Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Each human being’s complete genetic sequence reveals a significant amount of genetic variation. The collection of single nucleotide variations and copy number variations within an individual forms a genetic backdrop that influences how a person looks, grows, and physiologically responds to stressors such as a disease or medication. Population sampling has demonstrated that, among healthy individuals, the genetic sequence differs at around 10 million sites (out of 3.2 billion total DNA base pairs [bp]). These naturally occurring differences are called single nucleotide polymorphisms (SNPs). To be classified as an SNP, two or more versions of the nucleotide sequence must be present in at least 1% of the general population. Disease-causing nucleotide changes are relatively rare; thus the term single nucleotide polymorphism usually describes genetic variation of healthy individuals. Specific SNP-associated phenotypes are relatively trivial, such as SNPs in the trichohyalin gene (TCHH) that cause straight versus curly hair. Conversely, SNPs within the cytochrome P-450 isozyme 2D6 gene (CYP2D6) produce phenotypes with drastic modifications in an individual’s rate of opioid or antidepressant metabolism, greatly altering drug side effect profiles. , SNPs have been extremely useful in conducting genome-wide association studies (GWASs) to identify genomic regions that associate with particular phenotypes (preterm birth, preeclampsia, menopause, polycystic ovarian syndrome). A collection of SNPs that associate with a particular phenotype can be used to construct polygenic risk scores, an emerging science in predicting and preventing human disease.
In addition to individual sequence variation through SNPs, comparative genome studies between individual sequences have revealed a far more pervasive form of genetic variation, termed copy number variations (CNVs). , These are structural variants made up of relatively large DNA segments (ranging in size from 1000 to 500,000 bp or more) that can be duplicated or deleted at a given genetic locus and cumulatively affect 360 million nucleotides, or about 12% of the human genome. A CNV can be benign (no known effect on the phenotype) or pathogenic (well-documented effect on the phenotype), and a significant proportion of identified CNVs have as-yet unknown significance (may be changed to benign or pathogenic based on future data).
Thus whereas SNPs introduce genetic variation at the level of individual base substitutions, CNVs represent variation in the “dose” of a relatively large DNA segment. The collection of SNPs and CNVs influences how individuals respond to a challenge, such as an invading pathogen or ultraviolet ray exposure from the sun. Understanding genetic variation in the form of SNPs and CNVs and their biological influence can reveal predisposition toward disease, variable susceptibility to infections, and diverse responses to pharmacotherapy.
High-frequency sequence variants in the population are part of the normal backdrop of the human genome and for the most part are not thought to have functional consequences. Overt genetic pathology, in contrast, usually arises from genetic variants that disrupt the normal expression or function of one or more genes ( Table 2.1 ). Gene expression and function may be disrupted by less frequent gene variants in the coding portion of a gene, increases or decreases in the relative dose of a DNA segment (CNV), changes in the normal amount of a gene product, or sequence changes in regulatory regions that prevent the cell from normally expressing the intended gene product. The old term mutation has been replaced by variant, which can be benign, likely benign, of unknown significance, likely pathogenic, or pathogenic. Pathogenic and likely pathogenic variant designations are reserved for changes in the genetic code that lead to altered gene/protein function with clinical consequences. Pathogenicity is usually supported by the presence of the specific variant in multiple affected families, whether the variant is predicted to cause loss of function, and verification by functional studies, animal models, or both. A variant may involve changing a single nucleotide base or a larger DNA segment, in which bases are removed, duplicated, or inserted. For example, in sickle cell anemia there is only one identified disease-associated variant (G A G to G T G) in the hemoglobin gene (HBB) changing the protein’s sixth amino acid from glutamic acid to valine; alternatively, in cystic fibrosis (CF), more than 1000 disease-associated variants or alleles have been described to date in the CF transmembrane conductance regulator gene (CFTR). Both sickle cell anemia and CF are examples of single-gene disorders (also called mendelian disorders ) in which disease phenotype can be accounted for by the abnormal function of a single gene. Other human diseases or syndromes, such as autism, have a genetic basis, but the genotype-phenotype correlation is far more complex and may involve multiple genes in addition to environmental influences.
