Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter describes surgical aspects of acquired mitral valve disease, excluding ischemic mitral regurgitation (see Chapter 10 ). Associated or secondary tricuspid valve disease is also considered (see Section II of this chapter and Chapter 14 ), as is concomitant coronary artery surgery in patients with nonischemic mitral valve disease (see Section III of this chapter).
Sir Lauder Brunton was among the first to consider surgical treatment of mitral stenosis in his “preliminary note” in The Lancet in 1902. Cutler, then at Western Reserve University Medical School in Cleveland and later the Mosley Professor of Surgery at Harvard Medical School and Peter Bent Brigham Hospital in Boston, did experimental work on surgical approaches to mitral stenosis. In 1923, he and Levine reported an operation via median sternotomy in which a special curved knife was inserted through the left ventricular (LV) apex to cut a stenotic mitral valve. In 1925, Souttar digitally opened a stenotic mitral valve through the left atrial appendage.
An effective closed surgical approach to mitral stenosis began with Harken and colleagues and Bailey in the United States and Brock and colleagues in London. Harken had been doing animal experiments involving mitral valve surgery at the Boston City Hospital in 1939 before serving with the United States Army in World War II, during which time he became well known for successful removal of missiles and shell fragments from the heart. After the war he continued his work on mitral valve surgery at the Boston City and Peter Bent Brigham Hospitals. Bailey was working primarily at Hahnemann Hospital in Philadelphia. Although their techniques and terminology were somewhat different, their approaches to opening the valve through the left atrial appendage were similar. Technical modifications subsequently added to closed commissurotomy included Tubb's transventricular dilator, used with digital control by a finger inserted through the left atrial appendage.
In 1955, surgeons began to think of opening stenosed mitral valves by intracardiac techniques on cardiopulmonary bypass (CPB). However, closed heart operations produced such generally good results that the open heart technique did not come into wide use until after 1970.
Although a few ingenious closed methods of surgically improving mitral regurgitation were reported during the 1950s, particularly by Bailey, Davila, Nichols, and their colleagues, an effective open approach using CPB was not made until 1957 by Lillehei and colleagues and Merendino and Bruce. McGoon described an effective repair for mitral regurgitation due to ruptured chordae in 1960. In subsequent years, a number of surgeons have contributed technical advances in the repair of mitral regurgitation, particularly Carpentier, Duran, Frater, Reed, and their colleagues.
A number of surgeons realized very early the need for replacing at least some diseased mitral valves. However, it was Starr and Edwards from the University of Oregon Medical Center who, in 1961, first reported successful mitral valve replacement using a mechanical prosthesis.
Although the Starr-Edwards valve became the “gold standard” in prosthetic valves for most of the next decade, it soon became apparent that aggressive anticoagulation was necessary to control the marked thromboembolic tendency. Valve design focus subsequently was redirected toward lower-profile valves with novel occluder designs. The Bjork-Shiley prosthesis, designed by cardiac surgeon Viking Bjork of Sweden and Earl Shiley in California, was the first successful tilting disc valve. It emerged as the leading prosthesis in the 1970s. It was first marketed in 1971 with a carbon-coated disc and both inflow and outflow struts welded to the chromium alloy orifice. The hemodynamics and freedom from hemolysis were superior, but strict anticoagulation was required to prevent valve thrombosis. When a later design change (convexo-concave disc) was associated with strut fracture, the valve was eventually taken off the market. Enduring bileaflet valves would await the application of pyrolytic carbon technology from the space industry in about 1977. The bileaflet St. Jude Medical valve developed in the late 1970s became the dominant prosthetic valve of the 1980s, offering further improvement in hemodynamics, less blood stagnation, greater opening of the leaflets, and a lower risk of thromboembolism.
Biological or tissue prosthetic valves had been in development since the 1950s, and in the 1960s formalin fixation was introduced to sterilize and fix heterograft tissue. When investigators became aware of the tendency of formalin fixation to induce collagen breakdown in valve cusps—with resultant fibrosis, calcification, and degeneration—tissue fixation of porcine valves with glutaraldehyde rapidly became the standard. The first commercially available bioprosthetic valves were developed by Hancock in the United States (1970) and Carpentier in Paris.
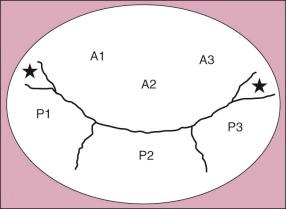
Anatomy of the mitral valve is discussed in detail in Chapter 1 . Several features are of particular importance in considering valve pathology and reparative techniques. The anterior leaflet is conveniently divided into three sectors: A 1 laterally, A 2 centrally, and A 3 medially. The posterior leaflet is also divided into three sectors: P 1 laterally, P 2 centrally, and P 3 medially ( Fig. 11-1 ). When the cause of regurgitation is prolapse of the posterior leaflet , sectors P 2 and P 3 are usually involved. Additionally, there is usually some degree of anular dilatation.
The right fibrous trigone is adjacent to the posteromedial commissure and is part of the central fibrous body located at the intersection of the membranous septum, mitral and tricuspid anulus, and aortic anulus ( Fig. 11-2 ). The left fibrous trigone is located near the aortic anulus under the left aortic cusp and adjacent to the anterolateral commissure. The posteromedial and anterolateral papillary muscles give rise to chordae tendinae going to both leaflets. The chords are generally categorized into three groups: first-order chordae originate near tips of the papillary muscles and insert on the free edge of the leaflets. These chordae prevent valve edge prolapse during systole and, when elongated or ruptured, produce mitral regurgitation. Second-order chordae (including two or more longer strut chordae) insert on the ventricular surface of the leaflets at the junction of the rough zone (closer to the free edge) and clear zone, which is demarcated by a ridge that corresponds to the line of leaflet coaptation. Third-order chordae originate from the underlying ventricular wall and insert on the posterior leaflet near the anulus. In addition, distinct commissural chordae exist at the commissures.

Acquired mitral stenosis usually results from rheumatic heart disease, as does mixed stenosis and regurgitation. It occurs as an isolated valvar condition in 40% of patients with rheumatic heart disease.
Commissural fusion and leaflet thickening are the dominant features in clinically important mitral stenosis. The characteristic fusion of the edges of the mitral leaflets in commissural areas is a complex process involving coapting edges of commissural, posterior (mural), and anterior (septal) leaflets at both the anterolateral and posteromedial commissures. Valve leaflets are thickened to varying degrees, particularly at their free edges and sites of fusion. Calcification often occurs in older patients, beginning at the commissures but at times extending posteriorly into the anulus.
Chordae tendineae are variably involved by the rheumatic process. Occasionally, in the presence of severe mitral stenosis, the chordae are nearly normal in appearance, and opening the valve commissures results in a wide orifice. More often, some degree of chordal thickening, fusion, and shortening is present in each commissural area. This process is extreme in some patients, particularly those in whom restenosis develops after an earlier commissurotomy, and results in an obstructing, tough, subcommissural mass on both sides of the narrow orifice. This advanced pathology may be purely rheumatic in origin or may partly result from hemodynamic and (in the case of restenosis) surgical trauma. Subcommissural pathology may render the mitral apparatus severely stenotic, even after commissurotomy. Rarely, mucopolysaccharidosis or severe anular calcification may cause pure mitral stenosis.
The left atrium is enlarged in pure mitral stenosis but usually not severely, and its wall is thickened. LV volume and mass are normal or slightly reduced. When fibrosis and particularly calcification involve the mitral anulus, regional wall motion at the LV base is impaired, but overall LV systolic and diastolic function are often normal. Late in the disease, however, LV function may be impaired.
Pulmonary vascular resistance (Rp) may increase in patients with severe mitral stenosis. This increase may be the result of spasm in the pulmonary arterioles, presumably a reflex from left atrial hypertension. Organic pulmonary vascular disease may also increase Rp and is often found in young Polynesian and Asian patients, as well as in a small proportion of other patients with long-standing mitral stenosis. Rarely, the vascular disease may progress to obliteration of pulmonary arterioles. Increased Rp produces a rise in pulmonary artery and right ventricular pressure out of proportion to the valve stenosis and left atrial pressure increase, which leads to right ventricular (RV) hypertrophy and secondary tricuspid regurgitation.
Mixed mitral stenosis and regurgitation is primarily rheumatic in origin. Stenosis is produced by varying degrees of commissural fusion and chordal thickening. Regurgitation results from fibrous retraction of the central unfused portion of the leaflets and either chordal shortening or chordal elongation. Shortening restricts leaflet motion and increases the gaping central orifice, whereas elongation permits cusp prolapse. Occasionally, chordae rupture as a result of the rheumatic process.
Endocarditis on a rheumatic stenotic valve adds regurgitation by eroding leaflet or chordal tissue.
Regurgitation may be due to rheumatic valve disease, but has numerous other causes and morphologic patterns.
Mitral regurgitation may occur as a severe lesion (sometimes combined with aortic regurgitation) during the acute rheumatic process in association with extensive myocarditis and sometimes pericarditis and pancarditis. Anular dilatation is the primary cause of regurgitation in this circumstance, with the valve leaflets frequently showing edema only and virtually normal chordae. After remission of the acute process, regurgitation may spontaneously regress, presumably because the myocarditis heals, the heart becomes smaller, and anular dilatation regresses. In most cases, however, there is progressive leaflet thickening, particularly of the posterior leaflet, which becomes retracted and rolled with shortening of chordae. The anterior leaflet is less thickened, and major chordae are frequently elongated, allowing leaflet prolapse. The posterior chordae may also elongate, and occasionally one or more may rupture. Commissural leaflets are obliterated and fused, but the commissures remain more or less open. Calcification is uncommon. Anular dilatation is almost invariably progressive and produces increasing regurgitation.
