Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mitochondria are very complex and highly dynamic organelles. Although responsible for only 10% of the cellular proteome, mitochondria serve not only as powerhouse of the cells but also as critical regulators of essential cellular processes including iron-sulfur cluster biosynthesis, calcium homeostasis, and cell death; hence they contribute to health and disease.
Mitochondria are surrounded by a double-membrane system, consisting of an inner mitochondrial membrane (IMM) and outer mitochondrial membrane (OMM) separated by an intermembrane space (IMS). The inner membrane forms numerous folds ( cristae ), which extend into the interior or the matrix of the organelle ( Fig. 10.1 ). Each of these subcompartments plays distinct functional roles.
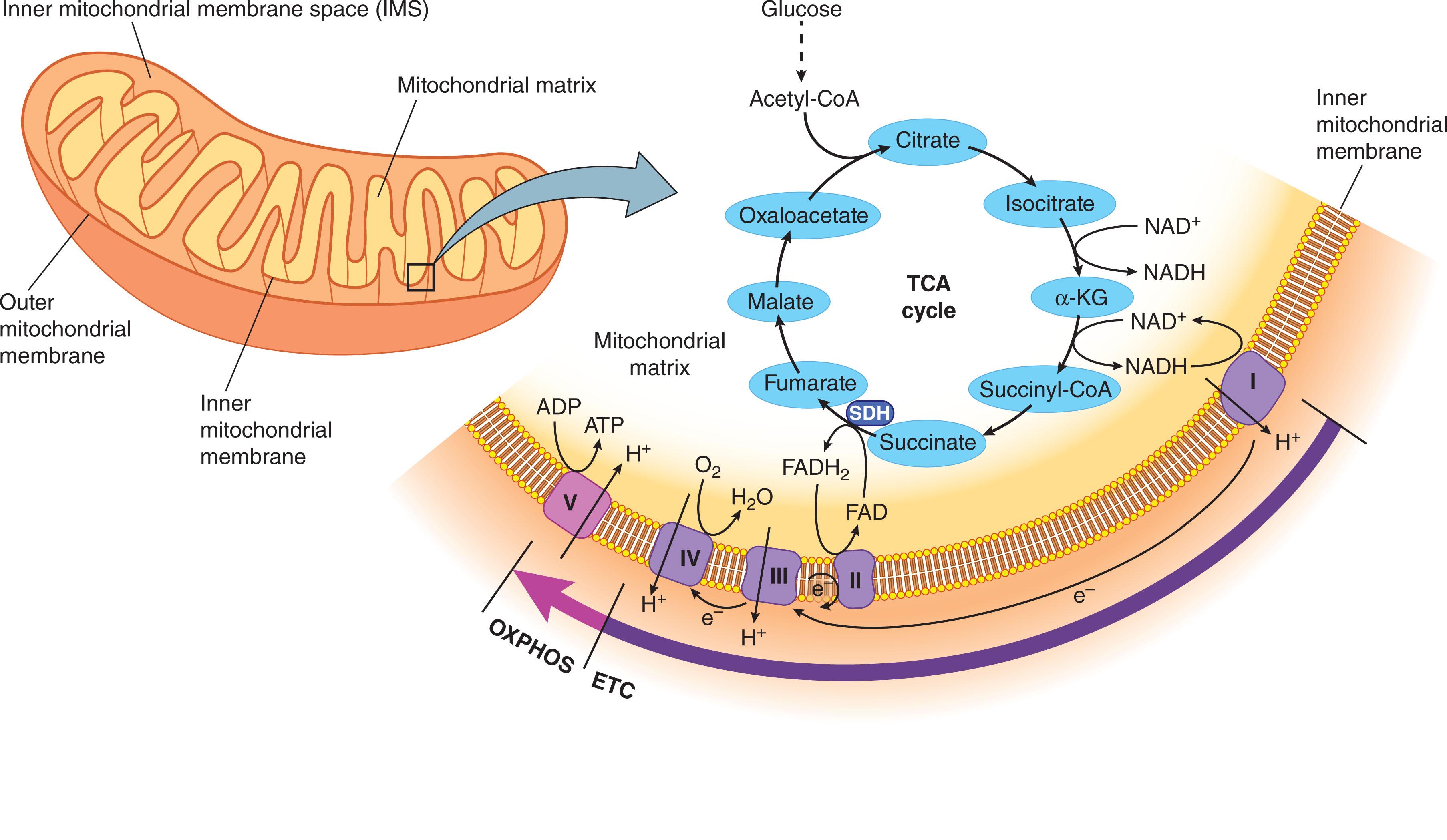
Mitochondria use almost 90% of O 2 consumed by the cell to generate adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS). During this process, pyruvate that is generated by conversion of glucose in the cytosol is transported into the mitochondria, where it enters the tricarboxylic acid (TCA) cycle and becomes oxidized to yield acetyl coenzyme-A (CoA). Acetyl CoA is also generated by oxidation of fatty acids via the TCA cycle. The series of reactions implicated in the TCA cycle, which occur in the mitochondrial matrix, result in generation of critical metabolites that are further transported into the cytoplasm, where they provide the building blocks for biosynthesis of macromolecules such as nucleotides, lipids, and proteins.