| Disorder | Risk Age | Prevalence of Disorder |
|---|---|---|
| Trisomies 21, 13, 18 | >35 years | 1/500 (0.2%) |
| Sex chromosome aneuploidies | Any | 1/1000 (0.1%) |
| Balanced chromosomal abnormalities | Any | 1/500 (0.2%) |
| Pathogenic (unbalanced) chromosomal abnormalities | Any | 1/10,000 (0.01%) |
| Mendelian disorders | Any | 1/280 (0.4%) |
| De novo variants | Any | ? |
| Pathogenic microdeletions/duplications | Any | 1/90 (1.2%) |
| Phenotypic Incidence | ||
| Structural/functional birth defects in newborns | 1/33 (3%) | |
| Autism | 1/88 (1.2%) | |
| Total prevalence of genetic pathology | 1/10 (10%) (30 million people in the United States) | |
Larger genetic alterations, which risk disruption or loss of multiple genes and their function, most commonly occur during cell division by mitosis or meiosis. A variety of chromosomal abnormalities may occur during chromosome alignment and segregation (see Chapter 1 ), leading to chromosome breaks or rearrangements or uneven distribution of chromosomes into daughter cells. Chromosome breaks and rearrangements may be genetically balanced (normal phenotype) or genetically imbalanced (producing abnormalities resulting from gain or loss of one or more genes) or may completely disrupt a critical gene at the breakpoint site on the chromosome, leading to loss of functionally relevant gene products. The molecular resolution of a regular karyotype (a technique for counting the chromosomal content of a cell under light microscopy) is above 5 megabases (Mb), which allows for detection of chromosome number changes and large structural chromosome rearrangements. In the past decade, advances in molecular cytogenetic technology, such as array comparative hybridization (also termed chromosomal microarrays ), have improved resolution for assessing cellular DNA content below 5 Mb, down to as low as 1 kilobase (kb), and revealed a new class of chromosomal syndromes caused by microdeletions and microduplications. These syndromes involve two or more contiguous genes (genes in adjacent locations on the chromosome). Table 2.2 lists some of the more commonly recognized syndromes caused by microdeletions and microduplications. The phenotypes of these conditions are the result of the absence (or duplication) of multiple contiguous (adjacent) genes within the involved region.
| Location | Genomic Disorder Associated With Deletions | Genomic Disorder Associated With Duplications | Size (Mb) |
|---|---|---|---|
| 1q21.1 | Thrombocytopenia–absent radius syndrome (TAR) region (OMIM 274000 ) | Not known | 0.347–0.357 |
| 1q21.1 | 1q21.1 microdeletion (OMIM 612474 ) | 1q21.1 microduplication (OMIM 612475 ) | 1.19 |
| 2q13 | Carrier juvenile nephronophthisis | Benign copy number variation | 0.150 |
| 3q29 | 3q29 microdeletion syndrome (OMIM 609425 ) | 3q29 microduplication syndrome (OMIM 611936 ) | 1.6 |
| 7q11.23 | Williams-Beuren syndrome (WBS) (OMIM 194050 ) | WBS duplication syndrome (OMIM 609757 ) | 1.5–1.8 |
| 15q11-q13 | Angelman syndrome (OMIM 105830 )/Prader-Willi syndrome (OMIM 176270 ) | 15q11-q13 duplication syndrome (OMIM 608636 ) | 0.50 to ∼6.0 |
| 15q13 | 15q11-q13 deletion syndrome (CHRNA7) (OMIM 612001 ) | Unclear (CHRNA7) (OMIM 612001 ) | 0.400 1.5–1.8 |
| 16p11.2 | 16p11.2 deletion syndrome (OMIM 611913 ) | 16p11.2 duplication syndrome (OMIM 611913 ) | 0.593–0.706 |
| 16p11.2-p12 | 16p11.2 deletion syndrome (OMIM 613604 ) | Pathogenic—Genoglyphix Chromosome Aberration Database (GCAD) (OMIM 23196) | 8.7 |
| 16p13.1 | 16p13.1 microdeletion predisposing to autism and/or mental retardation | 16p13.1 microduplication | 1.3 |
| 17p11.2 | Smith-Magenis syndrome (OMIM 182290 ) | Potocki-Lupski syndrome (OMIM 610883 ) | 3.7 |
| 17p12 | Neuropathy, hereditary, with liability to pressure palsies (HNPP) (OMIM 162500 ) | Charcot-Marie-Tooth disease CMT1A (OMIM 118220 ) | 1.4 |
| 17q11.2 | Neurofibromatosis type I (OMIM 613675 ) | NF1 critical region microduplication syndrome | 1.2–1.4 |
| 17q12 | 17q12 deletion syndrome (OMIM 614527 ) | 17q12 duplication syndrome (OMIM 614526 ) | 1.5 |
| 17q23 | 17q23.1-q23.2 deletion syndrome (OMIM 613355 ) | 17q23.1-q23.2 duplication syndrome (OMIM 613618 ) | 2.1 |
| 22q11.21 | DiGeorge syndrome/velocardiofacial syndrome (OMIM 188400 /192430) | 22q11.2 microduplication (OMIM 608363 ) | 1.5–3.0 |
| 22q11.23 | 22q11.2 distal microdeletion (OMIM 611867 ) | Unclear | ∼2.5 |
The focus of this chapter is to review the molecular (DNA-based) techniques available to identify disease-associated genetic variants, specifically pertaining to screening and diagnostic needs when planning pregnancy or during pregnancy. The extent and specificity of the resulting information depends on the source of genetic material, as well as the testing platform employed. Fig. 2.1 summarizes the potential sources of genetic material (DNA) for evaluation ( Fig. 2.1A ) and the currently available genetic assessments for preconceptional or prenatal screening or diagnosis ( Fig. 2.1B ).