Mitral valve prolapse is a billowing of one or both leaflets into the left atrium during ventricular systole, with or without mitral regurgitation. Prolapse of a mitral valve leaflet occurring as an isolated abnormality 1 is a relatively common and complex entity, occurring in 1% to 2.5% of the population. Familial mitral valve prolapse is inherited as an autosomal trait. Primary mitral valve prolapse occurs with increased frequency in patients with Marfan syndrome and certain other connective tissue disorders.
1 Variously termed myxomatous mitral valve prolapse, myxomatous mitral valve degeneration, floppy valve , “the symptom complex of midsystolic click and late systolic murmur,” and Barlow syndrome .
Our understanding of the anatomic details of prolapsing anterior and posterior leaflet components has been enhanced by the classification of Carpentier and others. In a pathologic study, Quill and colleagues identified abnormal (“deviant”) clefts in all valve segments, but most commonly in the P 2 region. Such abnormal clefts may contribute to mitral regurgitation observed in degenerative mitral valve disease.
Infrequently, in its severe form, mitral prolapse results in important mitral regurgitation (10% of patients ). Nonetheless, in the United States mitral valve prolapse has been reported as the most common cause of surgically treated isolated mitral regurgitation. The basic pathologic conditions are mitral leaflet redundancy and myxomatous leaflet thickening , resulting at least partly from myxomatous proliferation of the acid mucopolysaccharide within the spongiosa, which replaces collagen in the leaflets. The redundant and elongated leaflets no longer meet properly to support each other during systole, and they begin to overshoot into the left atrium. Not only is the valve thereby rendered regurgitant, but abnormal strain is placed on the chordae as well. The chordae elongate, and ultimately some rupture, producing more regurgitation. These histologic changes and severe valve redundancy are especially pronounced in younger patients with Barlow syndrome. Older patients with degenerative mitral regurgitation are more likely to have fibroelastic deficiency and less redundant valve tissue. These differences have important surgical implications because marked leaflet redundancy with more severe myxomatous changes requires more extensive reconstructive techniques. Calcifications may occur in the mitral anulus, but do not appear to contribute to mitral valve dysfunction.
“Idiopathic” and more or less localized chordal rupture is usually a variant of mitral valve prolapse syndrome, in which a considerable portion of leaflet tissue is uninvolved by the myxomatous process. In most cases, the posteromedial portion of the posterior leaflet is involved; after chordal rupture, this becomes redundant and flail. More extensive posterior chordal rupture sometimes occurs. Localized chordal rupture may also occur in patients with Marfan syndrome.
Mitral anular calcification may occur in older patients without evident disease of the leaflets or chordae, but it may be more common in patients with myxomatous degeneration and prolapse of the mitral leaflets. Anular calcification is probably a degenerative disease, more common in elderly patients and apparently more common in women. It is also seen in patients with LV hypertrophy, particularly those with hypertrophic obstructive cardiomyopathy (HOCM; see Chapter 19 ). The process involves the posterior or mural portion of the anulus more often than other portions. Degenerative anular calcification often extends into the adjacent ventricular myocardium, and it may secondarily produce mitral regurgitation or stenosis by displacing or immobilizing the mitral leaflets. Anular calcification considerably complicates mitral repair or replacement.
Papillary muscle dysfunction or rupture resulting from myocardial infarction or ischemic fibrosis can produce severe mitral regurgitation (see Chapter 10 ).
Endocarditis is a relatively uncommon cause of pure mitral regurgitation compared with its etiologic frequency in aortic regurgitation. When the aortic valve is infected and regurgitant, vegetations may drop down onto and infect the central portion of the anterior mitral leaflet, producing perforation and mitral regurgitation. In the absence of aortic valve disease, a normal or abnormal mitral valve may become infected, with destruction of cusps, chordae, or both (see Chapter 15 ).
Submitral LV aneurysms frequently result in mitral regurgitation. This unusual type of aneurysm is not ischemic in origin and occurs most often among the southern and western African black population. It may be multiloculated and have a well-defined neck situated immediately beneath the posterior mitral leaflet. Mitral regurgitation often coexists because of aneurysmal distortion of the posterior leaflet and leaflet prolapse. In rare instances, the aneurysm bulges into the left atrium from behind, partly obstructing the mitral orifice.
The normal mitral valve orifice area in an adult is 4.0 to 5.0 cm 2 . Most symptomatic patients have a mitral valve area less than 2.5 cm 2 . The diastolic transmitral gradient is the fundamental physiologic expression of mitral stenosis. At any given orifice size, the transmitral gradient is a function of the square of the transvalvar flow rate and diastolic filling time. Thus, for example, doubling the flow rate quadruples the transvalvar gradient. This explains the importance of exertion or other causes of increased cardiac output in development of dyspnea (induced by increased left atrial and pulmonary venous pressure) during the initial stages of mitral stenosis. As heart rate increases, during atrial fibrillation for example, diastolic filling time is greatly reduced, thereby increasing the gradient and left atrial pressure.
Effective atrial contraction during sinus rhythm has a major lessening effect on dyspnea with severe mitral stenosis, because the left atrial pressure is lower than during atrial fibrillation, and the loss of atrial contribution during atrial fibrillation results in about a 20% reduction in cardiac output.
Although the clinical condition of mitral stenosis is a continuum without discrete hemodynamic abnormalities corresponding to functional state, the following hemodynamic guidelines are useful in defining severity of mitral stenosis :
Mild: valve area 1.5 to 3.5 cm 2 and mean diastolic gradient less than 5 mmHg
Moderate: valve area 1.0 to 1.5 cm 2 and mean diastolic gradient 5 to 10 mmHg
Severe: valve area less than 1.0 cm 2 and mean diastolic gradient greater than 10 mmHg
Patients with moderate mitral stenosis are often asymptomatic at rest or with ordinary activities, particularly until the third or early part of the fourth decade of life. With severe exertion, pulmonary edema may develop suddenly.
Patients with severe mitral stenosis and without important elevation of Rp (“unprotected” mitral stenosis) have easy fatigability and effort dyspnea, orthopnea, and paroxysmal nocturnal dyspnea. As the disease progresses in duration and severity, structural changes (alveolar basement membrane thickening, increased lymphatic drainage, adaptation of neuroreceptors) may allow patients to remain functional for prolonged periods. When Rp rises, the alveolar bed is protected from sudden rises in capillary pressure with exertion, so pulmonary edema does not occur, and orthopnea and paroxysmal nocturnal dyspnea disappear. Hemoptysis is more common in this setting. When the reduction in mitral valve orifice size reaches severe levels, resting cardiac output becomes subnormal, which is usually accompanied by varying degrees of increased Rp (see also Morphology ). The patient with advanced mitral stenosis who has low cardiac output and chronic heart failure secondary to high Rp is seldom seen today in developed countries. These patients tend to be women who have marked mitral facies, peripheral coldness, cyanosis, hepatic enlargement and pulsation, high jugular venous pressure with waves of tricuspid regurgitation, and sometimes ascites and peripheral edema.
In most patients, mitral stenosis can be diagnosed clinically based on history, physical examination, chest radiograph, and electrocardiogram (ECG). Auscultatory findings provide good evidence of mitral stenosis when they include a loud first sound, an opening snap, and the characteristic diastolic rumble with a presystolic crescendo when sinus rhythm is present. In severe stenosis the mid-diastolic murmur occupies more than half of diastole, and the opening snap is early.
In surgical candidates with important mitral stenosis, the chest radiograph typically shows some left atrial enlargement, although it is often only about grade 2 (on a scale of 1 to 6, with 6 being most severe). The left atrial appendage may or may not appear prominent along the left upper border of the cardiac silhouette. The LV is normal in size, but the RV and pulmonary trunk are usually somewhat enlarged. When Rp is elevated, the pulmonary trunk, branches, and hilar arteries are more enlarged; once tricuspid regurgitation occurs, there is considerable right atrial and RV enlargement. The lung fields also show varying degrees of pulmonary venous hypertension on the plain chest radiograph (large pulmonary veins in upper lung fields, interstitial pulmonary edema, Kerley B lines, or alveolar pulmonary edema).
The ECG is not diagnostic but often shows P-wave abnormalities characteristic of left atrial enlargement (P mitrale) or atrial fibrillation, and evidence of RV hypertrophy when pulmonary hypertension is present.
Two-dimensional (2D) echocardiography is highly reliable for diagnosing and quantifying severity of mitral stenosis. It demonstrates degree of stenosis, leaflet mobility, thickening and probable calcification, and any subvalvar obstruction. Doppler echocardiography, enhanced by color flow imaging to identify precise flow direction, is valuable for estimating severity of stenosis. Currently these methods suffice for estimating mitral valve area as well as morphology and gradient across the valve. An echocardiographic grading system for mitral stenosis has been endorsed in the American College of Cardiology/American Heart Association (ACC/AHA) guidelines, which is useful for identifying patient suitability for valvotomy (balloon catheter), surgical commissurotomy, or valve replacement ( Table 11-1 ). Greater leaflet mobility, less subvalvular involvement, and less leaflet calcification (grade 1) increases the likelihood of successful valvotomy or commissurotomy.
| Grade | Mobility | Subvalvar Thickening | Thickening | Calcification |
|---|---|---|---|---|
| 1 | Highly mobile valve with only leaflet tips restricted | Minimal thickening just below mitral leaflets | Leaflets near normal in thickness (4 to 5 mm) | A single area of increased echo brightness |
| 2 | Leaflet mid and base portions have normal mobility | Thickening of chordal structures extending up to one third of the chordal length | Midleaflets normal, considerable thickening of margins (5 to 8 mm) | Scattered areas of brightness confined to leaflet margins |
| 3 | Valve continues to move forward to diastole, mainly from the base | Thickening extending to distal third of the chords | Thickening extending through entire leaflet (5 to 8 mm) | Brightness extending into midportion of leaflets |
| 4 | No or minimal forward movement of the leaflets in diastole | Extensive thickening and shortening of all chordal structures extending down to papillary muscles | Considerable thickening of all leaflet tissue (>8 to 10 mm) | Extensive brightness throughout much of leaflet tissue |
Cardiac catheterization is usually unnecessary for diagnosing mitral stenosis and estimating its severity. Catheterization is necessary in patients older than about age 35 to study the coronary arteries, however, because about 25% of patients older than 40 with mitral stenosis without angina have important coronary artery disease. When balloon valvotomy is used, prevalvotomy and postvalvotomy measurements are easily made by classic catheterization techniques or by echocardiography. Pulmonary capillary wedge pressure (P pcw ) is measured to determine severity of pulmonary venous hypertension. P pcw (which is similar to left atrial pressure) is compared with directly measured LV diastolic pressure to determine transmitral gradient; a resting end-diastolic gradient of 10 mmHg or more indicates important mitral stenosis. Mitral valve area is calculated from Gorlin's modified orifice equation.
