Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Among all of the capillaries in the body, the glomerulus is arguably the most unusual and important, if not the most aesthetically interesting. In this chapter, we review the morphogenesis of this unique capillary, discuss the origins of its cells and extracellular matrices, and describe some of the primary regulatory events that occur during glomerular development.
Among all of the capillaries in the body, the glomerulus is arguably the most unusual and important, if not the most aesthetically interesting. In this chapter, we review the morphogenesis of this unique capillary, discuss the origins of its cells and extracellular matrices, and describe some of the primary regulatory events that occur during glomerular development.
Formation of the permanent, metanephric kidney begins at embryonic day 11 in mice, day 12 in rats, and during the 4th-5th week of gestation in humans. As the ureteric bud projects from the mesonephric duct and enters the metanephric anlage, mesenchymal cells condense around the bud’s advancing tip. Soon thereafter, the condensed mesenchyme converts to an epithelial phenotype and proceeds through a developmental sequence of nephric structures, which are termed vesicle, comma-, and S-shaped, developing capillary loop, and maturing glomerulus stages. Bud tip stimulation of mesenchymal cell induction and aggregation, conversion to epithelium, and glomerular and tubule differentiation occur repeatedly until the full complement of nephrons has developed. Nephrogenesis concludes ~1 week after birth in rodents, and during the 34th gestational week in humans.
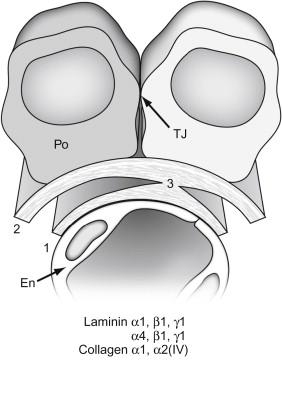
At the inception of the vesicle stage of nephron development, the aggregated mesenchymal cells near the ureteric bud tips convert to a cluster of epithelial cells (vesicle), and begin assembling a basement membrane matrix containing collagen type IV, laminin, and basement membrane proteoglycans around the basal surface of the vesicle. As development progresses through the comma- and then S-shaped stages, a groove (vascular cleft) forms in the lower aspect of the vesicle, into which endothelial precursor cells (angioblasts) migrate ( Figure 26.1 ). Two epithelial layers can be distinguished beneath the vascular cleft: visceral epithelial cells (which ultimately differentiate into podocytes); and parietal epithelial cells (which will become the thin epithelium lining Bowman’s capsule of the mature nephron). Epithelial cells above the vascular cleft ultimately develop into proximal, Henle’s loop, and distal tubule epithelium. During the S-shaped phase of nephron development, the distal segment fuses with the same ureteric bud branch tip that initially induced the nephric structure, so that the lumen of the forming nephron is now continuous with that of the developing collecting system. Continued growth and branching of the ureteric bud leads to the induction of new mesenchymal aggregates, and glomerulo- and tubulogenesis continues until the full complement of nephrons is achieved.
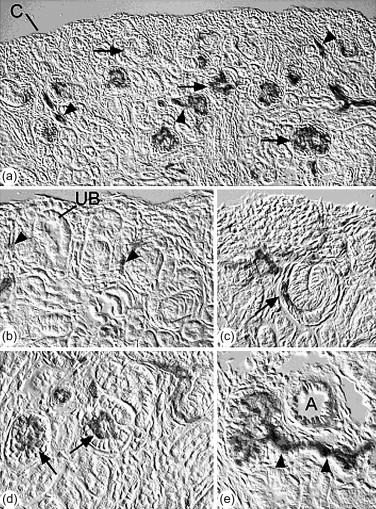
With the progressive invasion and differentiation of endothelial cells, the developing capillary endothelium assembles a subendothelial basement membrane matrix. Similarly, the developing podocyte cell layer assembles a subepithelial basement membrane, so that two distinct basal laminae can be seen between the endothelial and epithelial cells ( Figure 26.2 ). As nephrons develop further, these two basement membranes layers merge to form the glomerular basement membrane (GBM), with endothelial cells lining its inner surface, and podocytes adherent to its outer surface.