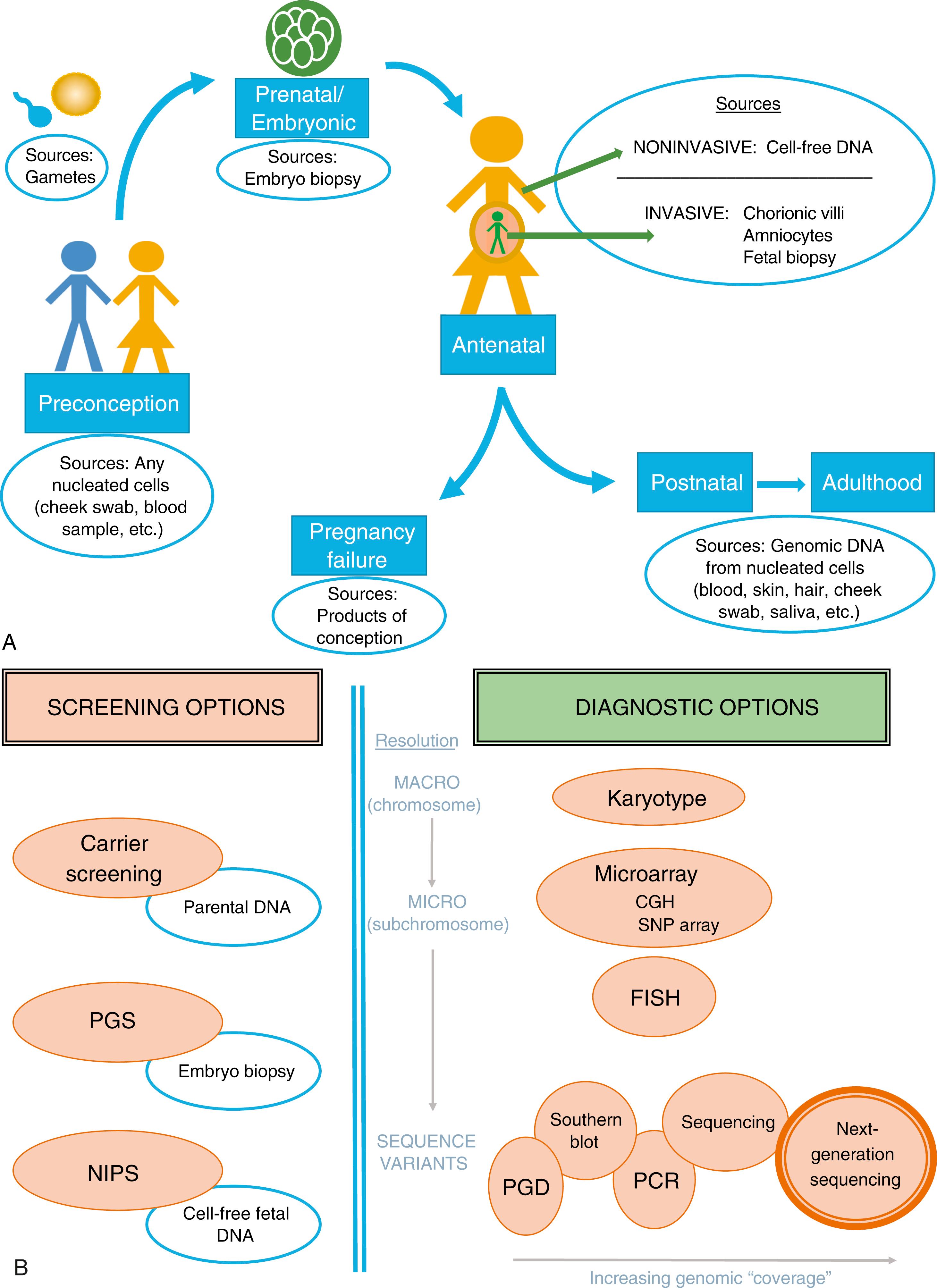
Genetic risk assessment begins with the evaluation of the biological parents. Family history and prior obstetric history may reveal indications for targeted testing, such as DNA sequencing or biochemical analysis for single-gene disorders, or parental karyotyping in the setting of recurrent pregnancy loss. Alternatively, indications to pursue screening or diagnostic testing may include increased risk of aneuploidy related to maternal age, specific populations with increased carrier frequency of certain diseases, or simply population-based screening risk. In addition, it is acceptable for any patient, regardless of risk, to choose diagnostic prenatal testing after informed consent. Prenatal diagnosis allows reproductive options or interventions before disease is clinically detected in a child. In some rare cases, such as inborn errors of metabolism, diagnosis in utero may allow the opportunity to prevent irreversible changes in early development by replacement of the affected gene product.