In addition to metabolites, the completion of the TCA cycle is also coupled to reduction of NAD + and FAD to NADH (nicotinamide adenine dinucleotide, reduced) and FADH 2 (flavin adenine dinucleotide, reduced), respectively. Both of these molecules are then transferred along the “respiratory chain,” also known as the “electron transport chain” (ETC). Specially, NADH and FADH 2 feed the ETC complex I and II, respectively, which then further transfer electrons to complex III and IV of the ETC. Such electron transfer through the four multisubunit protein complexes of ETC embedded in the IMM generates a proton gradient across the inner membrane that is used by complex V to synthesize ATP through OXPHOS (see Fig. 10.1 ).
Mitochondrial proteins and hence their functions are originated and controlled by two distinct genetic systems: nuclear deoxyribonucleic acid (nDNA) and mitochondrial (mt)DNA. The majority of the mitochondrial proteins (~1158) are nuclear-encoded proteins. They are imported as unfolded polypeptide chains into the mitochondria through complex mechanisms that mainly rely on the translocase TIM/TOM (translocase of the inner membrane/translocase of the outer membrane) system. These newly synthesized polypeptides contain specific signaling sequences, which direct and target them into distinct mitochondrial subcompartments (mitochondrial matrix, IMM, or IMS) where they undergo proper folding. Unlike the nDNA, mtDNA is simple and lacks both introns and histones. According to the endosymbiotic theory, the simplicity of mtDNA is a result of engulfment of a proteobacterium by another archeabacteria. The resulting double-membrane organelle was capable of producing ATP and providing the host with energy. Over time, however, most of the genetic material was either transferred to the host’s genome or lost. These events resulted in the double-stranded, circular mtDNA molecule, which consists of 16,569 base pairs. The main DNA coding sequence is the 1.1 kb loop and consists of two promoters for mtDNA transcription and an origin for replication. mtDNA encodes for only 37 genes of which 13 are structural subunits required for OXPHOS, 2 mitochondrial ribosomal (r)RNAs, and 22 mitochondrial transfer (t)RNAS, essential for synthesis of these subunits.
The pathophysiology of mitochondrial diseases is complex and is characterized by defects in OXPHOS. These defects can be triggered by mutations in either nDNA or mtDNA and hence in genes responsible for both the structure and function of mitochondria. As such, complex genetics that underlie mitochondrial diseases can display several patterns of inheritance, including autosomal and X-linked inheritance due to nDNA mutations and maternal inheritance for mtDNA mutations. Because multiple copies of mtDNA are present in each cell, mutations can affect all (known as homoplasmy) or a portion of mtDNA content (termed heteroplasmy). The levels of heteroplasmy vary between cells of the same tissue or organ and from organ to organ. Interestingly, 1 in 200 healthy individuals carry the 10 most common pathogenic mtDNA mutations, although at low levels of heteroplasmy. However, rare, de novo mutations have also been observed. Originally thought to be extremely rare, mitochondrial diseases have now been reported to affect 1 in 4300 people. Mitochondrial diseases are characterized by a high morbidity and mortality because they often involve multiple systems, particularly those that heavily rely on mitochondrial metabolism ( Fig. 10.2 ). However, in a relatively small number of cases, single organ involvement such as isolated ocular involvement in Leber hereditary optic neuropathy (LHON) has been also observed. The heterogeneity of the clinical manifestations makes both the diagnosis and treatment extremely challenging.
