Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Prostate cancer is the most common noncutaneous malignancy and the second leading cause of cancer death in men in the United States. Prostate-specific antigen (PSA) screening began in the late 1980s and dramatically increased the diagnosis of this disease. An almost-simultaneous decrease in disease-specific mortality has been noted. Whether this is a result of early and enhanced screening, early treatment of localized disease, early and aggressive treatment of micrometastatic disease, or other unknown reasons is a matter of considerable debate. Screening remains widespread but controversial because of conflicting evidence demonstrating overall survival benefit. Prostate cancer has a very heterogeneous natural history, and screening has resulted in overdetection and overtreatment of men with indolent prostate cancer. Prostate cancer is not, as yet, curable once it has metastasized; however, differentiation of early-stage disease that ultimately will progress from disease destined to remain indolent is a major research priority. The molecular genetics of prostate cancer hold promise for the development of new screening and diagnostic tests to resolve this issue.
Several risk factors have been associated with prostate cancer, including age, race, family history, and obesity. Subsequently, tumor suppressors, oncogenes, and polymorphisms have been analyzed to help explain these risk factors. Epigenetic alterations have been observed in prostate cancer affecting the expression and function of a large array of genes involved in tumorigenesis, tumor progression, and metastasis. The search for new and more specific biomarkers of disease continues with increased emphasis on epigenetic and genomic alterations predictive of metastasis and aggressiveness in this heterogeneous malignancy.
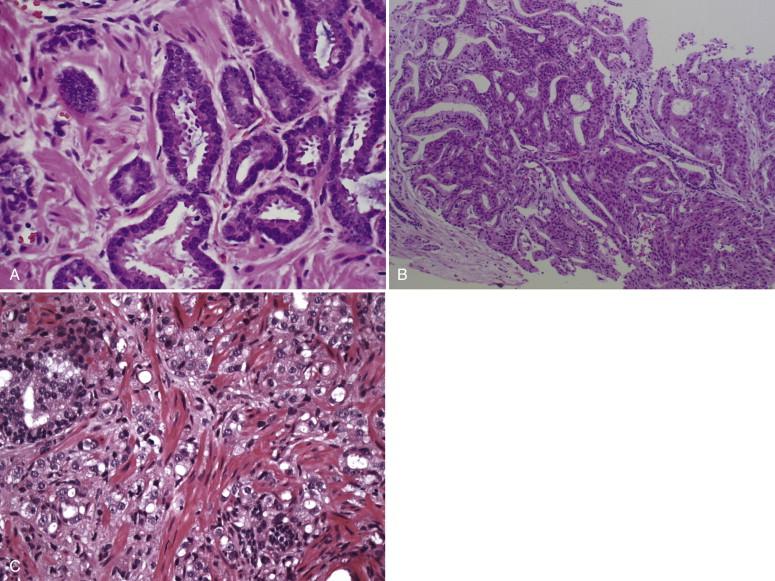
The Gleason grading system is the most commonly used pathologic grading system for adenocarcinoma of the prostate. Tissue samples are examined under low magnification, and the two most common gland architectural patterns are assigned a grade from 1 to 5 and reported as a Gleason score. Figure 38-1 demonstrates the three most common Gleason scores. Gleason 1 and 2 are rarely seen in contemporary series of patients. Pathologic Gleason grade is the most important prognostic variable to the clinical risk assessment of newly diagnosed prostate cancer, followed by tumor volume. The importance of Gleason pattern 4 and 5 volume has been correlated with subsequent pathologic stage, metastasis, and outcome. Despite the decrease in prostate cancer–specific mortality seen in contemporary series, there has been a paradoxical increase in the diagnosis of patients with higher Gleason grade disease. This may be due in part to changes in pathologic criteria for diagnosing Gleason 4 disease, rather than a true increase in the incidence of higher grade disease as a result of the International Society of Uropathologists (ISUP) modifying their recommendations on histological grading of prostate cancer by expanding criteria of grade 4 disease. After this recommendation was published, it was projected that many patients previously classified as 3+3 would be subsequently upgraded to Gleason 3+4.
Prostate cancer is often multifocal, meaning that usually, multiple, distinct areas of malignancy exist within the prostate gland. The largest focus of disease is often called the index tumor, and the size of this tumor has been used in disease prognostication and prediction of metastasis. An analysis of genetic alterations in tumor foci and metastases found that metastases were usually homologous with at least one tumor focus, but it was not always the index tumor. Several studies have described genetic heterogeneity within dominant tumor nodules and showed chromosomal differences between various areas of the same disease focus. Because of the genetic variability of prostate cancer and its multifocal nature, debate continues regarding the importance of smaller tumors and their impact on tumor progression and patient survival. There is genetic heterogeneity within dominant tumor nodules and chromosomal differences between various areas of the same disease focus. Because of the genetic variability of prostate cancer as well as its multifocal nature, debate continues regarding the importance of smaller tumors and their impact on tumor progression and patient survival.