Certain populations have an increased frequency of specific, identifiable disease-associated genetic variants. This may occur because the population has remained relatively isolated, because many individuals in the population are descended from a few common relatives having a specific variant (founder effect), or because the carrier state has a beneficial effect on survival in a particular environment (sickle cell carrier state granting protection from malaria). Carrier screening of couples refers to genetic testing of asymptomatic individuals to determine whether they carry pathogenic genetic variants that place their pregnancy at risk for prenatal and childhood genetic disorders. Traditionally, carrier screening has been targeted to specific ethnic populations known to be at increased risk for particular disorders (ethnic-based screening). However, as the population becomes more racially and ethnically diverse, pan-ethnic screening for a panel of disorders offered to all individuals regardless of ethnicity is currently available. Patients can undergo genetic carrier screening prior to conception (preferred) or during pregnancy.
The goal of carrier screening is to provide individuals with information that will permit them to make informed reproductive decisions. Table 2.3 lists the currently recommended ethnic-based or pan-ethnic carrier screening tests that should be discussed with patients considering pregnancy or who are currently pregnant, according to the American College of Obstetricians and Gynecologists (ACOG). , Screening for spinal muscular atrophy (SMA) and CF carrier status is recommended for all patients. Additional screening may be tailored by family history, increased risk for rare diseases (consanguinity), or ethnicity. Such testing is of maximal benefit when it is part of a comprehensive screening program including patient education, genetic counseling, timely disclosure of test results to patients, and availability of invasive diagnostic testing when needed.
| Group | Genetic Disorder | Carrier Frequency | Screening Test Available | Detection Rate (%) | |
|---|---|---|---|---|---|
| ALL WOMEN considering pregnancy or currently pregnant | Spinal muscular atrophy | Caucasian, 1:35 Hispanic, 1:117 Ashkenazi Jew, 1:41 Asian, 1:53 African-American, 1:66 |
DNA variants (carrier screening requires quantitative PCR) | Caucasian: 95% Hispanic: 91% Ashkenazi Jew: 90% Asian: 93% African-American: 71% |
|
| Cystic fibrosis | Caucasian, 1:25 Hispanic, 1:58 Ashkenazi Jew, 1:24 Asian, 1:94 African-American, 1:61 |
DNA variants (>1300 disease-associated alleles identified) Most common screen is panel of 23 pan-ethnic variants |
Caucasian: 88% Hispanic: 72% Ashkenazi Jew: 94% Asian: 49% African-American: 65% |
||
| Hemoglobinopathy (includes sickle cell disease [Hgb S], α-thalassemia, and β-thalassemia) | Hgb S African-American, 1:10 Also in high frequency: Mediterranean, Middle Eastern, Southeast Asian, or West Indian descent α-Thalassemia African, 1:3 Mediterranean, 1:30 Southeast Asian, Middle Eastern, 1:20 β-Thalassemia African-American, <1:8 Asian, 1:20 Mediterranean, 1:7 |
CBC with RBC indices for all women Hemoglobin electrophoresis if ethnicity-based risk or abnormal RBC indices |
|||
| Family history of fragile X–related disorders or intellectual disability suggestive of fragile X syndrome | Fragile X syndrome (related disorders include premature ovarian insufficiency and fragile X–associated tremor/ataxia syndrome) | 1:259 in general population | DNA-based molecular analysis (Southern blot and PCR) for triplet repeat | ||
| Ashkenazi Jewish (Eastern and Central European descent; should include Jews of unknown descent) | Tay-Sachs disease (Note: screening also recommended if patient is French Canadian or Cajun descent ) | Ashkenazi Jew, 1:30 French Canadian, Cajun, 1:30 to 1:50 Non-Jewish groups, 1:300 |
Biochemical: hexosaminidase A level | 98% | |
| Canavan disease | 1:41 | DNA variants | 97% | ||
| Familial dysautonomia | 1:31 | DNA variants | 99% | ||
| Additional autosomal recessive conditions for which screening should be considered in patients of Ashkenazi Jewish descent | Fanconi anemia group C | 1:89 | DNA variants | 99% | |
| Bloom syndrome | 1:100 | DNA variants | 95%–97% | ||
| Niemann-Pick disease type A | 1:90 | DNA variants | 95% | ||
| Mucolipidosis type IV | 1:127 | DNA variants | 95% | ||
| Gaucher disease | 1:15 | DNA variants | 95% | ||
| Familial hyperinsulinism | 1:52 | DNA variants | |||
| Glycogen storage disease type IA | 1:71 | DNA variants | |||
| Joubert syndrome | 1:92 | DNA variants | |||
| Maple syrup urine disease type 1B | 1:81 | DNA variants | |||
| Usher syndrome | 1:95 (type III) | DNA variants | |||