In a patient coming to treatment for mitral stenosis, the possibility of left atrial myxoma must always be considered. Echocardiography can detect a left atrial myxoma and is the type of screening performed when further information is needed (see Section I, , in Chapter 18 ).
Patients with mitral regurgitation are often asymptomatic for many years, during which time LV size may steadily increase and LV contractility decrease. Eventually, effort intolerance develops, and symptoms of pulmonary venous hypertension evolve. Fluid retention and chronic heart failure, occasionally with cardiac cachexia, are characteristic of the late stage of the disease; by then, secondary tricuspid regurgitation is usually evident.
As with mitral stenosis, important mitral regurgitation can usually be diagnosed based on history, physical examination, chest radiograph, and ECG. The classic apical systolic murmur of mitral regurgitation is pansystolic, loudest at the apex, and radiates to the left axilla and left lung base. Classical auscultatory findings in mitral valve regurgitation from prolapse include one or more midsystolic clicks and a late or holosystolic murmur of mitral regurgitation. When regurgitation is the result primarily of ruptured chordae and prolapse of the posterior leaflet, however, the regurgitant jet is directed toward the roof (superior aspect) of the left atrium and is transmitted to the aortic root. Therefore, the murmur is maximal in the parasternal aortic area and may radiate into the carotid arteries. In contrast to the anterior radiation of posterior leaflet prolapse, the murmur of anterior prolapse radiates posteriorly to the infrascapular and posterior cervical area. As a result of large and rapid mitral valve flow during diastole, an LV filling sound (S 3 ) and a diastolic rumble may be present. Two important signs of the severity of regurgitation are an overactive LV impulse at the apex (from LV enlargement) and a precordial lift, the latter the result of systolic pulsation in the enlarged left atrium and right ventricle. There is no correlation between intensity of the murmur and severity of mitral regurgitation, but a true holosystolic murmur is indicative of more severe regurgitation.
In severe chronic mitral regurgitation, the chest radiograph usually is highly characteristic. The left atrium generally is more enlarged than in patients with mitral stenosis, and the left atrial appendage is usually prominent. The LV may be enlarged, and there may be varying degrees of right atrial enlargement, depending on the amount of associated tricuspid regurgitation.
The ECG may remain normal even in the presence of severe mitral regurgitation. However, a pattern of LV hypertrophy is common.
As with mitral stenosis, 2D echocardiography demonstrates the details of leaflet pathology. Both transthoracic (TTE) and transesophageal echocardiography (TEE) are useful. Echocardiographic diagnosis of mitral valve prolapse requires prolapse of 2 mm or more above the anulus in the long-axis parasternal view. Leaflet thickness of 5 mm or more increases the likelihood of mitral valve prolapse. Prolapse of a specific leaflet can be visualized, and Doppler color flow imaging can identify the location and magnitude of the mitral regurgitant flow. Echocardiography may also be used to estimate both the degree of LV enlargement and, by quantification of shortening fraction, ventricular contractility. Newer echocardiographic methods using 3D techniques offer precise and accurate evaluation of leaflet physiology and specific areas of regurgitation within the valve apparatus. The effective regurgitant area and regurgitant volume can be measured echocardiographically and have been identified as predictors of outcome following mitral valve repair (see Indications for Operation later in this section) . The American Society of Echocardiography has recommended grading mitral regurgitation as mild, moderate, and severe (grades 1-3). For refinement of intermediate levels, the terms mild to moderate and moderate to severe can be used.
Left ventriculography also demonstrates the regurgitant process at the mitral valve and can show leaflet prolapse. Degree of regurgitation can usually be estimated with reasonable accuracy, although if left atrial or LV enlargement is severe, the estimate is less valid. Fairly accurate calculations of regurgitant flow can be made from measurements of LV stroke volume by quantitative left ventriculography, and of forward flow by some measurement of cardiac output. LV ejection fraction (EF) can also be calculated by quantitative cineangiography or radioisotope techniques. Decisions about treatment of patients with mitral regurgitation may require additional studies from which inferences can be made about LV contractility (see “ Mitral Regurgitation ” under Natural History later in this section).
Mitral regurgitation may develop acutely as a result of chordal rupture or infective endocarditis or may complicate the course of acute myocardial infarction (see Chapter 10 ). Symptoms and signs of severe pulmonary venous hypertension suddenly appear. The left atrium and LV are normal in size or only slightly enlarged. The chest radiograph is dominated by signs of pulmonary venous hypertension, and left atrial pressure is high, as is the v wave. A mitral regurgitation murmur is often midsystolic and higher pitched compared with the pansystolic murmur of chronic mitral regurgitation.
Rheumatic mitral stenosis develops slowly after initial rheumatic involvement of the valve. In the New England area of the United States, the average age of the initial attack of rheumatic fever is 12 years, average age of onset of clinical signs of mitral stenosis 20 years, and age at onset of symptoms 31 years. Progression of valvar fibrosis and calcification is related in part to repeated episodes of rheumatic fever, but mechanical trauma and deposition of platelets and other blood substances resulting from stenosis-induced alterations of flow patterns also play a role. This progression is a major factor in increasing symptoms, ultimately causing death.
Most patients with mitral stenosis have normal LV wall thickness, volume, and systolic and diastolic function but do not increase cardiac output under stress, such as when LV afterload is acutely reduced by infusion of sodium nitroprusside. These findings suggest that the major cause of chronically reduced cardiac output in these patients is obstruction at the mitral valve. In some patients with long-standing mitral stenosis, however, minor posterobasal regional wall contraction abnormalities develop, perhaps from a rigid mitral valve complex.
In a small proportion of patients with apparently isolated mitral stenosis (usually older persons), EF is substantially reduced. Such patients have severe posterobasal segmental wall contraction abnormalities, sometimes anterolateral segmental contraction abnormalities, and occasionally diffuse hypokinesis. Anterolateral segmental contraction abnormalities may be caused by papillary muscle scarring and immobilization. Diffuse hypokinesia may result from scarring and decreased LV compliance secondary to chronic low cardiac output and low coronary blood flow.
Once symptoms develop after the so-called latent period, their progression to a state of total disability (New York Heart Association [NYHA] functional class IV) requires an estimated 7 to 10 years ( Fig. 11-3 ). Average age of death of patients with surgically untreated mitral stenosis is estimated to be 40 to 50 years.
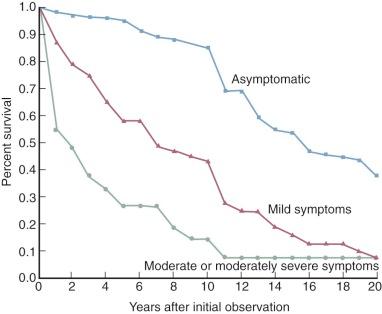
This general pattern of evolution is considerably shorter in some parts of the world and in some races. Polynesians in New Zealand, African Americans in south-central Alabama, Inuits in Alaska, and Asians experience a greatly accelerated evolution of signs, symptoms, and disability. Many reports suggest that in addition to possible genetic factors, economic underdevelopment may play a role.
Other events tend to occur during the lifetime of patients with surgically untreated mitral stenosis, and these in turn may alter the natural history of the disease. Atrial fibrillation usually develops, often occurring first in paroxysmal form. The first paroxysm, usually with tachycardia, may initiate symptoms because patients with mitral stenosis are particularly sensitive to loss of the atrial contribution to ventricular filling and to shortening of ventricular filling. Although originally incited by left atrial hypertension and hypertrophy, atrial fibrillation eventually becomes persistent because of disintegration of the architecture of atrial muscle. Because it reduces cardiac output and elevates left atrial pressure, atrial fibrillation accelerates clinical deterioration of patients with mitral stenosis and indicates a relatively advanced stage of the disease. It is an incremental risk factor for premature death of these patients. Olesen found that in patients with atrial fibrillation, 10- and 20-year survival was 25% and 10%, respectively, whereas in patients in sinus rhythm at initial observation, survival was 46% and 29%, respectively.
Systemic arterial emboli , most of which lodge in cerebral arteries, can suddenly complicate or kill patients with mitral stenosis. Most emboli originate in the left atrial appendage or left atrium, yet often no residual thrombus remains in the heart after embolization. Hoeksema and colleagues found that only 25% of patients with a history of arterial emboli had left atrial thrombi detected at closed commissurotomy. Also, some patients with large left atrial thrombi never have demonstrable embolization. Left atrial thrombosis and embolization are much more common when atrial fibrillation is present than in patients in sinus rhythm. At least 10% of surgically untreated patients develop arterial embolization during their lifetime, and a massive cerebral embolus may suddenly kill a mildly symptomatic patient.
