlying basis for greater genetic fragility in this genomic region, and the role of in utero exposure to exogenous substances that can potentiate KMT2A gene breakage and translocation, leading to rapid development of B-LBL. The concept of leukemia initiating in the prenatal period is supported by intriguing investigations “backtracking” identical KMT2A gene rearrangements in individual patient leukemias to their corresponding neonatal blood samples. This finding has similarly been demonstrated in older children with ETV6-RUNX1 B-LBL and now other types of ALL and AML in childhood. However, the latency period to presentation differs among subtypes of genetically defined B-LBL, suggesting that promoting mutations or environmental influences play an important role in leukemogenesis. In addition, healthy newborn or umbilical cord blood population screening studies have shown an appreciable prevalence (~0.1% to 1%) of leukemia-associated translocation events, albeit at very low quantitative levels (<10 −4 ); these data again point to the requirement for cooperating oncogenic mutations and a conducive background in the generation of overt leukemia. Nevertheless, the realization that at least some childhood leukemias have origins prenatally has spurred interest in identifying risk-modifying factors. In this regard, recent evidence shows that low penetrance population genetic variations in some genes (e.g., IKZF1 ) may increase the risk of developing B-LBL.
More comprehensive data from global genomic analyses have further advanced the characterization of childhood B-LBL. It is now apparent that translocation-type aberrations in B-LBL also encompass both cytokine receptor signaling deregulation and activating gene fusions involving signal transduction proteins, classically typified by BCR-ABL1 . For example, translocations of the IGH gene with the erythropoietin receptor ( EPOR ) or the type 1 cytokine receptor CRLF2 result in overexpression of these proteins, leading to autonomous receptor activity. Other gene rearrangements result in pathogenic activation tyrosine kinase genes (e.g., ABL1 , ABL2 , PDGFRB , JAK2 ). Single base mutations and small insertion/deletion (indel)-type alterations are also evident in a number of genes, including members of the Janus kinase ( JAK ) family. Importantly, many of these alterations affecting cell signaling and mitogenesis involve the JAK-STAT pathway, and these tumors share an overlapping gene expression signature similar to BCR-ABL1 –positive (Ph + ) B-LBL (so-called “Ph-like” B-ALL). Additional genetic deletion or mutation events in critical B-cell transcription factors, notably IKZF1 and PAX5 , are also observed in conjunction with both BCR-ABL1 –positive and “Ph-like” high-risk B-LBL. The proportion of B-LBL with Ph-like genetic changes increases with age, such that older children and young adult patients more frequently have these findings. Although typically considered a malignancy of “low mutation burden” (e.g., relative to many solid tumors), a greater degree of complexity is now apparent in B-LBL, with a spectrum of genetic changes spanning genomic instability (aneuploidy); transcription factor deregulation (effects on cell differentiation and proliferation); and aberrant kinase gene activation. This “mechanistic ontology” is useful for therapeutic management as well as risk stratification. The availability of JAK and tyrosine kinase inhibitor drugs has provided targets for rational treatment approaches in high-risk B-LBL patients, in addition to currently optimized chemotherapy or hematopoietic stem cell transplantation protocols. Although “genome-scale” NGS approaches (e.g., exome and RNA transcriptome sequencing) are often utilized in clinical research studies, efficient and cost-effective application of such methods in the routine molecular diagnostic setting is not optimal at present for clinical purposes. Conversely, comprehensive detection of this panoply of genetic aberrations in B-LBL is difficult using standard molecular diagnostic methods and instead requires a multimodal strategy including targeted NGS (for gene mutations, small indels, and specific translocations), FISH (for some translocations), RT-PCR (for selected expressed gene fusion mRNAs), chromosome arrays (for larger deletion/duplication analysis), and cytogenetics. A comprehensive but directed diagnostic testing approach can achieve a high rate of detection of most “favorable risk” and “high-risk” tumor genetic factors, including the more heterogeneous group of Ph-like cases. Nevertheless, a minority of these patients may still be missed in targeted analyses, and Ph-like B-LBL can possibly be more sensitively detected by a gene expression classifier method, as suggested in some pediatric hematology studies.