Although ethnic-based carrier screening has been accepted practice for decades, there are limitations to the ability of this approach to maximize identification of couples at increased risk for having a pregnancy affected by a mendelian disorder. First, patients may not have accurate awareness of their ethnic ancestry. Second, although the carrier frequency for disorders on ethnicity-based panels is individually high, these disorders account for only a small percentage of known mendelian disorders. For example, ACOG recommends at a minimum pan-ethnic screening for SMA, CF, and hemoglobinopathies, while currently over 3000 genes are known to cause mendelian disorders. Third, so-called next-generation sequencing technologies have enabled assaying hundreds or thousands of genes simultaneously in a cost-effective manner. These technological breakthroughs have led to widespread availability of expanded carrier screening, in which a large number of both common and rare disorders are screened for simultaneously in the general population.
Expanded carrier screening was initially introduced using a highly customized, multiple molecular inversion probe assay to convert the information content of a genetic variant into fluorescently labeled tag sequences. This approach identifies both the disease-associated and the wild-type alleles of each variant. With the development of SNP-based and sequencing technologies to rapidly assess an entire gene (see Cytogenetic Testing section later in this chapter), expanded carrier screening can now detect variants in all exons and potentially identify novel pathogenic variants in affected families. At least 200 conditions have available carrier screening, and that number is rapidly increasing. With such extensive availability of screening options, ACOG has provided consensus recommendations for the design of expanded carrier screening panels in order to maintain focus on the ultimate goal of providing patients with meaningful information to guide pregnancy planning. ACOG recommends that expanded carrier panels should only include disorders that meet at least several of the following criteria :
Have a carrier frequency of 1 in 100 or greater
Have a well-defined phenotype
Have a detrimental effect on quality of life
Cause cognitive or physical impairment
Require surgical or medical intervention
Have onset early in life
Have the ability to be diagnosed prenatally
As with all screening tests, expanded carrier screening is risk reducing rather than risk eliminating, because not all variants for any disorder can be identified. A common example of this is CF, in which a negative pan-ethnic variant panel significantly reduces the risk of a patient being a carrier but does not eliminate it given the large total number of variants in the gene. Furthermore, positive finding rates are high in expanded carrier screening panels, and many rare variants remain uncharacterized and thus are classified as variant of unknown significance. Srinivasan and colleagues have reported an expanded carrier panel for more than 100 mendelian disorders, with multiple variants tested per allele, in which 35% of individuals were found to be a carrier for at least one variant and the rate of carrier couples was approximately 0.6% to 0.8%. A study from a large repository of exome sequences from diverse populations showed that 32.6% (East Asian) to 62.9% (Ashkenazi Jewish) of individuals are variant carriers of at least one of the 415 autosomal recessive genes known to cause severe mendelian disorders. Their data showed that an ancestry-specific panel designed to capture genes with carrier rates >1.0% would include 5 to 28 genes, while a comparable pan-ethnic panel would include 40 genes. Although the carrier rate appears high, it should be considered that every individual is suspected to carry a dozen or more deleterious variants and that 0.17% to 2.52% of couples will be at risk of having a child affected by one of the screened conditions.
The appropriate extent of screening must be individualized for each patient and take into account both identified genetic risks and personal values after counseling by a qualified professional. Informed patient consent is recommended prior to offering expanded carrier screening and should meet the following ACOG guidelines , :
Carrier screening of any nature is voluntary, and it is reasonable to accept or decline.
Results of genetic testing are confidential and protected in health insurance and employment by the Genetic Information Nondiscrimination Act of 2008.
Conditions included on expanded carrier screening panels vary in severity. Many are associated with significant adverse outcomes such as cognitive impairment, decreased life expectancy, and need for medical or surgical intervention.
Pregnancy risk assessment depends on accurate knowledge of paternity. If the biological father is not available for carrier screening, accurate risk assessment for recessive conditions is not possible.