Infectious endocarditis is unusual in patients with mitral stenosis.
Massive pulmonary hemorrhage may occasionally develop in patients otherwise mildly symptomatic from mitral stenosis. The association between mitral stenosis and hemorrhage is strongly suggested by its prompt and long-standing remission after surgical treatment of the stenosis.
In fact, the aforementioned features and criteria do not truly represent the “natural” (i.e., untreated) history of mitral stenosis, but rather the spectrum of mitral stenosis in surgically untreated patients receiving medical treatment available in the mid-20th century. The end stage of the disease in many patients is characterized by cardiac cachexia , a state infrequently seen before diuretic therapy became available. The true natural history of mitral stenosis in the first quarter of the 20th century must have been different from that portrayed here, with the interval between onset of symptoms and death much shorter and with different patterns of death.
Likewise, evolution of the rheumatic disease complex is different in patients with mitral stenosis treated surgically vs. medically. The course of the disease varies because the increased lifespan due to surgery now results in development of hemodynamically important rheumatic aortic valve disease and important tricuspid regurgitation in a considerably greater proportion of patients with mitral stenosis ( Fig. 11-4 ).
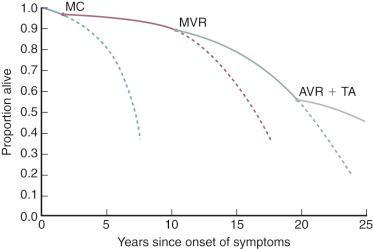
The natural history of mitral regurgitation is difficult to define because (1) etiology is variable, (2) age at onset is variable, (3) mitral regurgitation may be mild and nonprogressive for many years, and (4) LV function, an important determinant of symptoms and survival, deteriorates at different rates.
Patients with mild to moderate mitral regurgitation may remain asymptomatic for many years. Even when mitral regurgitation becomes severe, the effect of changes in global LV function is modified by the fact that total LV load resisting shortening (afterload) is abnormally low in these patients (see “” under Cardiac Output and Its Determinants in Chapter 5 ). Afterload is low as a result of ejection of part of the LV stroke volume into the low-pressure left atrium; because of this, the LV becomes small, and its wall thickens very early in systole. The resulting favorable relationships in the Laplace law reduce wall stress and afterload. Because of this, LV contractility may gradually deteriorate while LV systolic function (EF) is maintained, the mechanism being reduction in afterload. The EF may even increase during exercise under these circumstances while symptoms remain mild. This compensated period of mitral regurgitation may last for years. Later, as the regurgitation becomes severe and LV contractility decreases, EF may diminish during exercise, even in asymptomatic patients.
These considerations have led to development of techniques for estimating LV function that are independent of preload and afterload. Foremost among these are those that use ventricular end-systolic pressure-volume relations (see “” under Indications for Operation, Selection of Technique, and Choice of Device later in this section and “” in Section I of Chapter 5 ).
Patients with mitral regurgitation may develop tricuspid regurgitation, which also affects natural history (see Section II ).
Patients with rheumatic mitral regurgitation are more likely to have had a previous severe attack of rheumatic fever than those with mitral stenosis. The interval before the appearance of regurgitation also is shorter than for stenosis. Patients with surgically untreated but hemodynamically important rheumatic mitral regurgitation survive similarly to those with mitral stenosis. Furthermore, the curve is different in different environmental and genetic situations, as it is in mitral stenosis. In San Francisco, for example, survival of such patients 5 years after initial evaluation was 80%, with 10-year survival of 60%, whereas in Venezuela, 5-year survival was only 46%. Accelerated forms of rheumatic mitral regurgitation also occur in the same geographic areas where severe mitral stenosis appears in the pediatric population, with important symptoms by age 10 years.
The natural history of isolated mitral valve prolapse without regurgitation is highly variable, with the majority of patients having age-adjusted survival similar to that of the general population. However, the major predictor of cardiac mortality is moderate to severe mitral regurgitation. Mitral regurgitation associated with mitral valve prolapse has a complex natural history that entails more than leakage at the mitral valve. The mitral leaflets not only prolapse in this condition but are usually thickened as well. Mitral valve regurgitation is not the only event in patients with mitral prolapse. Serious but rarely fatal arrhythmias may occur in patients with only mild regurgitation, and psychiatric disturbances may develop. Symptoms may mimic thyrotoxicosis, hyperadrenergic states, or hypoglycemia. Patients often have higher than normal catecholamine levels and other evidence of high adrenergic tone. As a further complication, patients with hyperthyroidism have an increased prevalence of mitral prolapse.
Patients with the classic form of mitral prolapse, which includes thickening of the leaflets as well as prolapse, have an increased prevalence of infective endocarditis ; those with normal leaflets (in the absence of regurgitation) do not. Both groups have a higher prevalence of stroke.
Severe mitral regurgitation requiring valve repair or replacement rarely develops before age 50. Thereafter, the prevalence increases steeply, particularly in men. However, even men with mitral valve prolapse who have reached age 70 years have only about a 5% chance of requiring mitral valve repair or replacement. Once important mitral regurgitation appears, however, it tends to progress just as it does in patients with rheumatic mitral regurgitation. Presumably, as prolapse worsens, support in systole provided by closing of the two leaflets against each other (“stacked rifle effect”) is lost. This puts an abnormally large load on the chordae, which elongate, become thick, and eventually rupture. This process worsens the regurgitation and accelerates the natural history of the disease.
In view of the increasing ability of cardiac surgeons to repair mitral regurgitation, the natural history of severe mitral regurgitation secondary to mitral valve prolapse is of considerable importance. Two Mayo Clinic studies in the mid-1990s called attention to the increased mortality in patients with chronic flail leaflets treated medically. A 2008 multicenter European study examined the natural history of severe regurgitation caused by one or both flail leaflets (failure of leaflet coaptation with rapid systolic movement of the involved leaflet tip in the left atrium). Involvement was confined to the posterior leaflet in 314 patients (79%), anterior leaflet in 31 (8%), both in 46 (12%), and unspecified in 3 (1%). The long-term outcome with medical treatment included an important likelihood of major adverse events by 8 years, including atrial fibrillation, heart failure, and cardiovascular death ( Fig. 11-5 ). Need for mitral valve surgery (or death from cardiovascular causes) was nearly unavoidable by 8 years after diagnosis. A subgroup analysis of asymptomatic patients with normal ventricular systolic function revealed a 5-year survival of 97% with medical treatment, but the combined occurrence of atrial fibrillation, heart failure, or cardiovascular death was 42% at 8 years. A Mayo Clinic analysis of asymptomatic patients with severe organic mitral regurgitation identified an effective regurgitant orifice (by echocardiography) of at least 40 mm 2 as a major predictor of late mortality.
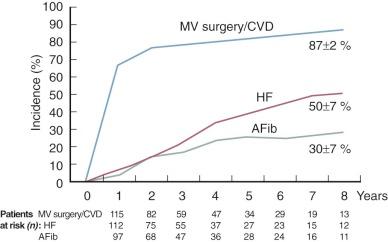
Patients with mitral regurgitation and ruptured chordae tendineae may have a slow, insidious development of symptoms. Ruptured chordae of the anterior or posterior leaflet or both are often found at operation, and the mitral valve leaflets have the appearance of myxomatous degeneration. These patients probably represent a subgroup of individuals with mitral valve prolapse. In fact, ruptured chordae may be present in patients with prolapse without important symptoms. Grenadier and colleagues found 11 (8%) of 134 patients with mitral valve prolapse to have ruptured chordae and few or no symptoms.
By contrast, important mitral regurgitation produced acutely by chordal rupture may occur in patients with no previous valve leakage. This happens predominantly in middle-age men. Often it is a complication in the life history of patients with midsystolic clicks and only trivial murmurs, but without previous evidence of mitral regurgitation. The anterior mitral leaflet and its chordae are frequently entirely normal, with the disease process limited to the medial aspect of the posterior mitral leaflet. In this group of patients with acute and severe symptoms, presumably initiated by sudden chordal rupture, the left atrium and LV are small, left atrial pressure is high, the v wave is greatly accentuated, and there is substantial clinical and radiologic evidence of pulmonary venous hypertension. TTE and TEE are diagnostic. The posteromedial segment of the valve (P 2 , P 3 ) prolapses, and the ruptured chordae are flail or seen as thickened stubs on the affected portion of the leaflet. If the patient survives the acute event, the LV and left atrium enlarge moderately over time, and symptoms may lessen with appropriate medical management (as has been shown experimentally as well ). The patient gradually regains a feeling of well-being. One year later, left atrial and ventricular enlargement may not have progressed, the LV and left atrium seemingly adapting to the volume overload. Years may pass before the self-aggravating tendency of mitral regurgitation results in increased regurgitation volume. After this, the classic natural history of important mitral regurgitation evolves.
In other patients with severe acute manifestations, symptoms improve only mildly with intense medical treatment. Although most patients survive, LV and left atrial enlargement progress steadily in the months after onset. Such patients have a large mitral regurgitant flow. When untreated surgically, they progress through the life history of severe mitral regurgitation more rapidly than most patients; without surgical intervention, they die within 2 to 5 years.
Infective endocarditis on a previously mildly abnormal mitral valve may produce acute mitral regurgitation. The natural history of that condition is similar to that described for acute chordal rupture, except that early mortality is higher. Infrequently, death is related to uncontrolled infection. Infective endocarditis is discussed in detail in Chapter 15 .
Tricuspid valve regurgitation may be associated with any type of mitral valve disease. Consequently, tricuspid anuloplasty or rarely replacement may have to be performed concomitantly with mitral valve surgery. Techniques for these operations are described in Chapter 14 .