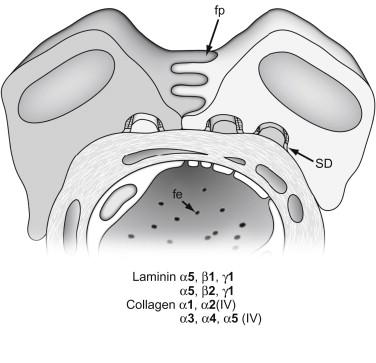
As glomerular capillary loops begin to form, the endothelial cells gradually flatten and become extensively fenestrated ( Figures 26.3 and 26.4 ). Initially, the fenestrations are spanned by diaphragms, but these structures soon disappear. The epithelial podocytes, which originally were columnar with apical junctional complexes, also begin to flatten and begin sending out basolateral cytoplasmic projections that interdigitate with similar projections from neighboring cells ( Figure 26.3 ). As glomeruli mature, these projections go on to develop into the podocyte pedicels or foot processes ( Figures 26.3 and 26.4 ). The apical junctional complexes migrate basolaterally between these cellular projections and, although the mechanism is not fully-understood (see below), convert into the slit diaphragm complex between foot processes ( Figure 26.4 ). Metabolic labeling studies, histochemical and immunohistochemical techniques, and inter-species transplantation experiments have all shown that both the endothelium and epithelium are actively synthesizing glomerular basement membrane (GBM) proteins at this time.
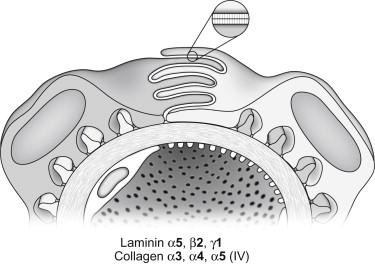
Here, capillary loop diameters expand, and endothelial and podocyte cell layers differentiate further until the fully mature glomerular morphology is achieved. Unfused basement membranes are rarely seen in maturing glomeruli. On the other hand, complex, irregular projections of basement membrane are commonly found beneath podocytes at this stage, particularly in areas where foot processes are broad and their formation into relatively narrow pedicels is still incomplete. In vivo labeling studies have shown that these subepithelial basement membrane segments are somehow spliced or inserted into the existing fused GBM, possibly to provide the additional GBM material necessary for the inflating capillaries. Shortly after the initial, vascular cleft stage, and continuing into maturing glomeruli, modification and remodeling of the GBM occurs, with the appearance of new basement membrane protein isoforms and the disappearance of earlier species. As discussed later, however, we do not understand how these events are regulated at either the gene or protein level. Additionally, once the glomerulus is fully mature ( Figure 26.4 ), matrix synthesis and cell morphogenesis virtually halt (except for some poorly-understood activities responsible for GBM “maintenance” or “turnover”), and how these processes are downregulated is also not known.
Although compelling evidence has accumulated showing that nephron epithelial cells, including the visceral and parietal epithelium of Bowman’s capsule, all derive originally from the metanephric mesenchyme, the origin of vascular endothelial cells during kidney organogenesis has been more difficult to understand. Studies conducted nearly 30 years ago convincingly showed that cells of extrarenal origin grew into the metanephros and established the microvasculature, including the glomerular capillary tufts. These studies involved the grafting of embryonic, avascular mouse or quail kidney rudiments onto avian chorioallantoic membranes. After culturing in ovo , the kidney grafts contained glomerular endothelial and mesangial cells stemming from host chorioallantoic tissues, therefore signifying the ingress of vessel progenitors from sites outside the kidney.
Contrary to the results discussed above, several lines of evidence from a number of more recent experiments have shown that the metanephros contains its own pool of endothelial progenitors (angioblasts) capable of vascularizing nephrons in vivo . The first clues about the existence of these intrinsic metanephric angioblasts came from transplantation studies between mice and rats. For example, when E12 mouse kidneys are grafted into anterior eye chambers of rats, the vascular and glomerular basement membranes that develop within the grafts after transplantation are almost entirely of mouse (graft) origin. Similarly, when E12 kidneys are transplanted under kidney capsules of adult ROSA26 mice (which bear a ubiquitously expressed LacZ reporter gene useful as a cell lineage marker), all of the microvascular and glomerular endothelial cells within grafts are derived from the engrafted kidney, not from the host. Furthermore, when kidneys from E12 ROSA26 mice are grafted into the nephrogenic renal cortices of newborn wild-type hosts, endothelial cells stemming from the grafts can be seen integrating into host vasculature. In additional experiments, when embryonic kidneys from Flk1 (VEGF receptor-2)–LacZ heterozygous mice are grown under routine organ culture conditions, Flk1-LacZ-positive microvessels do not develop in vitro , despite the extensive formation of metanephric tubules and avascular glomerular epithelial tufts. When these same cultured kidneys are then transplanted into anterior eye chambers of wild-type host mice, the grafts develop microvessels and vascularized glomeruli lined by Flk1-LacZ-expressing cells, indicating again that the endothelium originates from the engrafted kidney itself, and not from the host. Several other research groups have reached similar conclusions independently. For example, when avascular metanephroi from E11 Tie1/LacZ transgenic mice are transplanted into newborn wild–type hosts, widespread expression of Tie1/LacZ is found within glomeruli developing within grafts. Others have immunolocalized putative angioblasts in the metanephric mesenchyme of prevascular embryonic rat kidney.