A negative screen reduces but does not eliminate risk to offspring. This is referred to as residual risk .
Because expanded carrier screening includes a large number of disorders, it is common to identify carriers for one or more conditions. In most cases, being a carrier of an autosomal recessive condition has no clinical consequences for the individual carrier. If each partner is identified as a carrier of a different autosomal recessive condition, offspring are not likely to be affected.
In some instances, an individual may have two pathogenic variants for a condition (homozygous or compound heterozygous) and thus learn through carrier screening that they have an autosomal recessive condition that could affect current or future personal health. Some expanded carrier screening panels include selected autosomal dominant and X-linked conditions and, likewise, an individual may discover predisposition to these conditions. Referral to an appropriate specialist for medical management and genetic counseling is indicated in such circumstances to review the inheritance patterns, recurrence risks, and clinical features.
It is important for parents to understand that over 30% of genetic disorders are the result of de novo genetic variants. De novo variants arise in the germline or due to an error of somatic cell division. Therefore these variants will not be detected on parental carrier screens.
Downstream prenatal genetic testing on the offspring of carrier-positive parents is not available for all of the genes offered on expanded carrier screening panels. Therefore careful research on which laboratory can perform prenatal testing for a given condition is necessary prior to performing chorionic villus sampling (CVS) or amniocentesis.
A recent publication from American College of Medical Genetics and Genomics (ACMG) recommends a tiered system based on carrier frequency and defines the gene content in each tier. It is important to note that carrier frequency was used to mean in any ethnic group with reasonable representation in the United States. In this tiered approach, Tier 1 conveys the recommendations previously adopted by ACMG and ACOG, with universal screening for CF and SMA and additional carrier screening dependent on clinical risk assessment. Tier 2 includes genes that have ≥1/100 carrier frequency. Tier 3 includes conditions with a ≥1/200 carrier frequency and includes X-linked conditions. Tier 4 includes genes less common than those in Tier 3, does not have lower limit carrier screening frequency, and can greatly extend the number of conditions screened. Although there are many serious conditions at a carrier frequency of less than 1/200, the problem with Tier 4 screening is that there is less information about the natural history and poor genotype-phenotype correlation for the very rare conditions. ACMG recommends that all pregnant patients and those planning a pregnancy should be offered Tier 3 carrier screening. Tier 3 screening currently encompasses 97 autosomal recessive and 16 X-linked genes, with recommendation for continuous scrutiny and adjustment of this list of genes. This is especially important at the time when emphasis on sequencing genomes from diverse populations will bring additional information on the prevalence and carrier frequency of mendelian disorders.
ACOG and the Society for Maternal-Fetal Medicine (SMFM) recommend that all pregnant women be counseled, as early as possible in their prenatal care, about the opportunities for prenatal genetic assessment consisting of either aneuploidy screening or diagnostic testing. This recommendation is not dependent on maternal age or other risk factors. Furthermore, the same professional societies, in addition to the American College of Radiology, all recommend prenatal ultrasound for accurate determination of gestational age, fetal number, cardiac activity, placental localization, and diagnosis of major fetal anomalies. Fetal structural anomalies or multiple minor sonographic markers identified on ultrasound increase the likelihood of aneuploidy, DNA microdeletions, or other genetic syndromes. Prenatal genetic testing should be offered to further evaluate abnormal findings on prenatal ultrasound.
Comprehensive discussion of screening modalities and screening or testing indications for pregnancy is presented in Chapter 30 . Here we focus on molecular genetic technologies, which are laboratory-based techniques for evaluating DNA sequence variation in an embryo or fetus. The benefits and limitations of the various types of genetic assessment are discussed for each technique. General benefits of genetic testing include identification of disorders for which in utero treatment may provide benefit, optimization of neonatal outcomes by planning appropriate delivery staffing and location, providing family preparation for caring for a child with a genetic disorder, the option of pregnancy termination, future pregnancy planning, and providing reassurance when results are normal.
Genomic DNA is relatively stable; therefore it can be obtained from any cell with a nucleus, even if the cells are no longer viable. Samples for molecular testing can include blood lymphocytes, skin scrapings, hair, cheek cells or saliva, semen, urine, and paraffin tissue blocks. At the individual gene level, disease-specific testing for families that carry known genetic variants may be performed using standard polymerase chain reaction (PCR) amplification and Sanger DNA sequencing methods (see Hybridization Techniques: Southern Blot, Polymerase Chain Reaction, later). An updated list of relatively common genetic conditions for which DNA-based prenatal diagnosis is available is kept on the Genetic Testing Registry website ( www.ncbi.nlm.nih.gov/gtr ).