After the usual preparations, including placing an arterial catheter in the right radial artery, the patient is positioned in the right lateral decubitus position. The hips are rotated to the patient's left. The left arm is placed at the patient's side and hanging slightly over the edge of a well-padded portion of the operating table, with the left hand beneath the hips. This position permits good access to the operative area.
After appropriate skin preparation and patient draping, an anterolateral incision is made over the interspace, through which the impulse of the apex of the LV can be palpated. The incision in the interspace (typically the fifth or sixth) is carried posteriorly, but the latissimus dorsi usually does not require incision. At times, the costal cartilage above or below the incision is transected.
The pericardium is opened anterior to the phrenic nerve. A purse-string suture is placed just off, and usually just lateral and superior to, the LV apex. The purse string is threaded into a Rummel tourniquet or similar device. A similar purse-string suture is placed around the tip of the left atrial appendage. With both the assistant and surgeon grasping their own side of the appendage with a sturdy thumb (tissue) forceps, an incision is made just off its tip. The incision must be of a size to accommodate the surgeon's right index finger snugly. While bleeding is controlled by opposing the thumb forceps, blood is allowed to escape for two or three brief periods so that small clots may emerge. The surgeon's right index finger is then insinuated through the appendage into the left atrium and passed directly to the mitral valve. After the valve is evaluated, pressure is applied with the exploring finger against the anterolateral commissure. Occasionally, this maneuver opens a pliable but severely stenotic valve widely and essentially completely, with no further action needed. In most cases, however, a Tubbs dilator is required and is inserted through a small stab wound in the center of the LV apical purse string ( Fig. 11-6, A ).

An assistant gently secures the Rummel tourniquet that has engaged the apical purse-string suture. Guided by the intraatrial finger and with the setscrew on the dilator positioned so that the maximal opening is 2.5 cm, the dilator is passed through the valve. The dilator blades are opened moderately for a few seconds to ensure that each dilating blade is against a leaflet and not a commissure (see Fig. 11-3, B ). This and each successive dilatation last only 5 to 6 seconds, and the heart is allowed to recover completely between dilatations. Successive dilatations are then performed, first with the maximum opening set at 2.5 cm and then at about 2.9, 3.3, 3.7, and finally 4.0 cm. In small patients, the maximum dilator setting should be 3.3 to 3.5 cm. If important regurgitation develops after a dilatation, no larger settings should be attempted. Again, after the heart has recovered, the index finger is withdrawn as an assistant places a side-biting clamp across the lips of the incision in the appendage. At times, two Allis-Adair forceps close the incision well, and the clamp is not necessary. The purse string around the entrance of the Tubbs dilator is loosened slightly and then secured as the dilator is removed. No effort is made to tighten the purse string so that all bleeding stops, lest the purse string cut through the ventricular muscle. Digital control suffices for residual bleeding. Several full-thickness interrupted stitches are placed in the stab wound, and the purse-string suture is removed from the tourniquet and tied snugly but not tightly. The incision in the atrial appendage is closed with a few interrupted stitches. Usually the purse string on the appendage is removed rather than tied because of the risk of dislodging a small thrombus that may have formed. A large (2-cm) window is made in the pericardium posteriorly, behind the phrenic nerve, for drainage into the left pleural space. The pericardium is then closed with widely spaced interrupted sutures. A catheter is placed posterolaterally in the left pleural space, hemostasis is secured, and the incision is closed in layers.
After the usual preparations and median sternotomy, pericardial stay sutures are placed and a left atrial catheter inserted (see “” in Section III of Chapter 2 ). Using intraoperative TEE, chamber size is evaluated and mitral valve pathology confirmed, including degree of valve narrowing and any regurgitant jets. Often, subvalvar stenosis can be identified. Important tricuspid regurgitation or stenosis is noted.
The aortic cannula is inserted and venous cannulation accomplished with a single venous cannula, or bicaval cannulation may be used when the left atrium is small and exposure difficult. CPB is established and perfusate temperature lowered to 18°C to 20°C. A stab wound (part of the later left atriotomy) is made at the base of the right superior pulmonary vein for initial left atrial venting. A cardioplegic needle or aortic root catheter is placed in the ascending aorta, and a retrograde coronary sinus cardioplegic cannula may be introduced as well (see “” in Chapter 3 ). The aorta is clamped, cold cardioplegic solution infused, and perfusate temperature stabilized at 28°C to 32°C. The left atrium is opened vertically from the right side ( Fig. 11-7, A ). This may be done after developing the interatrial groove in cases of minimal left atrial enlargement, or the incision may be started medially on the right superior pulmonary vein without dissecting the groove. Superiorly, the incision is extended beneath the superior vena cava after the right pulmonary artery is dissected away from the superior aspect of the left atrium. Inferiorly, the incision may be extended by cutting behind the freed vena caval–right atrial junction. Rarely, when exposure remains poor because of the small size of the left atrium, the superior vena cava is transected on the atrial side of the cannulation site and the incision carried farther to the superior aspect of the left atrium. A Cooley left atrial retractor or Deaver retractor is inserted ( Fig. 11-7, B ). An intracardiac sump sucker placed through the incision is positioned in the orifice of one of the left pulmonary veins to keep the operative field dry.

The mitral valve is examined to determine its suitability for commissurotomy, and judgment is made as to whether the leaflets will be sufficiently pliable after commissurotomy to open adequately at a low left atrial pressure. Determination is straightforward when the leaflets are pliable and noncalcified and there is little or no coalescence of chordae. However, mitral commissurotomy can often yield reasonably good results when the valve is less ideal, and even when it is partially calcified. Thus, if some reasonable degree of mobility remains in the central portion of the anterior leaflet, persistent attempts should be made to open the valve widely by commissurotomy.
If commissurotomy is chosen, one stay suture can be placed in the midportion of the free edge of the anterior leaflet and another placed similarly in the posterior leaflet. Retraction on these sutures puts tension on the leaflets and their commissures. With a sharp-pointed scalpel (#11 blade), a stab incision is made in the fused anterolateral commissure next to the anulus (see Fig. 11-7, B ). The incision is extended with the scalpel toward the valve orifice, in the groove of the commissural fusion. A 3- to 4-mm incision reveals the fan of underlying chordae, making it easy to stay in the middle of the commissural tissue over the center of the fan. The incision is then carried into the valve orifice. Alternatively, a blunt-ended, long-handled hook is placed beneath each leaflet, and by trial and error these hooks are positioned exactly in the spot that provides the best exposure for division of each commissure. With the scalpel, an incision is made at the anterolateral commissure beginning at the valve orifice and extended toward the anulus. The surgeon takes care to follow the true line of the commissure, which extends more anteriorly than might be thought. With either method, when fused chordae are present beneath the commissure, they can usually be separated with the knife or scissors. When appropriate, the incision is carried down into the center of the papillary muscles, dividing them into anterior and posterior halves ( Fig. 11-7, C ).
The posteromedial commissure is usually less well defined and fused for a shorter distance than the anterolateral commissure. Using one of the techniques just described, this commissural leaflet tissue is incised. Chordae are often more fused beneath this commissure, and their separation by sharp dissection may be needed together with longitudinal division of the papillary muscle. If the chordae are fused together to form a fanlike fibrous sheet beneath either leaflet at one or both commissures, this sheet is fenestrated by removing a wedge of tissue from its center using a sharp-pointed scalpel. Localized but bulky calcium deposits are removed from the leaflets with bone-nibbling forceps.
A firm plastic catheter with multiple side holes (ideally a 24F chest drainage tube) is placed through the valve into the LV and tied to the left atrial vent snare to secure it. Catheter placement frustrates the valve, and the side holes lying in the left atrium are an additional safeguard to prevent ejection by the LV into the aorta until de-airing is complete. The left atrium is closed with continuous 3-0 polypropylene suture, but with the loops left loose where the catheter exits through the left atrial incision. The left atrial vent is removed during this procedure to allow the left side of the heart to fill with blood. If return to the left atrium is inadequate, right atrial pressure is raised by returning blood to the patient (see “” in Section III of Chapter 2 ). With the patient's head down, the aortic clamp is released while suction is applied to the aortic vent needle. The heart is defibrillated if necessary, and the ventricle is allowed to eject into the left atrium and pericardium via the transmitral catheter until all air is clearly eliminated.
Alternatively, after completion of the commissurotomy, the left atrium is completely closed before beginning aortic root reperfusion and later removing the aortic clamp. Closure with 3-0 polypropylene suture is started at the superior angle, carried partway down, and held. With another suture, closure is begun at the inferior angle and carried superiorly until the other suture is reached. The closure must be done accurately because it is difficult to see the angles later. The sump sucker is removed, and the left atrium is filled with saline solution just before the suture line is completed. A small stab wound is made through a purse string in the ascending aorta and a small curved clamp placed through the hole to allow egress of blood and air from the LV before removing the aortic clamp. The venous line is partially clamped, and while the lungs are inflated, air is evacuated from the ascending aorta stab hole. Suction is placed on the aortic root catheter in the ascending aorta, the aortic clamp is released, and rewarming is begun (see “” in Section III of Chapter 2 ).
If the period of global myocardial ischemia has exceeded 30 minutes, the technique of controlled aortic root or retrograde perfusion may be used (see “” in Chapter 3 ). These maneuvers may aid in evacuating air from the coronary arteries. If careful observation reveals LV distention, a 13-gauge needle is inserted into the RV and across the septum into the LV. After good cardiac action has returned, the usual de-airing procedures are followed (see “” in Chapter 2 ).
As rewarming progresses, two right atrial and two RV myocardial wires are placed. Before decannulation, examination by TEE should confirm no more than trivial or mild mitral regurgitation.