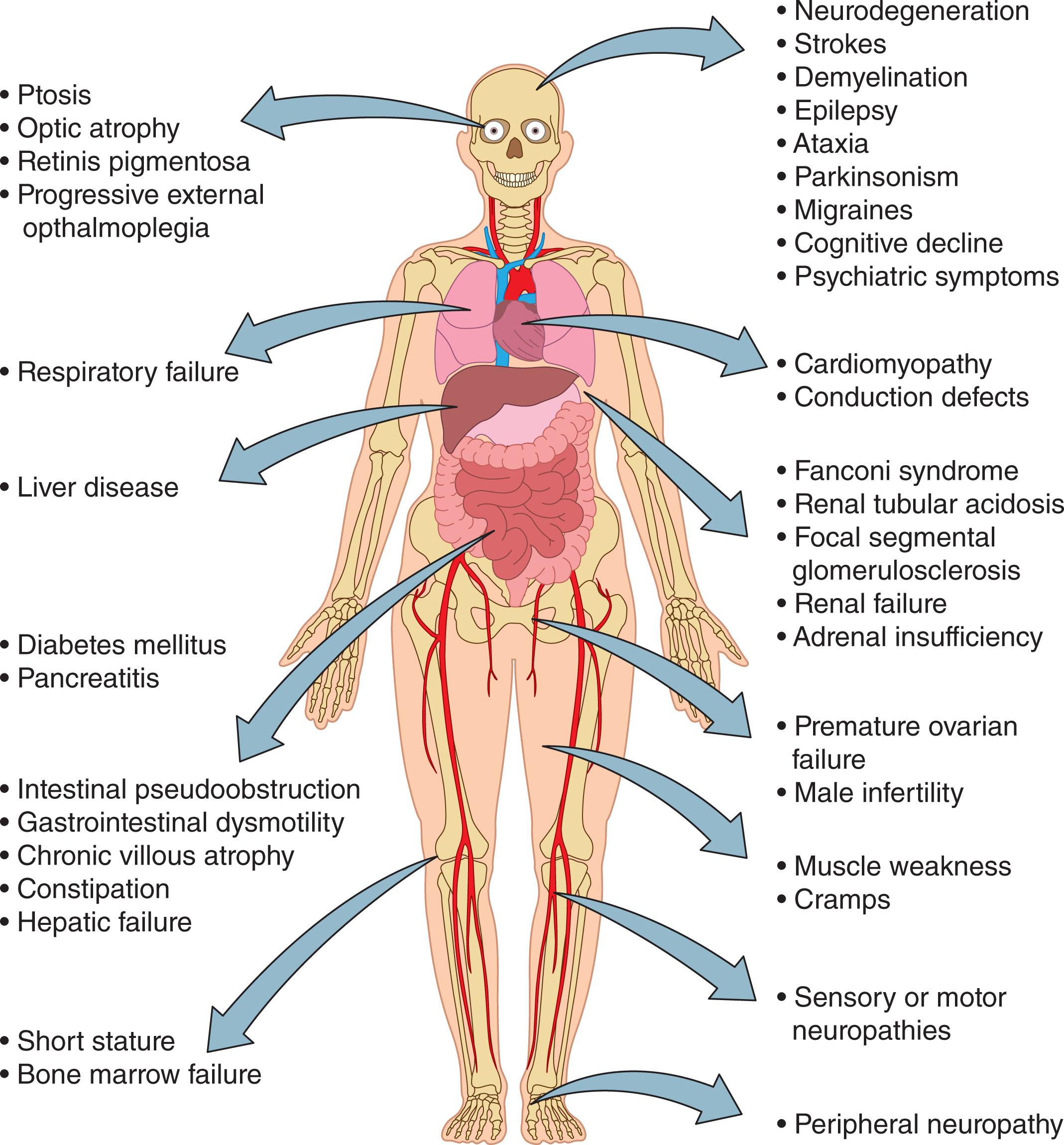
The onset of mitochondrial diseases has been reported to have a bimodal distribution reaching a peak in the first 3 years of life followed by a second peak that begins by the end of teenage years and extends to the fourth decade of life. Childhood-onset mitochondrial diseases are usually severe, although not always fatal. These diseases are often caused by recessive nDNA mutations or mtDNA mutations, which are present with very high levels of mtDNA heteroplasmy. Several syndromes that are due to mitochondrial abnormalities, including Leigh syndrome characterized by symmetric spongiform degeneration of the corpus striatum and brainstem with demyelination and vascular proliferation; Alpers-Huttenlocher characterized by epilepsy, psychomotor regression, and liver disease; Pearson syndrome and congenital lactic acidosis; and progressive pure myopathy or a spinal muscular atrophy-like phenotype, arise during childhood. In addition, some forms of renal cystic disorders, proximal tubulopathy, and hypertrophic cardiomyopathy that occur in children have also been attributed to mitochondrial defects.
