Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Original work described here was supported by NIH grants: DK069998 and DK105811 (PAF); DK087688 and DK102495 (JPV).
The authors declare that they have no financial or other conflicts of interest.
The chief cells of the parathyroid gland synthesize parathyroid hormone (PTH). It is initially translated as a 115-amino-acid peptide called preproparathyroid hormone (prepro-PTH). This single-chain peptide is sequentially converted to proparathyroid hormone (pro-PTH) by cleavage of the 25 amino-terminal residues (−6 to −31) ( Fig. 30.1 ) as the peptide moves through the Golgi complex. The principal biologically active PTH molecule [PTH(1–84)] is formed by cleavage of the six amino acids of the prosequence. Mature PTH(1–84) along with chromogranin-A (CgA) reside within dense core secretory granules until they are discharged into the circulation. Neither prepro-PTH nor pro-PTH appears in plasma. Further details of the molecular synthesis and processing of PTH are available. Once released into the general circulation, PTH(1–84) is rapidly cleared and metabolized. CgA is secreted in parallel with PTH in response to low extracellular calcium and can be processed to amino-terminal peptides that inhibit PTH secretion.
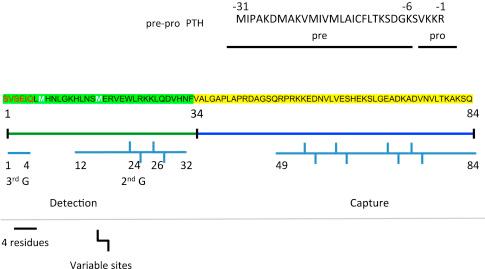
In addition to the full-length PTH(1–84), the parathyroids also generate a number of shorter fragments. PTH(7–84) is the major proteolytic product of PTH resulting from the action of cathepsins B and H in the parathyroids. The mechanism of regulation is presently unknown. However, it is clear that plasma calcium levels determine the relative proportion of intact PTH and amino-truncated peptide fragments. Amino-truncated PTH metabolites are also formed from intact, circulating PTH by hepatic Kupffer cells. Notably, PTH(7–84) and other peptide fragments were long assumed to be inactive degradation products that were inert by-products of the catabolism of PTH(1–84) and represented a means of reducing active protein. Such an interpretation was based on the competitive inhibition of PTH binding and action by PTH(7–34) and the inability of PTH(7–34) or PTH(7–84) to activate the type 1 PTH receptor (PTHR). More recent work shows that the concentration of PTH(7–84) and other amino-truncated PTH fragments increases significantly during renal failure representing as much as 95% of circulating peptide. The massive accumulation of such fragments in renal failure arises, in part, because they are cleared from the circulation predominantly by the kidneys and only minimally by the liver, whereas intact PTH is eliminated principally by extrarenal mechanisms. The clearance of amino-truncated PTH fragments is considerably slower than that for peptides with an intact amino terminus. The clinical significance of these peptides is of some considerable debate and uncertainty. In isolated kidney and bone cells, it is now apparent that PTH(7–84), along with a series of synthetic shorter PTH fragments, promotes PTHR downregulation without concomitant activation. The effect of PTH fragments on receptor endocytosis is governed by the cellular abundance of the adapter protein Na/H exchange regulatory factor (NHERF1). Notably, this selective action of PTH(7–84) is not due to competitive displacement of PTH(1–84) and suggests that amino-truncated PTH fragments may promote hormone resistance by causing receptor internalization from the plasma membrane. PTH(7–84) has been shown also to antagonize the effects of PTH(1–84) on bone in experimental renal failure.
PTH secretion is tightly regulated by changes of extracellular calcium through a negative-feedback mechanism that is primarily mediated by the calcium-sensing receptor (CaSR), a family C G protein–coupled receptor (GPCR) on parathyroid cells. Reductions of serum ionized calcium stimulate PTH secretion, whereas increases of calcium suppress PTH release and promote calcitonin secretion ( Fig. 30.2 ). The set point for this regulation is ∼1.3 mM, the normal value for extracellular calcium. CaSR regulation of PTH release is discussed further in physiological actions of PTH. The CaSR possesses two large exofacial lobes that detect changes not only of calcium but also of pH, salinity, amino acid concentration, and polyamines in the extracellular environmental fluid.