Repair rather than replacement is the procedure of choice for treating mitral regurgitation, and current trends favor this approach. The proportion of patients undergoing repair for isolated mitral regurgitation rose from 51% in 2000 to 69% in 2007, as recorded in the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database. However, not all cases are successfully managed in this fashion. Thus, at operation it must be determined whether repair will likely provide an acceptable result. This determination is aided appreciably by prebypass TEE. The magnitude and direction of the regurgitant jet can be assessed and the site of leaflet dysfunction accurately predicted. The abnormal valve physiology can often be characterized as restrictive, dilated, or prolapsing. These determinations allow informed decisions as to appropriate repair techniques. Repair can most confidently be performed when chordae are ruptured in a limited portion of the posterior leaflet and the anterior leaflet is essentially normal, or when there is simply prolapse of the posterior leaflet. Regurgitation from ruptured chordae to the anterior leaflet can be successfully repaired in most cases. Rheumatic mitral regurgitation can usually be repaired when distortion of the valve leaflets is minimal, as can certain cases of combined stenosis and regurgitation when commissurotomy precedes the repair. Nonrheumatic, noncalcific, almost pure mitral regurgitation in the adult, nearly always due to myxomatous degeneration, can usually be repaired. Regurgitation caused by infective endocarditis, with resultant chordal rupture or a perforated cusp, is reparable at times.
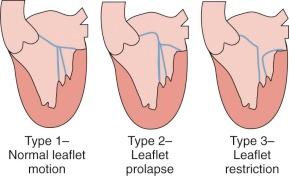
When regurgitation is associated with mitral valve prolapse, myxomatous degeneration, or chordal rupture, Carpentier's techniques have proven reproducible and durable. Carpentier and Kumar (of Duran's group) and their colleagues clarified the pathologic anatomy of the mitral valve. Their observations are fundamental considerations for a repair. As observed by echocardiography or at operation, the disease process of the mitral apparatus can be classified as restrictive, normal with anular dilatation, or degenerative (leaflet prolapse) ( Fig. 11-8 ).
Thus, with the mitral valve exposed as usual and using saline distention, areas of prolapse and regurgitation are identified. With forceps and blunt hooks (crochet hooks), each element of the valve apparatus (anulus, leaflets, chordae, papillary muscle) is examined for pathologic changes. In particular, the normal primary chordae are distinguished from the abnormal stretched or ruptured chordae to the offending prolapsed portion. A rectangular excision of the prolapsed sector of the posterior leaflet is done ( Fig. 11-9, A ). This can involve 15% to 25% of the posterior leaflet. Further mobilization of the remaining leaflet may be accomplished by resecting some adjacent secondary and tertiary chordae. The resulting gap is bridged by a compacting (compression) suture at the base of the resected leaflet, beginning in atrial tissue just below the anulus. Alternatively, with a sliding plasty, adjacent normal portions of the posterior leaflet are incised at their bases and moved centrally to obliterate the gap ( Fig. 11-9, B and C ).

If a sliding plasty has been used, reconstruction is performed by suturing the mobilized leaflet to the posterior anulus and then reapproximating the cut edges of the leaflets with interrupted nonabsorbable sutures. Other techniques for closing the gap between the resected leaflets have been described, including the folding plasty technique.
Usually an anuloplasty ring is then sutured into place to both support the repair and narrow the anulus ( Fig. 11-9, D and E ). Proper sizing of the anuloplasty ring (whether flexible or fixed) is essential to maintain competence and avoid postoperative LV outflow tract narrowing. Basically, the ring must recreate the normal anteroposterior depth of the anulus, which typically is the anteroposterior length of the splayed anterior leaflet. Thus, the size of the anterior leaflet rather than the intercommissural distance should be used to select the appropriate ring size. However, there is no uniform agreement about the technique or even the need to precisely measure anterior leaflet dimensions for ring selection. Brown and colleagues from the Mayo Clinic reported reproducibly good outcomes using a standard 63-mm posterior band in adult patients, without specific measurements.
Anterior leaflet prolapse repair (sector A 2 ) is more difficult and less often successful than posterior leaflet repair. A triangular wedge of the involved leaflet is resected, the leaflet is reconstructed, and a ring is placed ( Fig. 11-10 ). To preserve available anterior leaflet for proper coaptation, the triangular resection should extend no more than one third of the distance between the free edge of the anterior leaflet and the mitral anulus. Often the repair involves other techniques described by Duran, Carpentier, Antunes, and Orszulak and their colleagues. These maneuvers are technically demanding and not as reliable as those applied to the posterior leaflet. Techniques include chordal shortening, chordal transfer, and occasionally reimplantation of partially ruptured papillary muscles.

Chordal transfer involves excising the leaflet insertion of a normal intact chord from the posterior leaflet, flipping it anteriorly, and attaching it to the flail anterior prolapsing sector ( Fig. 11-11 ). Chordal shortening may also be used in such cases. The papillary muscle is split, and the chord is shortened by embedding it in the muscle.

Currently, however, chordal transfer techniques have been largely replaced by placing artificial chords. Diseased chordae tendineae may simply be replaced by 5-0 expanded polytetrafluoroethylene (PTFE) sutures ( Fig. 11-12 ). A double-armed suture is passed twice through the usually fibrous tip of the papillary muscle; each arm is then passed through the area of the leaflet where the native chordae are inserted, usually in a figure-of-eight fashion with each suture, and then tied. Intermediate-term results with this technique have been good.

Dreyfus and colleagues have described an extensive experience with papillary muscle repositioning for anterior leaflet prolapse. The anterior head of the anterolateral papillary muscle is repositioned for A 1 /A 2 prolapse, and the anterior head of the posteromedial papillary is repositioned for A 2 /A 3 prolapse ( Fig. 11-13 ).

Alfieri and colleagues have described a simple technique that may be useful for anterior leaflet prolapse ( Fig. 11-14 ). When the prolapse is near the commissure, the anterior and posterior leaflets are approximated “edge to edge” with one or two mattress sutures or, alternatively, with a figure-of-eight braided polyester suture. When the prolapse is in the central portion of the valve, one or two edge-to-edge leaflet-approximating sutures create a double-orifice mitral valve that functions adequately. Because mitral stenosis is a possible complication of this technique, it should probably be applied mainly in degenerative mitral valve disease. The resultant orifices should be at least 2 cm in diameter.

When there is bileaflet prolapse without anterior chordal pathology, often posterior leaflet resection and ring anuloplasty alone serve to correct the regurgitation, providing a durable repair without an additional procedure directed at the anterior leaflet.
When repair rather than replacement is done for rheumatic mitral regurgitation in adults, anuloplasty (generally using Carpentier's method, including an anuloplasty ring) has been the technique employed in most cases (see Fig. 11-9 ). When anuloplasty is necessary in young children with years of growth ahead, an anuloplasty ring is not used. Instead, an asymmetric measured anuloplasty is done by the technique described by Reed and colleagues ( Fig. 11-15 ). This operation, as with most repairs of mitral regurgitation, is based on the fact that the central portion of the anterior mitral leaflet is usually pliable and of good quality. Its leading edge forms the line of closure for the repaired valve, both in the Reed repair and after insertion of a Carpentier ring.

Determining competence of the repaired valve while the left atrium remains open is imprecise. Yacoub's maneuver involves perfusing warm blood into the aortic root proximal to the clamp through the cardioplegic needle or aortic root catheter so that function of the repaired valve can be inspected with the heart beating. A simpler method involves injecting saline under pressure through the valve into the LV. The distended leaflets and their opposing surfaces are examined, and areas of leakage, if present, are identified. The entire line of closure of the leaflets should be parallel to the mural part of the anulus—that is, to the hinge line of the posterior leaflet (or to this portion of the anuloplasty ring). If all this is satisfactory, the left atrium is closed, de-airing is performed as described under Open Mitral Commissurotomy, and CPB is discontinued. At this point, competence of the valve is assessed by TEE. If more than mild regurgitation is present, CPB is resumed, and the mitral valve is either re-repaired or replaced. In one institution, intraoperative TEE identified 26 of 309 patients (8%) undergoing repair for mitral regurgitation who had immediate failure of the procedure and required further repair or replacement at the same operation. Used in this manner, intraoperative TEE can limit the number of those who require late reoperation for failed repair.
CPB and myocardial management are as described for other mitral valve operations. The left atrium is opened from the right side. The aneurysmal sac is entered through an incision in the floor of the left atrium, parallel to and about 20 mm posterior to the hinge line of the posterior leaflet. The usually narrow pathway or “neck” between the LV and aneurysm is then easily visualized. This pathway can be closed with interrupted pledgeted mattress sutures, bringing them anteriorly through the hinge line of the posterior leaflet. The opening from the left atrium into the aneurysm is then closed, draining the now-evacuated and exteriorized aneurysmal sac through its free wall into the pleural space if desired. Reparative operations can be performed for residual mitral regurgitation if indicated.
Mitral valve replacement begins with exposure of the mitral valve as described for mitral commissurotomy, using one or two valve hooks to display the leaflets. To excise the valve, an incision is begun with the knife in the center of the anterior mitral leaflet at approximately the 12-o’clock position about 2 mm from the anulus, because at that point the leaflet tissue is typically pliable and free of disease ( Fig. 11-16 ). The incision is carried from this point leftward and rightward, either with the knife or scissors, and on to the commissural leaflet tissue at the anterolateral and posteromedial commissures. The incisions through the commissural leaflet tissue are kept next to the anulus so that the anterior and posterior leaflets stay together. The underlying papillary muscle and fused chordae are cut just in front of the incision for better exposure.

Classically, the excision is continued to the posterior leaflet from both sides. Ordinarily the posterior leaflet and its chordae are left in place when they are thin and pliable. Thus only the anterior leaflet is fully excised (but see “ Chordal Sparing Procedure ” later in this section). If this is not possible because of extensive disease, the secondary chordae that tether the posterior leaflet to the underlying ventricular myocardium are cut. When subanular calcification is present and can be excised without disturbing the anulus or the myocardium, it is removed. Otherwise, calcification is left in situ because overzealous efforts may damage the circumflex coronary artery or precipitate postrepair ventricular rupture.
