Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
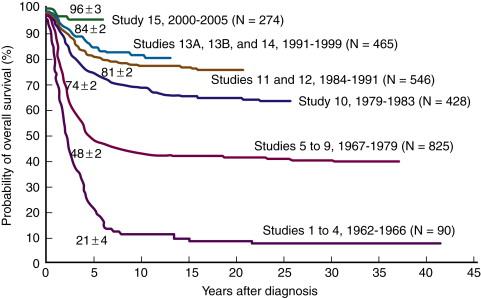
Acute lymphoblastic leukemia (ALL) is a sharply contrasting disease in the pediatric and adult populations. In children, it is both the most common leukemia and the most common malignancy. Childhood ALL has been emblematic of medical progress, with steady improvement over the past 50 years and a current 5-year event-free survival (EFS) rate of over 85% ( Figure 26-1 ). In contrast, in adults, ALL constitutes a minority of the leukemias and a tiny fraction of all malignancies. In addition, the long-term disease-free survival for adults with ALL is poor, in the range of 30% to 40%. An understanding of molecular genetics is playing an increasingly important role in optimizing therapy in pediatric ALL, defining distinct prognostic subgroups for which therapy can be tailored so that low-risk patients are spared unnecessary toxicity, while high-risk patients receive the intensive therapy most likely to effect a cure. Furthermore, some of the discrepancy between cure rates in childhood and adult ALL can be explained by noting that positive prognostic genetic lesions tend to be more common in children, whereas genetic lesions that are associated with more resistant disease tend to be more prevalent in older patients. New insights into the molecular biology of ALL may both increase the ability to more accurately risk stratify patients and identify targets for novel therapeutics that could increase survival and decrease toxicity in all patients with ALL.
The cornerstone of ALL therapy is stratification of patients into different risk groups based on a combination of clinical, laboratory, and molecular features, so that the type and intensity of therapy may be tailored appropriately. For example, in children, three major factors are used to assign risk-based therapy at diagnosis. First is the Rome-National Cancer Institute (NCI) risk status, which defines high-risk ALL as those with an age less than 1 year or more than 9.99 years, and/or initial white blood cell (WBC) count greater than 50,000/μL. In adults, older age and higher WBC are associated with increasing risk. Second is early response to treatment. Third, and increasingly important, is molecular genetic alterations of the tumor cells.
Cytogenetic analysis using karyotypic characterization and fluorescence in situ hybridization (FISH) is a crucial element in diagnostic evaluation. Translocations are relatively common in ALL and generally cause two types of events: a proto-oncogene may be brought into the proximity of a T-cell receptor or immunoglobulin locus, causing its overexpression; or the genes at the breakpoints of the rearranged chromosomes, often transcription factors, may fuse to form a new, chimeric protein that is oncogenic because of altered properties and/or expression patterns.
Although ALL has traditionally been defined by recurrent karyotypic changes, about one quarter of patients lack characteristic chromosomal rearrangements. In addition, none of the known rearrangements has been shown to be both necessary and sufficient for leukemogenic transformation. Recent technological advances in high-resolution genomic sequencing coupled with active large-scale support by initiatives such as the NCI TARGET (Therapeutically Applicable Research to Generate Effective Treatments; https://ocg.cancer.gov/programs/target ) project have led to large sample surveys identifying more than 30 validated recurring tumor-specific somatic subchromosomal mutations in a majority of patients with ALL. Among these, most occur in key signaling pathways such as B-cell development/differentiation, the TP53/RB tumor suppressor pathway, and Ras and Janus kinase signaling. These advances, along with microarray-based analyses of gene expression and epigenetic profiles, have elucidated important basic science aspects of leukemogenesis ( Table 26-1 ). In addition, some of these findings have already translated into several promising novel therapies.