Although current evidence shows that the embryonic kidney contains a pool of angioblasts capable of establishing the glomerular endothelium, whether these progenitors originate initially from outside the metanephric blastema or instead stem directly from metanephric mesenchyme is not yet clear. Nevertheless, immunolabeling experiments in developing rat kidney shows that endothelial, as well as mesangial, cell precursors share common markers during glomerulogenesis (RECA-1 and Thy1.1, respectively), suggesting that they may indeed derive from metanephric mesenchyme. Other immunolocalization and transplantation experiments have shown that juxtaglomerular cells in developing kidney also originate from metanephric mesenchyme, although they appear to stem from a different lineage than endothelial and mesangial cells.
Mechanisms controlling vascular development are highly complex and involve several different transcription factors, cell-cell and cell-matrix interactions, and many membrane receptor–ligand signaling cascades. Although our knowledge of these systems in a variety of vascular beds has improved dramatically during the past several years, many key questions regarding temporal and spatial controls still persist. With respect to the formation of glomerular capillaries, the process can be considered to progress through four interrelated events: (1) angioblast survival, proliferation and differentiation into endothelium; (2) glomerular endothelial cell recruitment; (3) initial assembly of the glomerular capillary tuft and associated mesangium; and (4) glomerular capillary stabilization and maturation.
Among all of the mechanisms involved with development of the systemic vasculature, signals evoked by binding of VEGF to its cellular receptors, VEGFR-1 and VEGFR-2, are singularly critical. Mice with homozygous Vegfa gene deletions die by E9.5 with severe vascular deficits. Remarkably, Vegfa heterozygote mutants also succumb by E12 with vascular phenotypes, indicating that a single Vegfa allele is insufficient to direct normal vascular development. Homozygous (but not heterozygous) Vegfr1 and Vegfr2 mutants die at mid-gestation, due to failure of endothelial differentiation and vessel integrity, respectively.
Developing podocytes are key sources of VEGF, and its secretion and binding to angioblasts bearing VEGF receptors may initiate their recruitment into the vascular cleft of comma- and S-shaped nephrons, which is the initial site of glomerulogenesis. Because Vegfa and Vegfr2 knockout mice die with vascular phenotypes before glomerulogenesis commences, the precise role of this ligand-receptor pair in mediating glomerular endothelial development has been difficult to analyze fully. Nevertheless, and underscoring the importance of the VEGF signaling system, injection of VEGF-blocking antibodies into developing mouse kidney cortex inhibits glomerular capillary formation in vivo . With the advent of cell selective and/or inducible gene deletion technologies, additional evidence for the importance of podocyte-derived VEGF has been obtained. For example, homozygous deletion of Vegfa selectively in podocytes (obtained in bi-transgenic mice carrying nephrin-cre recombinase and floxed Vegfa alleles) results in animals which die perinatally with non-vascularized glomeruli. Heterozygous deletion of Vegfa causes no evident phenotype initially. By 2.5 weeks of age, however, mice become proteinuric, and glomeruli contain swollen endothelial cells and hyaline deposits similar to those seen in patients with pre-eclampsia. By contrast, overexpression of the VEGF 164 isoform specifically in podocytes leads to collapsing glomerulopathy and death at ~5 days of age. When Vegfa is selectively deleted in podocytes of adult mice (using a Tet-On conditional expression model), mice become severely proteinuric and hypertensive, and glomeruli resemble those of humans with thrombotic microangiopathy (mesangiolysis, endothelial swelling, red cell fragmentation, and fibrin deposition). Clearly, the cellular controls for maintaining VEGF protein expression within an optimal range are critically important for the appropriate establishment and maintenance of the glomerular capillary.
