Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cancer is the most common cause of disease-related death in children beyond the newborn period. Although childhood cancers, as a group, account for only a small proportion of all human cancer, their unique biologic features, cell of origin, and response to therapy make them intriguing models with which to study and understand the process of human carcinogenesis.
Although most childhood cancers occur sporadically and their etiology remains unclear, hereditary or familial factors are evident in 25% to 40% of cases. Obvious environmental influences on cancer initiation are not generally apparent. Cancer predisposition syndromes manifesting in childhood in which nonmalignant phenotypic features are not observed include hereditary retinoblastoma (RB), Li-Fraumeni syndrome, and familial polyposis; others such as von Hippel–Lindau disease and Gorlin syndrome are associated with the coincident presentation of both benign and malignant neoplasms. Nonrandom molecular and cytogenetic alterations are frequently observed in most childhood cancers. These “markers” provide not only unique diagnostic identifiers but also frequently prognostic value with respect to disease outcome and anticipated response to therapy. Many of these genetic markers also recapitulate normal developmental processes and thus offer a window into the biologic mechanisms of carcinogenesis and normal embryologic growth and development ( Table 27-1 ). Importantly, the introduction of routine predictive genetic testing together with the development and implementation of clinical surveillance protocols has led to early tumor detection and improved survival for both children and adults with hereditary forms of cancer. In this chapter, we address the diversity of molecular mechanisms in several prototypical childhood cancers and cancer predisposition syndromes.
| Solid Tumor | Cytogenetic Abnormality | Genes ∗ |
|---|---|---|
| Ewing sarcoma | t(11;22)(q24;q12),+8 | EWS ( 22 ) FLi1 ( 11 ) |
| Neuroblastoma | del1932–36, DMs, HSRs, +17q21-qter | N-MYC |
| Retinoblastoma | del13q14 | Rb |
| Wilms tumor | del11p13, t(3;17) | WT1 |
| Synovial sarcoma | t(X;11)(p11;q11) | SSX ( X ) SYT ( 18 ) |
| Osteogenic sarcoma | del13q14 | ? |
| Rhabdomyosarcoma | t(2;13)(q37;q14), t(1;13)(1p36;q14), 3p-, 11p- | PAX3 ( 2 ) FKHR ( 13 ) ; PAX7 ( 1 ) FKHR ( 13 ) |
| Peripheral neuroepithelioma | t(11;22)(q24;q12),+8 | EWS ( 22 ) FLi-14 ( 11 ) |
| Astrocytoma | ¡(17q) | ? |
| Meningioma | delq22 | MN1, NF2, ? |
| Atypical teratoid/rhabdoid tumor | delq22.11 | SNF5/INI1SMARCB1 |
| Germ cell tumor | ¡(12p) | ? |
Retinoblastoma (RB) is a rare childhood tumor thought to arise in the embryonic retinal epithelium. Although the incidence of RB is only approximately 1 in 20,000 births, or some 200 new cases per year in North America, this tumor has been a target of intense research interest. RB is the prototype cancer caused by mutations of a tumor suppressor gene. Tumors are often bilateral and multifocal. In approximately 40% of RB cases, the disease is inherited as an autosomal dominant trait, with a penetrance approaching 100%. The remaining 60% of cases are sporadic (nonheritable). Fifteen percent of unilateral RB is heritable but by chance develops in only one eye. Survivors of heritable retinoblastoma have a 100-fold increased risk of developing mesenchymal tumors such as osteogenic sarcoma, fibrosarcoma, and melanoma later in life. RB is characterized by the rapid growth of undifferentiated neuroblastic precursors derived from various layers of retinal ganglion cells. The cells are small and round with a high nuclear:cytoplasmic ratio, exhibiting numerous mitoses that reflect their rapid proliferative rate. RB cells appear undifferentiated, with evidence of ganglionic differentiation, including the presence of Flexner-Wintersteiner rosettes.
Tumors that are limited to the globe are staged according to the schema of Reese and Ellsworth, which is based on the number and size of the tumors. These classification systems predict the likelihood of obtaining local tumor control and preservation of vision. Each eye is staged individually. RB can spread beyond the orbit by direct invasion of adjacent tissue and hematogenous spread. The treatment of patients with RB depends on the size of the tumor and the extent of tumor invasion at the time of diagnosis. Surgery is the mainstay of treatment for children with unilateral RB. Large intraocular tumors as well those with bilateral multiple tumors are treated with adjuvant chemotherapy.
The RB gene maps to chromosome 13q14. Biallelic disruption of the RB gene leads to disease—an observation consistent with Knudson’s “two-hit” theory of carcinogenesis. RB consists of 27 exons and encodes pRB, a 105-kDa nuclear phosphoprotein plays a central role in the control of cell cycle regulation, particularly in determining the transition from G 1 through S (DNA synthesis) phase in virtually all cell types.