| Chromosomal Abnormality | Genes Involved | Pediatric (%) | Adult (%) | Mechanism of Transformation | Prognostic Impact |
|---|---|---|---|---|---|
| Pre-B ALL | |||||
| t(12;21)(p13;q22) | ETV6-RUNX1 | 25 | 2 | Represses AML1 function as transcriptional activator | Favorable |
| t(1;19)(q23;p13) | E2A-PBX1 | 6 | 3 | Promotes PBX1 function as transcriptional activator | Formerly poor; negated by intensive therapy |
| t(17;19)(q23;p13) | E2A-HLF | <1 | <1 | Repression of E2A and antiapoptotic effects (?) | Poor |
| t(9;22)(q34;q11) | BCR-ABL | 3 | 25 | Increased tyrosine kinase activity | Poor; partially ameliorated by imatinib (Gleevec) |
| t(4;11)(q21;q23) | MLL-AF4 | 8 | 10 | Disruption of Hox expression patterns | Poor |
| iAMP21 | RUNX1, mir-802, and DSCR | 2 | Initiates development of secondary lesions (in, e.g., IKZF1 , ETV6 , RB1 ) | Poor | |
| PAX5 mutation | PAX5 | 30 | 12 | Disrupts normal B-cell maturation process | No known significance |
| EBF1 mutation | EBF1 | 4 | 1.5 | Disrupts normal B-cell development/decreases PAX5 expression | No known significance |
| IKZF1 mutation | Ikaros | 80 (Ph + ) 7 (Ph − ) |
63 (Ph + ) 19 (Ph − ) |
Activation of JAK/STAT and increased expression of Bcl-xL | Poor |
| CRLF2 overexpression | CRLF2 | 8 (15% in high risk and 50% of DS-ALL) | 10-15 | Upregulation of JAK/STAT and PI3K/mTOR pathways | No significance in DS; controversial in SR, poor in HR |
| JAK mutation | JAK1 , JAK2 , JAK3 | 10 in B cell (mostly JAK2) | 17 in T cell (mostly JAK1) | Constitutive activation of the JAK tyrosine kinase | Poor |
| CREBBP mutation (relapsed ALL) | CREBBP | 19 | N/A | Impaired transcriptional regulation of CREBBP targets | No significance |
| T ALL | |||||
| NOTCH mutation | NOTCH1 | >50 | >50 | Constitutively activated NOTCH1 causing activation of downstream targets (e.g., c-Myc, cyclin D, and NFκB) | Improved |
| t(1;14)(p34;q11) | TAL1, TCRα/δ | 7 | 12 | Repression of E2A transcriptional activity | Poor vs. no prognostic significance |
| t(11;14)(p15;q11) | LMO1 , TCRα/δ | <1 | <1 | LMO1 activation; repression of E2A transcriptional activity | Unknown |
| t(11;14)(p13;q11) | LMO2 , TCRα/δ | 1 | <1 | LMO2 activation; repression of E2A transcriptional activity | Unknown |
| t(10;14)(q24;q11); t(7;10)(q35;q24) |
HOX11 , TCRδ , or TCRβ |
0.7 | 8 | Dysregulated expression of intact HOX11 | Favorable if intensive therapy |
| t(5;14)(q35;q32); t(5;14)(q35;q11) | HOX11L2 , BCL11B , or TCRδ | 2.5 | 1 | HOX11L2 activation | Poor vs. no prognostic significance |
| t(8;14)(q24;q11) | MYC , TCR | <1 | <1 | MYC overexpression | Unknown |
| t(7;19)(q35;p13) | LYL1 , TCRβ | 1.5 | 2.5 | Unknown | |
| Mature B ALL | |||||
| t(8;14)(q24;q32) | MYC , IgH | 2 <1 <1 |
4 <1 <1 |
MYC overexpression | |
| t(8;22)(q24;q11) | MYC , Igλ | ||||
| t(2;8)(p12;q24) | Igκ , MYC | ||||
Ploidy can be assessed either by chromosome number or by flow cytometry using the DNA index (DI), the ratio of fluorescence in leukemic blasts compared to normal cells. Normal diploid cells have 46 chromosomes and a DI of 1.0, hyperdiploid cells have higher values, and hypodiploid cells lower. Hyperdiploidy is further classified as “low” and “high” (greater than 50 chromosomes).
Hypodiploid cases constitute approximately 6% of pediatric and 2% to 8% of adult ALL. Those with fewer than 45 chromosomes have significantly worse outcome, with the worst outcome occurring in near-haploid cases (24 to 28 chromosomes). The adverse prognostic impact in adults is somewhat weaker. “Pseudodiploid” cases, with normal chromosome number but structural abnormalities, also do relatively poorly. Rare cases with near triploidy or near tetraploidy (more than 80 chromosomes) have traditionally been associated with poor outcome. However, more recent reports analyzed from studies using modern therapies refute this claim, showing that these lesions should be classified prognostically as neutral or even favorable.
Hyperdiploidy occurs in about 35% of pediatric and 25% of adult ALL cases. In children, ALL with more than 50 chromosomes, or simply the simultaneous trisomies of 4 and 10 (and less importantly 17), is an independent positive prognostic indicator. In adults, the prognostic implications are less clear. Although the biologic basis of hyperdiploidy is poorly defined, it often co-occurs with other favorable risk factors as well as Ras and FLT3 mutations and FHIT hypermethylation.
The ETV6-RUNX1 ( TEL-AML1 ) fusion protein formed by the t(12;21)(p13;q22) translocation is the most frequent abnormality in children (25%), whereas it is much rarer in adults (2%). ETV6 (ETS variant gene 6) is also known as TEL (translocation-ETS-leukemia). RUNX1 (runt-related transcription factor 1) is also known as AML1 or CBFA2 (core binding factor A2). In nearly all cases the translocation is cryptic, involving a region too small to be detected by karyotype.