Advancement of in vitro fertilization techniques has allowed optimization of methods to remove or biopsy small numbers of cells from an in vitro fertilized embryo for genetic assessment prior to implantation. The techniques available to retrieve preimplantation cells include polar body biopsy of prefertilized oocytes, biopsy of one or two cells (termed blastomeres ) from the six- to eight-cell early-cleavage-stage embryo on day 3, or removal of 5 to 12 cells from the trophectoderm of the 5- to 7-day blastocyst. In all cases, removal of the cells does not appear to cause any cellular damage, with continued development of the embryo and no increased risk for congenital anomalies.
Early attempts at noninvasive genetically based prenatal screening were focused on isolation of intact fetal cells within the maternal circulation. To date, this technology has proven unsuitable for clinical application due to multiple technological obstacles, such as limited numbers of fetal cells, unreliable recovery of fetal cells, and evidence that these cells persist long after pregnancy, thus complicating specificity in the setting of subsequent pregnancies.
In contrast, identification of fetal-derived cell-free small DNA fragments (<200 bp) in maternal plasma has been highly successful. In 1997, Dr. Dennis Lo and colleagues published the first report of identifiable cell-free fetus-derived Y chromosome sequences in the plasma of pregnant women carrying male fetuses. Subsequent work has established that approximately 5% to 20% of the cell-free DNA circulating in maternal plasma originates from the fetus (derived from the trophoblast) and provides 25 times more fetal DNA present in a pregnant woman’s plasma than could be extracted from intact circulating fetal cells. Massive parallel sequencing of cell-free DNA derived from maternal plasma has led to the development of algorithms to determine fetal ploidy (chromosome count) in noninvasive prenatal screening (see details later).
Circulating fetal DNA is predominantly a product of placental apoptosis. This cell-free DNA consists of small fragments (fewer than 200 bp), undergoes a rapid turnover, and may appear in apoptotic bodies or nucleosomes. Fetal SRY gene sequences are present in the circulation as early as 18 days after embryo transfer, before the definitive fetoplacental circulation, which is not established until 28 days after conception. Fetal DNA is continuously liberated into the maternal circulation, with a mean half-life estimated to be 16 minutes at term. Levels increase until approximately 10 weeks’ gestation, remain stable between 10 and 21 weeks, and then continue to increase until the third trimester. Fetal DNA levels are undetectable about 2 hours after birth.
For the goal of prenatal diagnosis (not screening), the most commonly employed forms of genetic testing are CVS and amniocentesis to obtain cellular samples from the placenta and fetus, respectively, for cytogenetic analysis and molecular genetic testing (see also Chapter 30 for full discussion of indications and techniques). ACOG recommends that all women, regardless of age or risk, be offered biochemical and/or ultrasound screening and invasive testing. The decision to pursue invasive testing needs to incorporate considerations of level of risk that the fetus is affected, level of risk associated with the procedure, and the patient’s impression of the impact of having an affected child.
Of note, the risks involved with invasive testing may be much lower than estimates that have been previously quoted since the advent of invasive testing in the 1970s. Results of a meta-analysis, which included only contemporary large studies (published after the year 2000, reporting greater than 1000 total procedures), demonstrated no significant difference in the risk of miscarriage prior to 24 weeks’ gestation for women undergoing amniocentesis or CVS compared to those who do not have invasive testing. Procedure-related risk of miscarriage for amniocentesis was 0.1% and for CVS was 0.2%. Nevertheless, rates of performance of these invasive procedures are significantly declining as screening tests with higher detection rates and lower false-positive rates have become available (see Noninvasive Prenatal Screening, later). Thus it will be more difficult in coming years for practitioners to be adequately trained in these invasive techniques. It is important to note that the range of genetic abnormalities that can be detected is far greater with invasive testing than with any available noninvasive screening tests.