In the developing retina, inactivation of the RB gene is necessary and sufficient for tumor formation. It is now clear, however, that these tumors develop as a result of a more complex interplay of aberrant expression of other cell cycle control genes. In particular, a tumor surveillance pathway mediated by Arf, MDM2, MDMX, and p53 is activated after loss of pRB during development of the retina. In a small fraction of RB tumors, no RB1 mutations are detected; in the majority of these, high-level amplification of the MYCN oncogene is observed, suggesting a novel mechanism of tumorigenesis in the presence of nonmutated RB1 genes. Not only do these observations provide a provocative biologic mechanism for tumor formation in retinoblastoma, but they also point to potential molecular targets for developing novel therapeutic approaches to this tumor. For example, the MDM2/MDMX antagonist, Nutlin-3a, efficiently targets the p53 pathway and is effective as an ocular formulation in treating RB in orthotopic xenografts.
Wilms tumor (WT), or nephroblastoma, is an embryonal malignancy that arises from remnants of immature kidney. It affects approximately 1 in 7000 children, usually before the age of 6 years (median age at diagnosis, 3.5 years). Five percent to 10% of children present with synchronous or metachronous bilateral tumors. WT typically presents as an asymptomatic abdominal mass, although a small fraction of children have symptoms such as hematuria or hypertension. Approximately 20% of children present with metastatic disease.
The relationship between WT and aberrations of normal development is striking. In early development, the embryonal mesonephros emerges from a complex interaction between epithelial-derived ureteric bud tissue and mesenchymal-derived metanephric blastema through a series of differentiation events. Mature nephrons derived from the mesonephros are composed of nephroblasts, tubules, and stromal tissues that ultimately form the adult kidney. These different tissues together confer the distinctive “triphasic” histologic features of WT that arise in the intralobar area. Tumors that arise in the perilobar area tend to be biphasic or monomorphic, typically epithelial. This presentation suggests that these tumors arise from a cell that is more prevalent later in development, having a more limited potential to differentiate along multiple lineages.
A peculiar feature of WT is its association with nephrogenic rests, foci of primitive but nonmalignant cells whose persistence suggests a defect in kidney development. These precursor lesions are found within the normal kidney tissue of more than one third of children with WT. Nephrogenic rests may persist, regress spontaneously, or grow into large masses that simulate true WT and present a difficult diagnostic challenge. Another intriguing feature of WT is its association with specific congenital abnormalities, including genitourinary anomalies, sporadic aniridia, mental retardation, and hemihypertrophy. A genetic predisposition to WT is observed in two distinct disease syndromes with urogenital system malformations—the WAGR (Wilms tumor, aniridia, genitourinary abnormalities, mental retardation) syndrome and the Denys-Drash syndrome (DDS )—and in Beckwith-Wiedemann syndrome (BWS ). WT was the first of the solid tumors of childhood recognized as being curable even in the setting of metastatic disease. Sequential clinical treatment protocols evaluated by the National Wilms Tumor Study have led to effective multimodality approaches that cure up to 90% of children with WT. The cornerstone of therapy is surgery; chemotherapy agents with or without radiotherapy are used to treat minimal residual or metastatic disease.
The WAGR syndrome is associated with constitutional deletions of chromosome 11q13. Whereas it is now known that the WAGR deletion encompasses a number of contiguous genes, including the aniridia gene Pax6 , cytogenetic observation in patients with WAGR was also important in the cloning of the WT1 gene at chromosome 11p13. WT1 spans approximately 50 kb of DNA and contains 10 exons that encode the WT1 protein transcription factor. DDS, the second syndrome closely associated with this locus, is a rare association of WT, intersex disorders, and progressive renal failure. Virtually all patients with DDS carry germline WT1 point mutations.
WT1 is altered in only 10% of Wilms tumors. This observation implies the existence of alternative loci in the etiology of this childhood renal malignancy. One such locus also resides on the short arm of chromosome 11, telomeric of WT1, at 11p15. This gene, designated WT2, is associated with BWS. Patients with BWS are at increased risk of developing Wilms tumor, as well as other embryonic malignancies, including rhabdomyosarcoma (RMS), neuroblastoma, and hepatoblastoma. The putative BWS gene maps to chromosome 11p15. Whether the BWS gene and WT2 are one and the same or two distinct yet closely linked genes remains to be determined. Using long-oligonucleotide array comparative genomic hybridization (array CGH), a novel gene termed WTX was identified on chromosome Xq11.1. WTX is inactivated in one third of WTs, and tumors with WTX mutations lack WT1 mutations. Bilateral WT or a family history of WT occurs in 1% to 5% of patients. Although linkage studies have indicated that the gene for familial WT must be distinct from WT1 and WT2 , and from the gene that predisposes to BWS, this gene has been neither cytogenetically localized nor isolated.
Neuroblastoma (NB) is the most common tumor of the peripheral sympathetic nervous system in children. The embryonic neural crest gives rise to the peripheral nervous system including cranial and spinal sensory ganglia, autonomic ganglia, the adrenal medulla, and other para-endocrine cells distributed throughout the body.