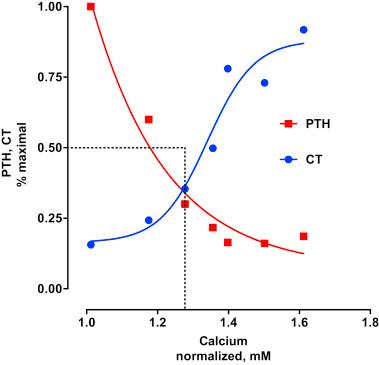
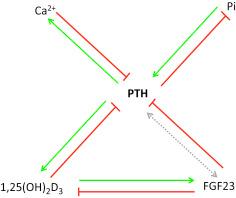
A second mechanism regulating PTH secretion involves fibroblast growth factor 23 (FGF23). Parathyroid glands express FGF receptors FGFR1 and FGFR3 and Klotho. FGF23 decreases PTH secretion and PTH mRNA. The inhibitory action of FGF23 on PTH secretion involves both Klotho-dependent and Klotho-independent pathways. Klotho-dependent inhibition is mediated through a canonical mechanism involving MAPK/ERK, whereas Klotho-independent FGF23 regulation of PTH secretion acts through calcineurin-NFAT signaling. Taken together, these results suggest a more comprehensive model for PTH secretion that has a calcium limb regulated by the CaSR and a phosphate-sensing branch that is controlled by FGF23 acting through FGFRs ( Fig. 30.3 ).
The amount of preformed PTH is limited and, as noted earlier, its degradation rapid. The availability of sufficient PTH to meet changing demands on extracellular mineral ion homeostasis is achieved by the coordinated regulation of PTH formation by controlling gene expression. PTH synthesis and secretion are regulated by extracellular Ca 2+ , vitamin D, and phosphate. Reductions of extracellular calcium increase the synthesis of PTH mRNA, whereas elevations of calcium decrease PTH formation. In a reciprocal manner, increases of serum phosphate enhance PTH synthesis, whereas decreases of phosphate reduce its formation. The regulation of PTH synthesis by serum phosphate occurs independently of calcium and vitamin D. Whether such control by Ca 2+ and phosphate is exerted at transcriptional or posttranscriptional levels remains controversial. 1,25(OH) 2 D 3 directly regulates PTH transcription. 22-oxacalcitriol, a vitamin D analog exhibiting few effects on serum calcium, also suppresses PTH formation.
More PTH is secreted and less is hydrolyzed during periods of hypocalcemia. In this setting, PTH(7–84) release is augmented. PTH synthesis also increases, and the gland hypertrophies in prolonged hypocalcemia.
Secretion of PTH(1–84) is favored at normal or low calcium levels, whereas amino-truncated PTH fragments are preferentially secreted at elevated extracellular calcium concentrations. PTH(1–84) has a half-life in plasma of about 2–4 min. The liver, primarily, and kidneys, secondarily, account for about 90% of its clearance. By contrast, the half-life of PTH(7–84) and other carboxy-terminal PTH fragments are 10 min or longer. Furthermore, unlike PTH(1–84), PTH(7–84) is cleared primarily by the kidneys. Thus, formation of PTH(7–84) in chronic kidney disease (CKD) is elevated and elimination is reduced resulting in profound accumulation of carboxy-terminal PTH fragments in the peripheral circulation. The level of these fragments may approach or exceed that of PTH(1–84). The physiological consequences and recommended interventions for such situations are presently conflicting and uncertain as they remain ambiguous whether low- or high-turnover bone disease can be explicitly related to the absolute or relative abundance of PTH(1–84) and carboxy-terminal PTH fragments, i.e., peptides lacking the amino terminus.