Transcription of VEGF and VEGFR genes is activated by hypoxia-inducible transcription factors (HIFs), which consist of heterodimers of HIF α- and β-subunits. Under normal oxygen concentrations, the HIF α-subunit undergoes prolyl hydroxylation, binding to von Hippel Lindau protein (VHL), polyubiquitination, and proteasomal degradation. In hypoxia, the prolyl hydroxylase enzyme is inhibited, and HIF α chain degradation is avoided. Hypoxia-stabilized HIF α/β heterodimers bind to hypoxia-responsive elements (HREs) located in promoter/enhancer regions of inducible genes, many of which are proteins expressed in response to hypoxic stress. For example, erythropoietin, transferrin, VEGF, VEGFR1, and VEGFR2 are among the more than 70 distinct genes known to be transcriptionally activated by HIFs.
There are at least three distinct HIF α- and two β-subunits known at present, making a variety of different HIF isoforms possible. Because HIF stabilization is enhanced in cells experiencing subnormal oxygen tensions, such as those in rapidly growing tissues, robust VEGF and VEGFR synthesis commonly occurs during organogenesis. Increased VEGF/VEGFR signaling stimulates mitosis in endothelial progenitor cells, phosphorylation of the antiapoptotic kinases Akt/PKB and focal adhesion kinase (FAK), and upregulation of the survival factors Bcl2 and A1. In time, these events can lead to the creation of new blood vessels, which can then provide appropriate levels of oxygen specifically to the formerly hypoxic tissue sites.
The expression patterns for several of the different HIF α- and β-subunits have been documented in developing human, rat, and mouse kidney using in situ hybridization and immunohistochemistry. In general, both HIF-1 and HIF-2α are found in glomeruli, with specific immunolocalization of HIF-2α protein to immature podocytes (which are rich sources of VEGF). HIF-2α is also expressed by developing vascular endothelial cells in the kidney (most of which express VEGFR-2), whereas HIF-1α is found in cortical and medullary collecting duct epithelium. HIF-1α and HIF-2α protein are undetectable in fully mature glomeruli. Mice with a global deletion of Hif2α show no defects in glomerular development or function, and no deficits in VEGF or Flk1 expression. Interestingly, HIF-1β is apparently ubiquitously expressed by all cells in the kidney, but HIF-2β distribution is greatly restricted during development and, in mice, becomes confined to nuclei in cells of the thick ascending limb of Henle’s loop.
The selective expression of certain HIF isoforms in different tissue compartments of developing kidney may reflect the coordinated regulation of different sets of HIF target genes. Importantly, individuals with mutations in VHL, a key protein in mediating HIF α chain degradation, and thereby reducing expression of HIF target genes, are prone to developing hemangioblastomas and clear cell-renal cell carcinomas. Some studies have shown that HIF-1α and HIF-2α had differential and sometimes antagonistic effects on the growth of clear cell-renal cell carcinomas, with HIF-1α retarding and HIF-2α promoting tumor growth. These findings provide further evidence for differential effects of different HIF isoforms, and call for more studies examining the expression of HIF and HIF gene targets in the developing kidney. Surprisingly, when Vhl is selectively deleted in podocytes, glomerular vascularization patterns are not affected, and kidneys develop normally. On the other hand, mice become proteinuric by 4 weeks of age, and there is ectopic deposition of collagen (α1) 2 α2(IV) in peripheral loop GBMs, and upregulation of an ancient oxygen-binding protein, neuroglobin, specifically in podocytes.
Once glomeruli are vascularized and fully mature, podocytes still continue VEGF synthesis. Likewise, expression of Flk1 is also maintained by glomerular endothelial cells of mature kidneys. VEGF-Flk1 signaling in glomeruli therefore probably exerts functions extending well beyond those needed for mobilization of angioblasts and initial formation of the capillary tuft. For example, in co-cultures of epithelial cells with endothelium, epithelial-derived VEGF has been shown to induce fenestrae formation in the endothelium. When Vegfr2 is inducibly deleted in adult mice, podocytes appear normal, but there is loss of viable glomerular endothelial cells. Perhaps the continued expression of VEGF by podocytes and Flk1 by glomerular endothelial cells in vivo is necessary for maintenance of the highly-differentiated state seen in the endothelium.