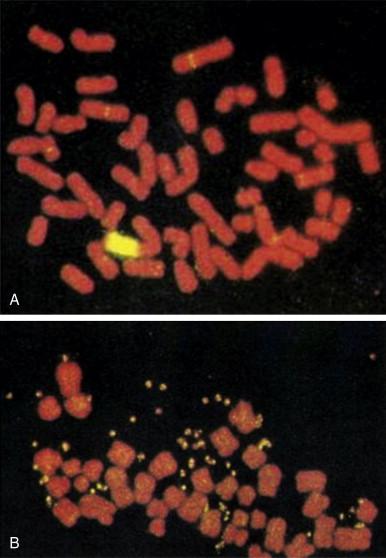
NB most commonly arise in cells of the adrenal medulla and at other abdominal retroperitoneal sites of the known peripheral nervous system. Approximately 15% of cases occur in the paravertebral thoracic cavity in close association with the dorsal root ganglion. Most cases of NB (60% to 70%) present with metastatic disease, most commonly involving bone, bone marrow, and liver. NB is characterized histologically by the presence of small, round cells with hyperchromatic nuclei and stippled chromatin. At a cytogenetic level, homogeneously stained regions and double-minute chromosomes are typically observed ( Figure 27-1 ). A hallmark of its light microscopic appearance is the presence of Homer-Wright rosettes characterized by tumor cell clusters around a central mesh of cell processes, termed neuropile .
The clinical stage and age of onset are highly significant independent prognostic variables. For example, a unique presentation of NB, stage IV-S (IV-special) is frequently associated with spontaneous remission. This form of the disease typically presents in infants younger than 1 year of age with evidence of remote disease in the liver and bone marrow, though sparing bone. It is not known whether IV-S NB represents metastatic disease or a multifocal nonclonal disorder of neuroblast development. Stages I and II NB can generally be effectively managed with surgical resection alone, although those rare patients with low-stage disease and adverse biologic markers often require adjuvant chemotherapy. Multimodality therapy, including high-dose chemotherapy, hematopoietic stem cell harvest and rescue, radiation therapy, 123 I-MIBG therapy, and Ch14.18 immunotherapy are required to achieve remissions in stage IV (and to a lesser extent stage III) NB, although remission is maintained in less than 40% of patients.
Nonrandom cytogenetic abnormalities are observed in more than 75% of neuroblastomas. The most common of these is deletion or rearrangement of the short arm of chromosome 1, although loss, gain, and rearrangements of chromosomes 10, 11, 14, 17, and 19 have also been reported. Two other unique cytogenetic rearrangements are highly characteristic of neuroblastoma: homogeneous staining regions and double-minute chromosomes (see Figure 27-1 ). These contain regions of amplification of the N-myc gene, an oncogene with considerable homology to the cellular proto-oncogene c-myc. N-myc amplification is associated with rapid tumor progression, and virtually all neuroblastoma tumor cell lines demonstrate amplified and highly expressed N-myc . Decreased N-myc expression is observed in association with the in vitro differentiation of neuroblastoma cell lines. This observation formed the basis for therapeutic trials demonstrating a survival advantage to patients treated with cis -retinoic acid.
Neuroblastoma cells that express the high-affinity nerve growth factor receptor trkA can be terminally differentiated by nerve growth factor and demonstrate morphologic changes typical of ganglionic differentiation. Tumors showing ganglionic differentiation and trkA gene activation have a favorable prognosis. Expression of trkB receptor is associated with poor prognosis tumors and appears to mediate resistance to chemotherapy.
In addition to chromosomal loss on chromosome 1p36, unbalanced loss of heterozygosity at 11q23 is independently associated with decreased event-free survival. Alterations at 11q23 occur in almost one third of neuroblastomas, being most commonly associated with stage IV disease and age at diagnosis greater than 2.5 years. Telomerase expression and telomere length are yet other valuable markers of clinical significance. In particular, short telomere length is predictive of favorable prognosis, regardless of disease stage, whereas long or unchanged telomeres are predictive of poor outcome. Both in vitro and in vivo studies suggest that telomerase inhibition may represent a unique mechanism for novel biological treatment of NB. A small subset of neuroblastomas is inherited in an autosomal dominant fashion. Until recently, the only gene definitively associated with neuroblastoma risk was PHOX2B , also linked to central apnea. Utilizing high-resolution microarray and next-generation sequencing approaches, de novo or inherited missense mutations in the tyrosine kinase domain of the ALK (anaplastic lymphoma kinase) gene on chromosome 2p23 have been observed in many hereditary neuroblastoma families, as well as in sporadic cases, although no clear correlation with stage of disease has been observed. Current Phase I/II clinical trials with ALK inhibitors substantiate the value of such target identification for novel therapies. However, combination whole-exome, genome, and transcriptome sequencing of neuroblastoma identifies few recurrently mutated genes ( ALK , PTPN11 , ATRX , MYCN , and NRAS ) or pathogenic germline variants ( ALK , CHEK2 , PINK1 , and BARD1 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here