Family history is one of the strongest risk factors for the development of prostate cancer, with a two- to eightfold higher risk of prostate cancer in men with an affected first-degree relative. Prostate cancer associated with familial clustering and high incidence of cancer among multiple first-degree relatives with a diagnosis before age 60 is considered hereditary and/or familial prostate cancer (HPC/FPC). Approximately 9% of all cases are attributable to hereditary prostate cancer following an autosomal dominant susceptibility pattern. Prostate cancer susceptibility genes have been identified using lineage analysis of affected families. Significant linkage between chromosome 1q24-25, the HPC1 locus, and hereditary prostate cancer has been established. RNASEL, which lies within the HPC1 locus, encodes an endoribonuclease that mediates the activities of an interferon-inducible RNA degradation pathway. Polymorphisms of the RNASEL gene have been associated with increased prostate cancer risk. However, not all studies have confirmed these findings. Mutations in the RNASEL gene do not occur at a greater frequency in patients with familial prostate cancer compared with patients with sporadic prostate cancer. Recent genome-wide association studies (GWAS) consistently identified that several single-nucleotide polymorphisms (SNPs) in the 8q24 locus are associated with risk of HPC/FPC22. Genetic variants caused by polymorphisms or mutations in other genes, such as PALB2, BRCA2, the androgen receptor (AR), 5-α-reductase type II (SRD5A2), and CYP17, have also been implicated in the development of HPC/FPC.
Early candidate gene approaches have implicated many different genes in prostate cancer. Germline mutations involving ELAC2 ( HPC2 ), MSR1 , and RNASEL genes have been reported in familial prostate cancer. The most common somatic mutations found in sporadic prostate cancer include TP53 , PTEN , and AR . Recent whole-genome exon sequencing analyses identified significant mutated genes, including TP53, AR, ZFHX3, RB1, PTEN, APC, MLL2, OR5L1, and CDK12. Of these genes, MLL2, OR5L1, and CDK12 have unknown tumor suppressor functions in prostate cancer. The most commonly affected signaling pathways by genetic alterations are the WNT signaling (TP53, APC, CTNNB1, MYC, and SMAD4) and the PTEN interaction network (PTEN, MAGI3, and HDAC11).
DNA copy number variation (CNV) is DNA structure alteration involving relatively large (at least 1 kb) regions. CNV can manifest as loss or gain of chromosomal regions. Earlier studies using fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH) identified common losses at 1p, 6q, 8p, 10q, 13q, 16q, and 18q and gains at 1q, 2p, 7, 8q, 18q, and Xq. For example, MSR1, NKX3.1, and N33 are candidate tumor suppressor genes in prostate cancer lying within the most commonly deleted regions on chromosome 8p. MSR1 encodes a receptor on the macrophage cell surface that induces binding of oxidized low-density lipoprotein and other polyanionic ligands. Mutations, polymorphisms, or loss of the MSR1 gene may compromise global macrophage function, thereby exposing organs, including the prostate, to oxidative stress and damage. Although this gene does not code for prostatic proteins directly, oxidative stress has been implicated in the initiation of prostate carcinogenesis.
Loss of 8p23, a region that harbors the CUB and Sushi multiple domains 1 gene ( CSMD1 ), has been associated with advanced prostate cancer. The retinoblastoma (RB1) gene is also a tumor suppressor gene and lies within the 13q locus. It is deleted in early prostate cancer development in animal models, prostate cancer cell lines, and some human prostate cancer specimens. RB1 inactivation in prostate cancer is the result of loss of heterozygosity and mutation. The 10q locus is lost in up to 45% of prostate cancers examined, and MXI1 and PTEN are two putative tumor suppressors in this region.
Regions of chromosome amplification in advanced prostate cancer include 8q containing the MYC gene and Xq11-13 encoding the AR gene. Gene amplification at 11q13.1 has been associated with disease recurrence. There are several candidate genes in this location ( Table 38-1 ), but only MEN1 and MAP4K2 correlate with disease progression.
| Gene | Chromosome/Locus | Function | |
|---|---|---|---|
| PCA3 (DD3) | 9q21–22 | ncRNA with unknown function | Oncogene |
| EZH2 | 7q35 | Gene silencing by histone modification | Oncogene |
| NKX3-1 (NKX3.1) | 8p21 | Homeobox gene, regulates epithelial growth and differentiation | Tumor suppressor |
| PTEN | 10q23 | Dual specificity protein/3-lipid phosphatase | Tumor suppressor |
| CDKN1B (p27) | 12p11–13 | Cyclin-dependent kinase inhibitor | Tumor suppressor |
| KLF6 | 10p15 | Zinc finger transcription factor | Tumor suppressor |
| ERG/ETV1 ( ETS family TMPRSS2-ERG ) | 21q22.3/7p21.2 | Androgen-responsive fusion protein | Fusion oncogene |
| RB1 (RB) | 13Q14–1-14–2 | Suppress cell division | Tumor suppressor |
| TP53 (p53) | 17p13 | Cell cycle control | Tumor suppressor |
| CSMD1 | 8p23 loss | CUB and Sushi multiple domains 1 | Tumor suppressor |
| MAP4K2 | 11q13.1 gain | Mitogen-activated protein kinase 2 | Oncogene |
| MEN1 | 11q13.1 gain | Multiple endocrine neoplasia | Oncogene |
| SF1 | 11q13.1 gain | Splicing factor 1 | Oncogene |
| PPP2R5B | 11q13.1 gain | Protein phosphatase2, regulatory subunit B isoform | Oncogene |
| NAALADASEL | 11q13.1 gain | N -acetylated α-linked acidic dipeptidase-like | Oncogene |
| EHD1 | 11q13.1 gain | EH-domain containing 1 | Oncogene |
Recent prostate cancer genome sequencing studies consistently show that the genes most commonly affected by loss of copy number are CHD1, NTSE, PTEN, RB1, and TP53, and the most gained genes are AR, MYC, PIK3CA, and the HOXA3 cluster. These aberrations are more prominent in castration-resistant prostate cancer compared to localized disease.