Beyond VEGF and VEGFR, several other growth factor-receptor signaling systems important for vessel development systemically are also crucial for glomerular capillary formation, including the Tie/angiopoietin and PDGFR/PDGF families. Developing glomerular endothelial cells express Tie-2, and and one of its ligands, angiopoietin-1, is important for vascular organization and remodeling. Another Tie-2 ligand, angiopoietin-2, may mediate vascular integrity and permeability. The coordinated expression of these two angiopoietins may therefore regulate the maturation and stabilization phases of glomerular development (reviewed in ). Additionally, Tie-2 and at least some members of the angiopoietins contain defined HREs in their promoters, making their transcriptional regulation by hypoxia/HIFs seem likely. Similarly, an HRE is found in the PDGFB gene promoter, although this may not necessarily be responsive to hypoxia. During early glomerular development, PDGFB protein is expressed by podocytes. This may be important for the glomerular recruitment of immature mesangial cells, which express the PDGFB receptor, PDGFRβ. In later developmental stages, both PDGF and PDGFRβ expression becomes confined to the mesangium, which may provide autocrine signals required for mesangial cell proliferation and/or maturation (see below).
Like other developing vessels, at least some neuronal axon guidance receptors and ligands are also found in developing glomeruli. For example, neuropilin-1 (Np1), which is a co-receptor with VEGFR2 for VEGF 164 (but lacks a cytoplasmic signaling domain), immunolocalizes to glomerular endothelial cells. Semaphorins-3A and -3F, which are ligands for Np1, have been found on podocytes, suggesting that semaphorin-Np1 signaling between podocytes and endothelium may help pattern glomerular morphogenesis. One study, however, has also reported that Np1 is expressed by podocytes in vivo . Recent experiments also showed that podocyte-derived VEGF may act as an autocrine survival factor for cultured podocytes in vitro . Additionally, these same studies found an upregulation of VEGFR2 in cultured podocytes, suggesting that VEGF/VEGFR2 signaling is important not only for glomerular capillary formation and maintenance, but also for podocyte differentiation.
Other receptor-ligand signaling systems probably crucial for glomerular capillary formation include members of the Eph/ephrin receptor/counter-receptor families. Specifically, the receptor tyrosine kinase EphB1 and its ligand, ephrin-B1, which itself is also a transmembrane protein receptor, are both expressed in similar distribution patterns in developing kidney microvasculature. Although the precise roles for Eph/ephrin signaling in the glomerulus are still uncertain, knockout mice display lethal vascular phenotypes, including defects in vessel patterning, sprouting, and remodeling (reviewed in ). Reciprocal gradients of Eph and ephrin protein concentrations have been identified in the developing brain, where they appear to direct accurate neuronal patterning in the visual system. Perhaps analogous events take place in the developing glomerulus, where spatial signals conveyed between endothelial cells help target them to correct microanatomical domains.
Fundamentals regarding the development of the intercapillary mesangium, as well as the origin and recruitment of mesangial cell progenitors, are still largely unresolved issues in glomerular biology. Nevertheless, we have known for some time that PDGFB and its receptor, PDGFRβ, are both expressed by mesangial cells of mature glomeruli. Additionally, studies in developing kidney have shown that immature podocytes produce PDGFB which may help recruit mesangial cell progenitors expressing PDGFRβ into glomeruli. Later, podocyte expression of PDGF declines, and the synthesis of both PDGF and PDGFRβ becomes confined to the mesangial cells, perhaps to promote their proliferation or maturation. Gene deletion studies in mice have conclusively shown an absolute requirement for PDGFB/PDGFRβ signaling. Null mutants for either genes die perinatally, with massive hemorrhaging systemically. Importantly, glomeruli in these mutants entirely lack mesangial cells and consist of one or only a few large, swollen capillary loops.
Interestingly, once the glomerulus has fully matured, there appears to be a small population of extraglomerular mesangial cells capable of completely repopulating the glomerulus if the intraglomerular mesangium becomes severely injured. These mesangial reserve cells reside in the juxtaglomerular apparatus, and are distinct from renin-secreting cells, macrophages, vascular smooth muscle cells, and endothelial cells. In an anti-Thy-1 model of proliferative glomerulonephritis in rats, these extraglomerular mesangial reserve cells migrate into the glomerulus and entirely restore the depleted intraglomerular mesangium. Alternatively, some studies suggest that bone marrow hematopoitic stem cells can be a source of mesangial cells. Perhaps additional studies based on these fascinating observations can shed more light on the origin and development of mesangial cells during glomerulogenesis. Similarly, much more work needs to be done on the assembly and maintenance of the mesangial matrix. Although this matrix undergoes morphologic and compositional changes throughout glomerulogenesis, it has not yet been the topic of thorough study. Whether the mesangial matrix is produced exclusively by mesangial cells or whether glomerular endothelial cells and podocytes also contribute components, are also not understood. On the other hand, and as discussed later, considerable progress has been made in understanding the assembly of the GBM (see below).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here