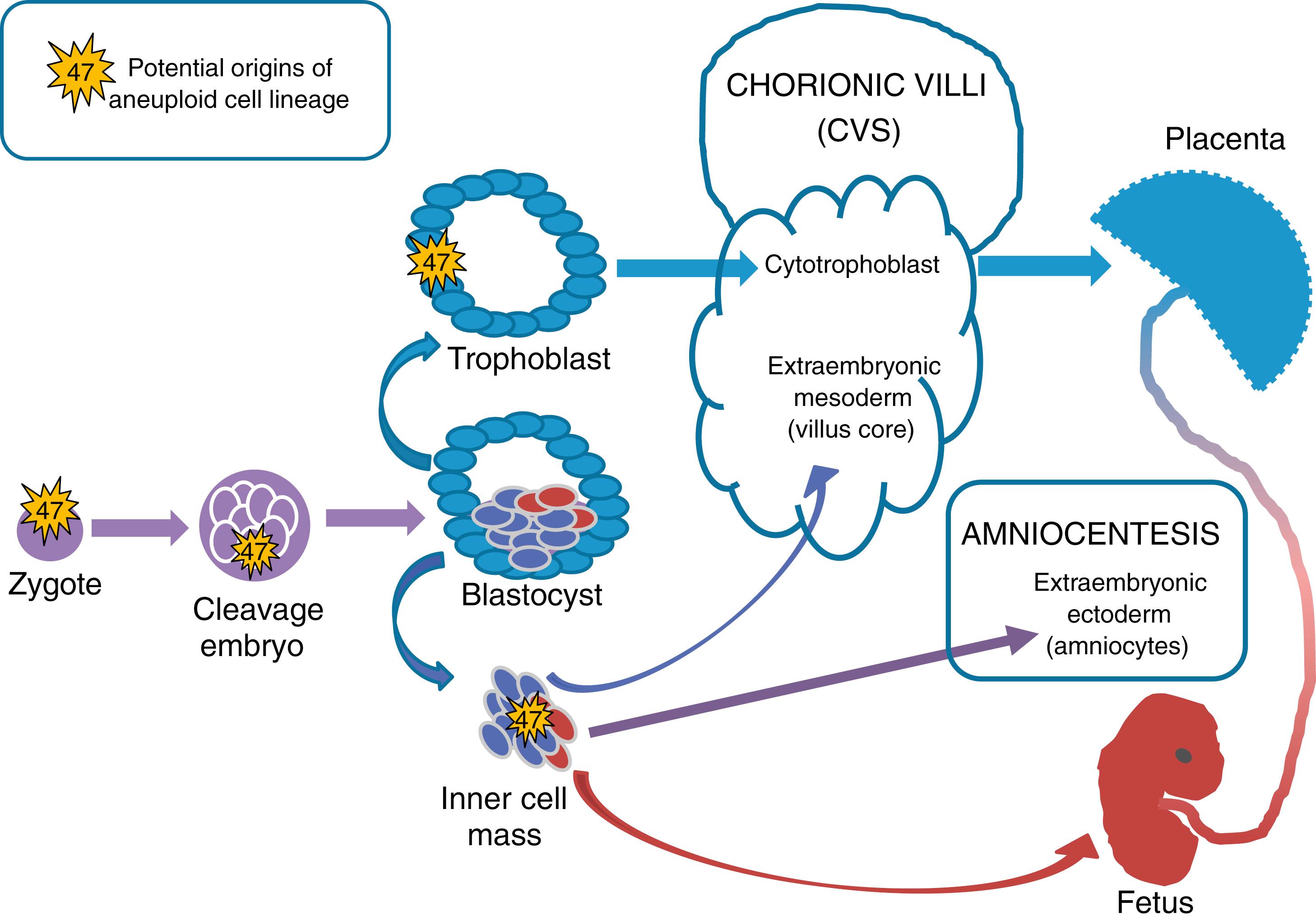
To understand the clinical implications of abnormal chromosomal content in some or all of the cells obtained from prenatal sampling (CVS or amniocentesis), it is important to review the different cellular origins and early development of the fetus and placenta ( Fig. 2.2 ). Chromosomal mosaicism refers to the presence of two or more cell lines with different karyotypes in a single sample. Chromosomal mosaicism becomes generalized (present in the entire organism) or confined to a specific compartment (placenta, fetus, specific fetal organ system), depending on the developmental stage at which improper chromosome segregation occurs, including meiosis during germline development, early postzygotic mitosis in early embryos, or late postzygotic mitosis (also refer to Chapter 1 for review of mechanisms of chromosome segregation). The most commonly encountered aneuploidy in prenatal diagnostic testing is trisomy. Note that if trisomy originates in the zygote, the organism becomes mosaic by way of trisomy rescue, or elimination of the extraneous chromosome copy in a subset of cells. Generally speaking, the earlier in development that an abnormal chromosome segregation event such as nondisjunction or trisomy rescue occurs, the more widespread the mosaicism may be in the organs of the differentiated organism (i.e., more likely to affect both the chorion/placenta and the fetus). Later chromosome segregation events are more likely to be confined to specific cell types, giving rise to clinical findings such as confined placental mosaicism, which leads to discordant karyotypes found with CVS versus amniocentesis. By understanding the developmental origin of the cells biopsied via CVS, amniocentesis, or postnatal karyotyping, the clinician can use appropriate testing methods to distinguish between placental mosaicism and generalized mosaicism.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here