Both intact PTH(1–84) and an array of smaller PTH fragments are produced both by the parathyroids glands and peripheral metabolism. The nature of these PTH fragments has been determined mostly by a combination of immunochemical reactivity, biochemical separation, and classical amino acid sequencing. This information has been comprehensively reviewed. Using separated serum fractions and multiple PTH assays, PTH(1–84) forms some 20% of total circulating PTH under normocalcemic (1.22 mM) conditions, increases to 35% in hypocalcemia, and decreases to 4%–15% in response to progressively elevated calcium.
Although the precise identity of the various PTH fragments remains uncertain, it is clear that the majority of circulating PTH forms are non-PTH(1–84) fragments, e.g., PTH(39–84) and PTH(7–84). These peptide fragments represent 80% of circulating PTH in normal individuals and up to 95% in patients with CKD. Recent application of immunoseparation and mass spectrometry to identify PTH fragments is generated by trypsin digestion. Curiously, no peptide with a starting at position 7 could be found, implying the absence of PTH(7–84) in circulation. As similar analytical approaches become more routinely applied, a clarification of this discrepancy will emerge, along with a better understanding of the various PTH fragments present under physiological and pathological settings.
PTH(1–84) harbors methionines at positions 8 and 18 ( Fig. 30.1 ). When oxidized, as occurs normally to some degree but increasingly in patients with advanced kidney disease, the resulting structural changes reduce or eliminate PTH biological activity. Nonetheless, these inactive molecules are detected by commonly employed third-generation PTH assays and may partially account for discrepancies that arise in disorders affecting PTH release and its metabolism. These considerations underscore the need for reliable measurements of circulating, biologically active forms of PTH that are essential to the diagnosis and clinical management of patients with renal disease or mineral ion disorders.
Assays for the determination of serum PTH have evolved considerably since their introduction in 1963. First-generation assays of PTH employed radioimmunoassays (RIAs) based on the principle of competitive inhibition of binding of [ 131 l]-labeled PTH to specific hormone antibodies. These assays recognized only a single epitope within the middle or carboxy regions ( Fig. 30.1 ) of the peptide and therefore could not discern the presence or absence of the amino terminus. Furthermore, the presence of PTH fragments was not appreciated or measured by the original PTH RIAs. The development of two-site immunometric assays (second-generation assays) was thought to overcome the limitations of the earlier RIAs. These second-generation immunometric assays employed an immobilized “capture” antibody located in or near the amino terminus and a second, higher affinity antibody present between residues 49 and 84 for detection. These assays were based on saturation rather than competitive binding as in RIAs, while the detection antibody was in or near the amino terminus. Several observations, however, suggested the presence of anomalous peptides when these assays were employed. Compared with healthy subjects, patients with CKD exhibited significant increases in the nonsuppressible fraction of PTH, i.e., the portion that is not attenuated by increasing serum calcium when parathyroid function is measured dynamically and serum PTH is calculated as a function of serum Ca 2+ using a four-parameter curve fitting procedure. Precise analytical characterization of circulating PTH unambiguously confirmed that first-generation immunometric assays failed to detect the presence of these PTH fragments. The terminology describing the second immunometric assays is somewhat confusing because they are defined as measuring “intact PTH,” whereas in fact they detect both PTH(1–84) and large amino-truncated PTH fragments that lack biological activity.
Third-generation PTH assays now employ a detection antibody located within the first three or four amino acids ( Fig. 30.1 ). These new assays distinguish between bioactive PTH [PTH(1–84) + amino-terminal peptides] and other circulating PTH forms and are referred to as measuring “whole” PTH. The ability to measure whole PTH separately from large amino-truncated PTH fragments is thought to increase the accuracy of laboratory testing of parathyroid status and distinguishing between high- and low-turnover bone status in patients with renal failure. Direct comparisons of various assays individually or as ratios as a function of serum calcium do not seem to provide improved predictive behavior, and the significance of employing ratios of bioactive PTH to amino-truncated fragments for therapeutic intervention has been questioned.