The mechanical prosthesis or bioprosthesis can be sewn into place with interrupted simple sutures, but preferably with horizontal mattress sutures using pledgets on the atrial side, or with a continuous size 0 polypropylene suture ( Figs. 11-17 and 11-18 ). All methods are associated with an extremely low prevalence of periprosthetic leakage (see “ Periprosthetic Leakage ” under Modes of Death later in this section). A technique employing interrupted pledgeted mattress sutures with the pledgets on the ventricular side may be chosen when heavy calcification remains in some areas of the anulus or, rarely, when exposure is particularly difficult. With this technique, all sutures are placed in the heart first and then passed through the valve sewing ring. The prosthesis can then be lowered into position and the sutures tied. Protruding suture ends can damage the leaflets of bioprostheses.


After completing the valve insertion, a catheter is passed through the valve into the LV to act as a frustrator. The steps for exiting from the left side of the heart and de-airing are as described for mitral stenosis.
Information based on experimental observation and clinical experience indicates that retention (rather than resection) of the mitral tensor apparatus at mitral valve replacement results in better LV function postoperatively (see “ Cardiac Performance ” later in this section). To insert a prosthesis while retaining both the anterior and posterior chordal attachments, the anterior leaflet may be split centrally and folded laterally and the posterior leaflet left as is. Alternatively, both the intact anterior and intact posterior leaflets can be folded toward their bases, then a prosthesis inserted ( Fig. 11-19 ). These maneuvers (including folding the anterior leaflet) do not result in LV outflow tract obstruction or prosthetic obstruction when done for mitral regurgitation.

In both the operating room and intensive care unit, patients may display a large v wave in the left atrial pressure pulse. This is not a reliable indicator of mitral valve regurgitation, because the height of the v wave is related primarily to the level of mean left atrial pressure, and in patients who have just undergone mitral valve repair or replacement, mean left atrial pressure is usually elevated. TEE can settle this issue.
Care of patients after mitral valve procedures is as described in Chapter 5 . Ventricular unloading is an important aspect of the postoperative maintenance of optimal cardiac output in patients operated on for mitral regurgitation. Restoring mitral competence increases load-resisting shortening. Thus, by removing a parallel low-resistance circuit, myocardial oxygen requirement increases along with impairment of myocardial contractile reserve. Nitroglycerin, nitroprusside, and phosphodiesterase inhibitors are useful for decreasing afterload and improving cardiac performance.
Patients undergoing mitral valve replacement, including those receiving bioprostheses and often those undergoing commissurotomy or repair of mitral regurgitation, are begun on anticoagulant therapy using warfarin the evening of postoperative day 1 or 2 (day of surgery is postoperative day 0). For adults with a normal prothrombin time (PT), the initial dose is generally 7.5 to 15 mg, followed by daily doses in the hospital guided by daily PT measurements. The goal is prothrombin activity 20% to 30% of normal or PT twice the control value. This is best monitored and regulated using the international normalized ratio (INR) terminology. The INR allows standardizing the determination of PTs by accounting for differences among commercial thromboplastin reagents. Optimal INR for patients after mitral valve replacement is 2.5 to 3.5.
When a mechanical valve has been inserted, this program is continued indefinitely, and the patient is educated about its extreme importance. Warfarin appears to be associated with a greater risk of excessive anticoagulation (hemorrhage) than reduced anticoagulation (thromboembolism and thrombosis). Thus, an appropriate recommendation is an INR of about 2.5 to 3.5. (See Special Situations and Controversies for additional details on long-term anticoagulation.)
When a bioprosthesis is inserted or anuloplasty or valvotomy performed, anticoagulation is continued for 2 to 3 months. Lower INR values (1.5-2.0) are acceptable during this period. Patients who undergo mitral valve repair and have paroxysmal or persistent atrial fibrillation should continue long-term anticoagulation with warfarin. Long-term treatment with low-dose aspirin (75-100 mg/day) is probably advisable for patients who remain in sinus rhythm after mitral valve repair.
Patients who have undergone mitral valve repair or replacement should receive anti-endocarditis prophylactic antibiotics for medical procedures as indicated.
Evaluation of mitral valve repair or replacement by 2D and Doppler echocardiography should be routinely performed before hospital discharge or at first follow-up clinic visit. Assessment of right and left ventricular systolic function and identification of any residual mitral regurgitation will help guide afterload reduction therapy.
In an earlier era, mortality after closed commissurotomy was high. In the current era, hospital mortality after either closed or open commissurotomy approaches zero, and late survival is similar in risk-adjusted comparisons. A difference exists only in prevalence of postcommissurotomy mitral regurgitation (see “ Mitral Regurgitation ” in text that follows).
In many institutions, percutaneous catheter valvotomy has almost completely replaced surgical commissurotomy for isolated mitral stenosis. Procedure mortality approaches zero, and risk of complications (bleeding, severe regurgitation) requiring urgent operation is low.
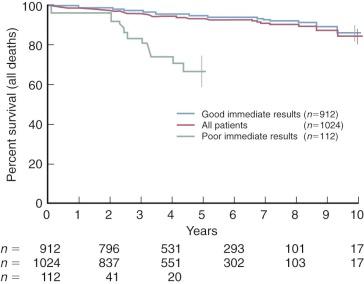
Intermediate-term survival is good in those with favorable immediate hemodynamic results ( Fig. 11-20 ).
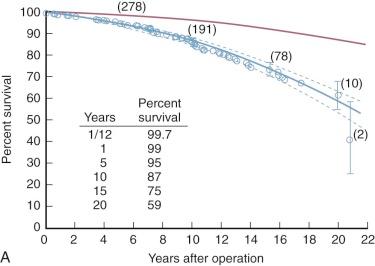
Mitral commissurotomy is not curative with either open or closed (or balloon) commissurotomy, with survival progressively diverging from that of the general population ( Fig. 11-21 ). However, few late deaths result directly from the effects of recurrent or residual mitral stenosis or regurgitation. Rather, they result from thromboembolism or early or later sequelae of reoperation and mitral valve replacement.
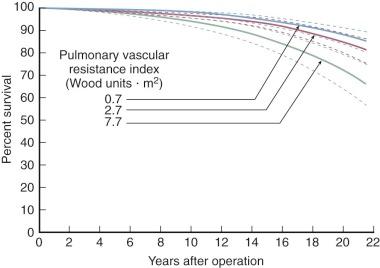
Few deaths occur early after mitral commissurotomy, and no risk factors are associated with these. Risk factors associated with later deaths include elevated Rp before commissurotomy, which directly affects survival rather than leading to reintervention, but its effect is moderate ( Fig. 11-22 and Table 11-2 ). The aforementioned morphologic risk factors lead to recurrent symptoms and reintervention, usually valve replacement, and affect survival in this manner. Although not evident in a risk factor analysis using only preoperative variables, development of important mitral regurgitation after commissurotomy and occurrence of a thromboembolic event both adversely affect survival.
| Hazard Phase | |||
|---|---|---|---|
| Risk Factor b,c | Early | Late | |
| Demographic | |||
| (Older) | Age at commissurotomy | • | |
| African American | N | • | |
| (Higher) | Pulmonary vascular resistance index | O | • |
| Morphologic | N | ||
| Leaflet calcification | E | • | |
| (Greater) | Left ventricular enlargement (grades 0-6) | • | |
a UAB group, 1967 to 1988; n = 339; deaths = 65.
b Variables entered into the analysis, coefficients, and P values of the risk factors are provided in original article. Postcommissurotomy variables such as residual mitral regurgitation were excluded from this analysis.
c Type of commissurotomy (open or closed) was not a risk factor.
Mitral regurgitation is a risk of mitral commissurotomy by any technique, but occurs in only 2% to 5% of patients who undergo open commissurotomy and in about 10% of patients who undergo closed commissurotomy. Rarely does the newly developed regurgitation require immediate operation, but it may lead to reoperation within a few months. Mild postcommissurotomy mitral regurgitation has little effect on survival or need for mitral valve replacement, but important postcommissurotomy regurgitation adversely affects both ( Fig. 11-23 ).
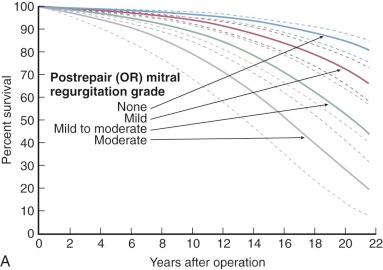
Prevalence of new important mitral regurgitation is about 10% after percutaneous balloon mitral commissurotomy.
Increase in calculated mitral valve area (or orifice size) produced by commissurotomy varies greatly and depends not only on the surgical opening but also on leaflet pliability and extent of subvalvar obstruction from fused chordae. Feigenbaum and colleagues found that closed mitral commissurotomy, usually with a transventricular dilator, increased mitral valve area by an average of 1.3 to 2.6 cm 2 , with postoperative mitral valve areas ranging from 0.7 to 5.8 cm 2 . Younger patients tend to have a better functional result and experience greater increases in calculated valve area than older patients, perhaps because younger patients tend to have more pliable valves. The same general results are achieved by both open and closed commissurotomy and percutaneous balloon mitral valvotomy ( Fig. 11-24 ). Enlargement after percutaneous balloon valvotomy results primarily from splitting of fused commissures.