The identification of prognostic predictive molecular markers in pediatric B-LBL has significantly augmented the development of refined risk-adapted therapy practices for lower risk and particularly high-risk patients. This approach is based on the premise of achieving maximum cure rate while minimizing toxic effects and late sequelae such as treatment-related neoplasms. For B-lineage ALL in particular, initial clinical risk assessment (e.g., age, white blood cell and blast count, extramedullary involvement) is further modified by the presence and type of tumor genetic abnormalities, as outlined above. The BCR-ABL1 chimeric gene fusion, hypodiploid karyotype, KMT2A rearrangement, Ph-like genotype or phenotype, iAMP21, IKZF1 deletion (often seen in Ph-like disease), or therapeutic induction failure each constitutes very high-risk patient subtypes. Conversely, B-ALL cases with hyperdiploidy and “favorable” trisomies, or the ETV6-RUNX1 gene fusion are considered good prognostic factors. Early genetic classification (i.e., before the end-induction treatment phase) thereby influences the chemotherapy protocol and duration for a given patient. Most large cancer groups for children utilize this strategy of tumor genetic risk stratification in slightly different formulations. Useful as these parameters have been for more appropriately stratifying patients earlier to receive optimized therapy, a significant subset of children experiences treatment failure and leukemic relapse, indicating some enduring lack of precision in the risk assessment models. Better predictive surrogate markers of biologic response and disease clearance have therefore been sought, and minimal residual disease detection has emerged as a very powerful method of post-therapeutic prediction, contributing to a more “individualized” approach of risk evaluation. Many large clinical studies have now confirmed the utility of MRD in determining relapse potential in childhood B- and T-lineage B-LBL. Levels of MRD greater than 10 −4 at the end of induction therapy have been shown to be highly correlated with risk of relapse compared to MRD-negative status, and this relationship has proved to be strongly independent of nearly all currently applied clinical and cytogenetic/molecular genetic factors in multivariate analyses. Targets for molecular MRD measurements include clone-specific antigen receptor gene rearrangements (e.g., IGH , IGK , TRG , TRB ), tumor-specific single gene mutations, and chimeric fusion gene mRNA transcripts.
The clonal nature of immunoglobulin or T-cell receptor gene rearrangements confers a unique and ubiquitous marker for MRD assessment in lymphoid malignancies relative to leukemia-specific fusion gene transcripts, which are present in less than one third of cases. Notably in B-LBL, “illegitimate” or cross-lineage gene rearrangements also occur frequently at the T-cell receptor genes, such that most B-lineage tumors have at least one and as many as two to three unique gene rearrangements available for MRD monitoring. The highly specific nature of rearranged antigen receptor Vn(D)nJ regions in lymphoblastic leukemia cells can be amplified, then sequenced for the design and synthesis of tumor-specific PCR primers and probes for use in targeted quantitative PCR analysis following therapy induction. Poor technical reproducibility and an overall labor-intensive approach are significant limiting factors to routine implementation, despite the attractiveness of the actual tumor markers. Recent developments using NGS technology for deep sequencing of B-cell and T-cell receptor genes have indicated an alternative and very powerful means of detecting tumor-specific clones at very high analytic sensitivity (10 −6 ). Using a comprehensive consensus PCR primer strategy, a library pool of rearranged antigen receptor gene products can be prepared for high-throughput NGS using Illumina or Ion Torrent platforms. Relying on the highly specific nature of V-(D)-J segmental recombination and especially the unique CDR3 region sequence, the presence of tumor DNA can be directly and specifically queried following therapy in a single rapid assay, as long as the initial clonal rearrangement(s) of the tumor cells were determined at diagnosis. The development of commercial platforms (e.g., Lymphotrack, Invivoscribe, San Diego, CA; clonoSEQ, Adaptive Biotechnologies, Seattle, WA) has greatly facilitated clinical molecular laboratory access to this technology, as well as the related bioinformatics software to effectively analyze the large amount of sequence data generated. Other investigators have established the use of flow cytometric evaluation of MRD, based on quantitative or qualitative alterations in antigen expression by the leukemic blasts. Flow cytometric protocols can achieve comparable analytic sensitivity to molecular methods and have a potential advantage of being more easily standardized between laboratories, although at the extremes of analytic sensitivity (e.g., 10 −5 to 10 −6 ), NGS methods appear to be more reliable. Whether a molecular- or cell marker–based technology is employed, the profound effect of early MRD detection on relapse prediction is evident, and childhood leukemia cooperative groups have begun to incorporate MRD data as a routine component of risk assessment, enabling higher risk MRD-positive patients to be rapidly stratified into more intensive therapy arms. Timing of MRD assessment is critical for the derivation of meaningful prognostic data in childhood ALL. In general, the end of induction therapy is a significant and highly informative time-point, although ongoing serial measurements can further increase the predictive value. End-induction MRD status, in conjunction with “rapid” versus “slow” blast clearance from the bone marrow has been described as further improving relapse risk prediction for individual patients.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here