ETV6 encodes a widely expressed nuclear protein belonging to the Ets family of transcription factors, which are involved in diverse developmental processes including the establishment of embryonic and adult hematopoiesis. A helix-loop-helix (HLH) region known as the ETS domain allows DNA binding for transcriptional regulation, and an HLH region known as the pointed domain appears to facilitate self-association ( Figure 26-2 , A ). RUNX1 is a transcription factor with highly restricted expression in hematopoietic cells and developing ganglions but that is required for transcription of several hematopoietic-specific genes, and may organize the factor complex necessary for lineage-specific transcription.
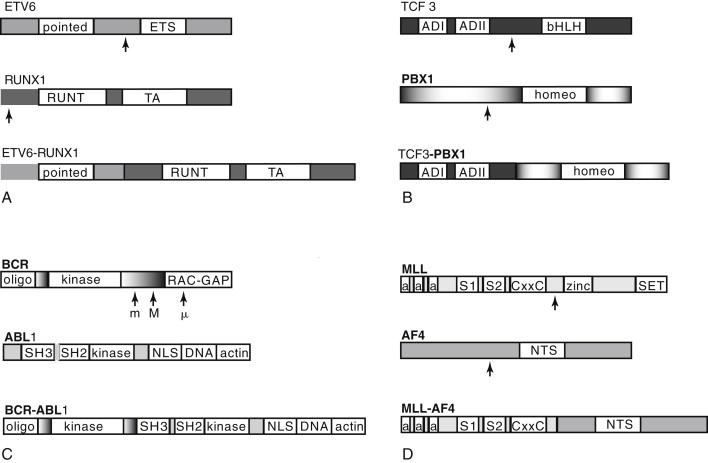
The ETV6-RUNX1 fusion protein is widely expressed because of the ETV6 promoter and converts RUNX1 from a transcriptional activator to a repressor. The exact mechanism of repression is unclear, as is the manner in which this mediates leukemogenesis. ETV6-RUNX1 overexpression causes leukemia in only a minority of mouse models, with low penetrance and prolonged latency, suggesting that additional events are crucial for full transformation. A frequent secondary event in ETV6-RUNX1 + ALL is loss of heterozygosity (LOH), deletion, or otherwise downregulated expression of the remaining normal copy of ETV6 , suggesting a potential role for ETV6 as a tumor suppressor.
ETV6-RUNX1 positivity tends to occur in children 1 to 10 years of age, and nearly exclusively in CD10 + B-precursor ALL. In the past, cases characterized by ETV6-RUNX1 + ALL had a hallmark tendency to relapse late, with excellent chemosensitivity and salvage rate. On modern treatment regimens ETV6-RUNX1 relapsed disease is extremely rare and, although survival for all subtypes of ALL in children with contemporary chemotherapy strategies has improved, ETV6-RUNX1 has retained positive prognostic significance. When relapse does occur, evidence suggests that it may represent evolution of a new leukemic clone from the preleukemic ETV6-RUNX1 + cell of origin.
The TCF3-PBX1 fusion protein, associated with the t(1;19)(q23;p13) translocation, is the second most common translocation in pediatric ALL, occurring in approximately 6% of all pre-B ALL. It is a rare (3%) and adverse feature in adults. The fusion protein combines the two activation domains of the basic helix-loop-helix (bHLH) transcription factor TCF3 (previously E2A ) on chromosome 19 with the homeobox ( HOX ) gene PBX1 (for pre-B cell homeobox 1) on chromosome 1, resulting in a strong transcriptional activator effect on PBX1 (see Figure 26-2 , B ). TCF3 is a transcriptional activator critical in lymphocyte development, as well as widely expressed and influential in diverse cellular processes. PBX1 belongs to the TALE (three amino acid loop extension) class of atypical homeodomain proteins. The homeodomain mediates both DNA-binding and HOX gene interaction.
The TCF3-PBX1 chimeric transcription factor strongly activates a subset of HOX genes normally regulated by PBX1 . The basis for its transforming ability may be reduction of wild-type TCF3 levels; aberrant activation of PBX1 targets in pre-B cells; or activation of targets not normally regulated by PBX1 that are affected by the TCF3-PBX1 fusion protein. Fusion protein overexpression in mouse models causes a variety of leukemias, although not B-lineage ALL, suggesting potent non–lineage-specific transforming activity. Unlike other ALL translocations, t(1;19) + ALL does not show evidence of in utero origin.
TCF3-PBX1 positivity often coincides with other high-risk factors. Early studies indicated an independent adverse prognostic impact, but on modern intensive pediatric regimens, survival is equivalent. The t(1;19) occurs most often as an unbalanced translocation. Cases with a balanced translocation do more poorly in some studies but not others.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here