Jüppner and colleagues recently identified a homozygous arginine-to-cysteine mutation at position 25 in siblings with idiopathic hypoparathyroidism. Serum PTH levels measured with antisera capturing the 39–84 region and then detected with an antibody in the 1–34 (PTH-S assay), 13–34 (ntPTH assay), or 1–3 region (whole PTH assay) ( Fig. 30.1 ). PTH-S and ntPTH assays showed normal to slightly elevated or subnormal levels, respectively, whereas PTH levels measured with the whole PTH assay were extremely elevated. The divergent results of the PTH assays would lead to different diagnoses: ntPTH and PTH-S assays with essentially low to normal levels implicating hypoparathyroidism, whereas the strikingly elevated levels reported by the whole PTH assay would suggest the presence of pseudohypoparathyroidism. Recombinant R 25 C-PTH(1–34) exhibited negligible binding or bioactivity, and the missense mutation evidently lowers affinity for detection by PTH-S and ntPTH assays but not the whole PTH assay. These important findings further emphasize the need for additional refining of the immunometric assays and new and sequence-based determinations. As noted earlier, mass spectrometry analysis reveals a variable component of inactive, oxidized PTH that is detected by the “whole” PTH(1–84) assay. Based on these findings, Hocher et al. proposed a fourth-generation assay, where oxidized forms of PTH are first removed by affinity chromatography before measurement of “whole” PTH.
The canonical type 1 PTHR mediates PTH actions. This GPCR is abundantly expressed in the kidney and bone, where it transduces signals from PTH and the PTH-related peptide (PTHrP). Two other PTHRs have been identified. The type 2 PTH receptor (PTHR2), which exhibits about 50% sequence identity with the PTHR, responds virtually uniquely to PTH and not PTHrP. The structural determinants that account for the ability of the PTHR2 to discriminate between PTH and PTHrP have been identified. Histidine at position 5 (Ile in PTH) and Phe at position 23 (Trp in PTH) account for the differential binding affinity and activation. In the kidney, PTHR2 expression is thought to be limited to glomerular and other vascular cells and is not expressed on tubular epithelial cells. It has not been implicated in renal calcium or phosphate transport. The 39-amino-acid tuberoinfundibular neuropeptide (TIP39) is the cognate PTHR2 ligand.
A third, nonmammalian, PTH receptor (PTHR3) has been identified in zebrafish. It shares higher homology with the PTHR than with the PTHR2. However, the PTHR3 displays greater affinity for PTHrP than for hPTH and exhibits more robust activation of adenylyl cyclase.
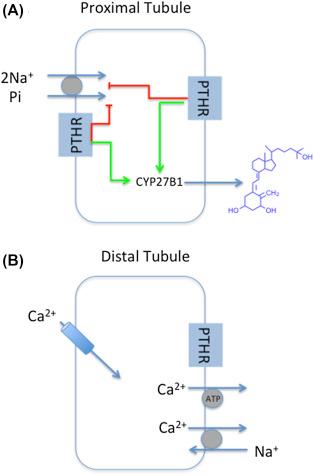
RT-PCR and in situ hybridization were used to define the nephron sites of PTHR mRNA expression. General consensus pointed to conspicuous PTHR expression in the glomerulus, PCT and PST, CAL and DCT. Some differences may also be noteworthy. Riccardi and Hebert additionally reported PTHR expression in the rat CCD, a finding not confirmed by Yang et al. Immunoelectron and immunofluorescence microscopy localization detected PTHR on apical and basolateral membranes of proximal convoluted tubule cells. Other studies employing immunofluorescence reported both luminal and basolateral PTHR expression in proximal tubules and thick ascending limbs but only on basolateral surfaces of distal convoluted tubules. The physiological actions of PTH are generally ascribed to the presence of PTHR on proximal and distal tubules ( Fig. 30.4 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here