The prostate cancer genome can harbor an average of 90 chromosomal rearrangements involving many genes. One of the most frequent gene fusion events in prostate cancer is the fusion of the TMPRSS2 and ERG genes, which are located 3 Mb apart on chromosome 21q22.2. TMPRSS2 is an androgen-responsive gene, and ERG encodes an erythroblast transformation-specific (ETS) transcription factor. Their fusion is believed to be stimulated by androgens that recruit AR and TOP2B topoisomerase to chromosomal sites where TOP2B introduces double-strand breaks in DNA. TMPRSS2-ERG fusion results in overexpression of ETS genes in prostate cancer. TMPRSS2-ERG has been identified in 40% to 70% of prostate cancer and correlated with metastasis and disease-specific mortality. Gene fusion events such as TMPRSS2-ERG may be used for prostate cancer diagnosis through simple PCR detection of gene fusions in urine sediment.
The Wnt signaling pathway plays a key role in embryonic development and is essential for the maintenance of stem cells. Wnt is an extracellular protein that interacts with the membrane-bound frizzled receptor to initiate its biologic activity. Wnt signaling leads to stabilization of CTNNB1 and its nuclear accumulation. Nuclear CTNNB1 converts the TCF/LEF DNA-binding protein complex from a transcriptional repressor into a transcriptional activator. Inappropriate activation of the Wnt pathway is observed in many cancers and is putatively associated with tumor development.
In mice, CTNNB1 stabilization through targeted excision of CTNNB1 exon 3 induces prostate intraepithelial neoplasia (PIN)-like lesions that are similar to the early stages of human prostate cancer. In human prostate cancers, high levels of nuclear CTNNB1 are detectable by immunohistochemistry, whereas their levels are undetectable in normal prostate tissue. High levels of CTNNB1 expression are associated with the more aggressive prostate tumors. Together, these findings imply that inappropriate activation of the Wnt signaling pathway can contribute to prostate cancer and progression.
There are several mechanisms by which the Wnt pathway may be inappropriately activated in prostate cancer; DNA methylation plays a key role in several of these processes. The adenomatous polyposis coli (APC) gene is hypermethylated in prostate tumors relative to samples of benign prostatic hyperplasia (BPH; 64.1% vs. 8.7%). APC is a key component of the CTNNB1 degradation complex. Thus, methylation-dependent silencing of APC can lead to CTNNB1 accumulation and Wnt pathway activation.
E-cadherin (CDH1), a cell membrane protein, interacts with CTNNB1 and sequesters it at the inside surface of the cellular membrane. However, CDH1 expression is often lost in prostate cancers because of chromosomal loss or promoter hypermethylation. Thus, because CDH1 is no longer present, CTNNB1 is released into the cytoplasmic and nuclear compartments, leading to Wnt pathway activation. Finally, the secreted-frizzled related proteins (SFRPs) and Wnt inhibitory factor-1 (Wif-1) sequester Wnt and antagonize Wnt signaling. In this manner, loss of SFRP/Wif-1 expression can lead to Wnt pathway activation. The genes encoding several of the SFRPs and Wif-1 are epigenetically silenced by DNA methylation in colorectal, lung, bladder, and kidney cancers and lymphocytic leukemia. In prostate cancer, Wif-1 expression is strongly suppressed. The SFRP1 gene is also aberrantly hypermethylated in prostate tumors relative to BPH tissue and is partially to completely methylated in several human prostate cancer cell lines. These findings suggest that silencing of genes antagonist to Wnt may play a role in prostate cancer development.
The Wnt signaling pathway may interact with other signaling pathways such as the AR-CTNNB1, which in turn can upregulate AR transcriptional activity in an androgen-dependent manner. Subsequently, AR enhances nuclear translocation of CTNNB1. In addition, PI3K/Akt can modulate the activity of CTNNB1 by phosphorylation of CTNNB1 by GSK3B, a substrate of Akt.
Only a small fraction of the transcription output in human genome encodes for proteins. Noncoding RNAs (ncRNAs) are arbitrarily classified into two major classes based on their size: small (microRNA) and long ncRNA (lncRNA).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here