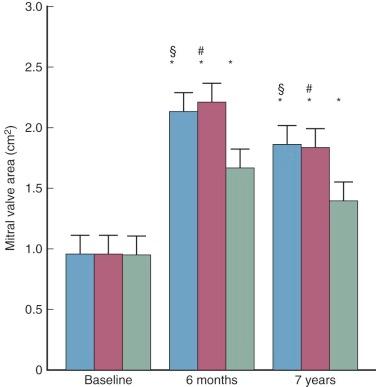
Somewhat better orifices can be obtained by open than closed commissurotomy. Nakano and colleagues achieved valve areas of 2.52 ± 0.19 cm 2 in patients with pliable valves and minimal subvalvar disease, as well as good but smaller orifices in patients with extensive subvalvar diseases. Ben Farhat and colleagues reported an equivalent increase of mitral valve area (0.9-2.2 cm 2 ) for open commissurotomy and percutaneous balloon valvotomy that was greater than for closed commissurotomy (0.9-1.6 cm 2 ). The superior hemodynamic results continued over a 7-year follow-up period (see Fig. 11-24 ).
A secondary effect of increased orifice size is lowering of left atrial pressure, although it frequently remains above normal. On average, left atrial pressure at rest is about 12 mm Hg after valvotomy, increasing to about 17 mm Hg on exercise. LV end-diastolic pressure often is modestly higher after commissurotomy.
Cardiac output is usually increased by operation, and the amount of increase at rest and exercise correlates well with the increase in calculated valve area. Rp usually falls immediately, especially in young patients, as verified by Block and Palacios during percutaneous balloon commissurotomy. Pulmonary artery pressure usually falls, which correlates well with the decrease in left atrial pressure and Rp.
Successful mitral commissurotomy may reduce the likelihood of thromboembolism, although accurate comparisons are not available. About 90% of patients are free of a thromboembolic event 8 to 10 years after open commissurotomy. The linearized rate of thromboembolism has been reported at 1% to 2% per patient-year. This statistic is not very useful, however, because the hazard function for a first or subsequent thromboembolic event is not constant ( Fig. 11-25 ). Atrial fibrillation, older age, and a history of thromboembolism preoperatively are reported risk factors for postcommissurotomy thromboembolism. Also, a postcommissurotomy thromboembolic event predisposes the patient to further events ( Table 11-3 ). The type of surgical commissurotomy, open or closed, has not been shown to be a risk factor.
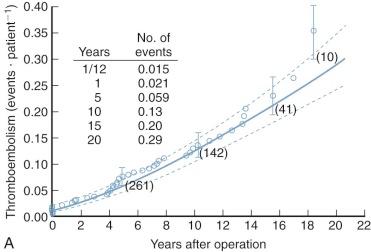
| Hazard Phase | |||
|---|---|---|---|
| Risk Factor | Early | Late | |
| Demographic | |||
| (Older) | Age at commissurotomy (years) | • | |
| History of thromboembolism before commissurotomy | • | ||
| (Greater) | Number of previous postcommissurotomy thromboembolic episodes (0, 1, 2) | • | |
| Morphologic | |||
| (Greater) | Leaflet calcification (grade 0-5) | • | |
| Pliable leaflet | • | ||
a Thromboembolism was considered to be a modulated renewal process (event), and thus the first, second, or third events (total of 44) are included.
Successful open or closed mitral commissurotomy, in properly selected patients, results in dramatic relief of symptoms. More than 90% of patients are in NYHA functional class I or II during the first 1 or 2 postoperative years ( Fig. 11-26 ). Lack of permanent favorable results from mitral commissurotomy is evident in the gradual decline in functional status. Redevelopment of symptoms results from gradual loss of leaflet pliability as well as progression of subvalvar pathology and increase of valvar calcification. Although recurrence of rheumatic fever may accelerate this pathology, progression seems to occur even without further rheumatic episodes. Thus, NYHA functional class correlates well with estimated area of the mitral orifice late postoperatively; the mean value is 2.0 cm 2 in class I patients, 1.7 in class II, and 1.6 in class III. Immobility of valve leaflets and obstructive subvalvar agglutination of chordae are the main risk factors for a poor early functional result. Calcification of a portion of the leaflet(s) does not preclude a satisfactory result.
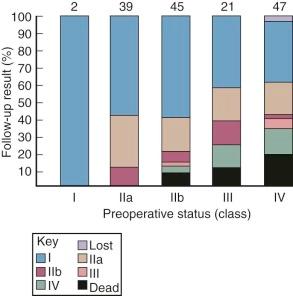
Despite excellent early and intermediate results in the current era, durability of results is limited. Scarring from the rheumatic process in the valve seems to progress gradually. Eventually, although not until 20 years postoperatively in some patients, most patients lose their good functional status and return with restenosis or new-onset regurgitation ( Fig. 11-27 ). Absence of leaflet pliability in the presence of valvar calcification is a risk factor for the rate of decline in functional status after all types of commissurotomy. Type of surgical commissurotomy (open or closed) has not been shown to be a risk factor ( Table 11-4 ).
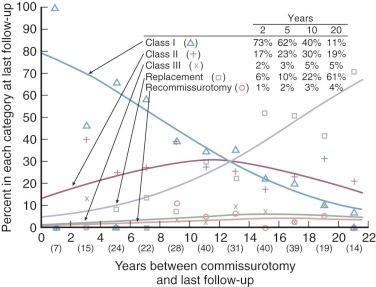
| Risk Factor | P Value | |
|---|---|---|
| (Older) | Age at commissurotomy | .03 |
| (Higher) | Precommissurotomy NYHA class | .0001 |
| Morphologic | ||
| (Greater) | Valve calcification (grades 0-5) | .0003 |
| (Greater) | Leaflet immobility (grades 0-5) | .002 |
| (Longer) | Follow-up interval | <.0001 |
Most patients undergoing mitral commissurotomy by any technique will require another procedure at some time, generally mitral valve replacement because of gradual loss of leaflet pliability, progression of subvalvar pathology, and increase of valvar calcification (see Fig. 11-27 ). About 20% of patients undergoing surgical commissurotomy require valve replacement within 10 years, and about half require it by 20 years ( Fig. 11-28 ).
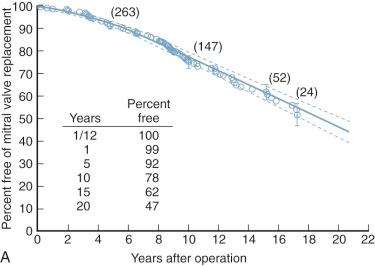
The same variables that cause survival to vary probably cause prevalence of postcommissurotomy mitral valve replacement to vary ( Table 11-5 ). Again, type of surgical commissurotomy (open or closed) has not been demonstrated to be a risk factor for subsequent mitral valve replacement. Morphology of the mitral valve powerfully affects the time-related prevalence of mitral valve replacement after surgical commissurotomy ( Fig. 11-29 ) as well as after percutaneous balloon valvotomy. At 7 years, freedom from restenosis was 93% for the open and balloon procedures vs. 63% after closed commissurotomy.
| Risk Factor | Late Hazard Phase | |
|---|---|---|
| Morphologic | ||
| (Smaller) | Preoperative mitral valve area | • |
| (Greater) | Leaflet immobility (grades 0-5) | • |
| Leaflet calcification | • | |
| (Greater) | Postrepair (OR) mitral valve regurgitation (grades 0-5) | • |
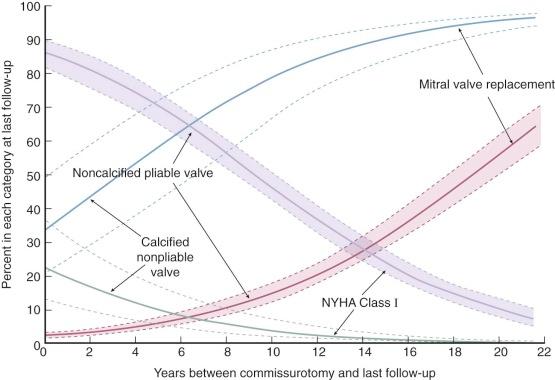
Early hemodynamic, functional, and anatomic improvement after percutaneous mitral valvotomy is, as noted earlier, nearly equivalent to results following open surgical commissurotomy. Long-term outcomes are not available. There is a progressive decrease in valve area that closely parallels that seen historically and in concurrent studies for surgical approaches. Degree of deterioration can be predicted by variables related to preoperative functional class and morphologic features of the mitral valve ( Table 11-6 and Fig. 11-30 ). Thus, long-term survival and need for reintervention after the catheter approach should be similar to results after the open surgical approach.
| Risk Factor | P Value |
|---|---|
| Age at intervention | .12 |
| Gender | .7 |
| Cardiac rhythm | .09 |
| Interval to follow-up | .03 |
| Type of intervention | .2 |
| Mitral valve morphology | |
| Thickening | .08 |
| Mobility | .09 |
| Calcification | .08 |
| Subvalvar disease | .04 |
| Composite valve score | .04 |
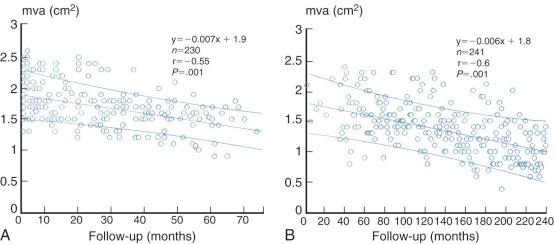
Survival after a second mitral commissurotomy is surprisingly good in view of the more advanced valvar pathology usually present at reoperation. Peper and colleagues report 5-, 10-, and 15-year survival of 96%, 83%, and 63%, respectively, after repeat open commissurotomy. Also, 16 (31%) of 52 hospital survivors underwent still another mitral operation, usually a replacement, an average of 8.2 years later.
In general, early, intermediate, and long-term results of repair of mitral valve regurgitation have been good.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here