In this context, Pearson syndrome that was first described in 1979 by Howard Pearson at Yale University (see Chapter 30 ) is of special interest to hematologists. It is characterized by a mutation in mitochondrial DNA that leads to transfusion-dependent sideroblastic anemia, vacuolization of marrow precursor cells, and exocrine pancreas dysfunction. Affected individuals experience failure to thrive, pancreatic fibrosis with insulin-dependent diabetes and exocrine pancreatic deficiency, muscle and neurologic impairment, and frequently, early death during infancy. The few patients who survive into adulthood often develop symptoms of Kearns-Sayre syndrome. Less than 100 cases of Pearson syndrome have been reported. In infants, Pearson syndrome is associated with large mtDNA deletions ranging in size from 1.1 to 10 kb. Such mtDNA deletions may occur de novo or be transmitted in a maternal inheritance pattern. The mother of the child, although clinically unaffected, may potentially harbor a great degree of mtDNA deletions in a population of her oocytes. If the mother is clinically unaffected, the risk for the other siblings is estimated to be 1% to 4% because of the possibility of maternal germline mosaicism. Because mitochondria are maternally inherited, offsprings of a male with an mtDNA pathogenic variant are not at risk of inheriting the condition. Prenatal testing for pregnancies at increased risk is available, but results are not reliably predictive of disease severity. Intriguingly, several isogenic immortalized pluripotent stem cell (iPSCs) lines derived from cells of a patient with Pearson lacked detectable levels of the deleted mtDNA, whereas other lines had a great degree of deletions. The iPSCs carrying a high burden of deleted mtDNA displayed differences in proliferation, mitochondrial function, and hematopoietic phenotype when differentiated in vitro, as compared with isogenic iPSC which lacked the deleted mtDNA.
Interestingly, a study of a cohort of 178 infants with Diamond-Blackfan anemia showed that 4.8% of them actually had Pearson syndrome. The identification of Pearson syndrome is not insignificant because patients with Diamond-Blackfan anemia are empirically treated with corticosteroids, which do not benefit patients with Pearson syndrome and may lead to infectious and metabolic complications.
Mitochondrial diseases can also present and progress in several ways during adult life. The abnormalities in an affected adult patient often do not fit into a specific syndrome because mitochondrial disease can encompass multiple systems of the body. Patients with Kearns-Sayre syndrome, a progressive cradioencephalomyopathy, in infancy can be clinically manifested as Pearson syndrome, whereas in middle age the patients develop chronic progressive external ophthalmoplegia (CPEO). Multiple endocrinopathies involving the adrenal gland, pancreas, thyroid, and parathyroid gland frequently develop in patients with more severe disease.
Neurogenic muscle weakness, ataxia, and retinitis pigmentosa (NARP) syndrome, a progressive neurologic disorder, forms a clinical continuum with maternally inherited Leigh syndrome. NARP is caused by a mutation in mtDNA involving specifically the MT-ATP6 gene. Individuals with heteroplasmy levels of greater than 70% to 90% clinically manifest NARP, whereas those with greater than 90% present Leigh syndrome. Leigh syndrome becomes apparent during the first year of life, whereas the first symptoms of NARP syndrome begin in childhood or early adulthood. Together, this pattern of disease indicates the complexity and the marked clinical variation of disease manifestations observed in patients, which frequently delays both diagnosis and appropriate clinical care. Next-generation sequencing has dramatically advanced the diagnosis and increased our understanding of the molecular landscape and the genetic basis of these disorders. Many novel therapeutic approaches are being explored in preclinical models, and several new therapies are being evaluated in ongoing clinical trials. Despite this success, many challenges remain, including understanding of the mechanisms implicated in the phenotypic expression of a particular genetic defect as well as the optimal care for patients with mitochondrial diseases. In this chapter, we will review the role of mitochondria in both normal and abnormal hematopoiesis, as well as in aging.
Hematopoiesis relies on the integrity of a unique pool of hematopoietic stem cells (HSCs). HSCs and specifically those with long-term (LT) hematopoietic reconstitution capabilities occupy the apex of the hematopoietic system. They sustain LT hematopoiesis throughout the lifespan of an individual by constantly replenishing the hematopoietic system with committed progenitors and differentiated blood cells. Vast numbers of all types of adult mature hematopoietic cells (10 11 to 10 12 ) are continuously generated each day through a series of lineage-committed progenitor cells. The LT regeneration capacity is due to HSCs’ ability to balance the self-renewal with differentiation. Such balance is central to blood cell homeostasis and is regulated by the complex interplay of cell-intrinsic and cell-extrinsic signaling networks. This interplay is orchestrated and controlled by the mitochondrial network, which recently has emerged as a critical determinant of HSC homeostasis, commitment, and differentiation.
During homeostasis, HSCs are predominantly quiescent. To meet the physiologic demands for mature cells during steady-state condition, HSCs exit the quiescent state and become activated into slowly dividing cells. Upon hematopoietic stress that occurs after acute depletion of progenitors and mature hematopoietic cells, HSCs rapidly enter the cell cycle and proliferate to replace the depleted cells. The exit of HSCs from quiescence, and their proliferation and commitment to multiple different cell fates, are intrinsically coupled with altered cellular metabolism and mitochondrial activity ( Fig. 10.3 ). It has been postulated that a metabolic switch from glycolysis to mitochondrial OXPHOS precedes the transition of HSCs from quiescence to proliferation. However, evidence has demonstrated that priming of quiescent HSCs and their entry into the cell cycle appears to be accompanied by upregulation of glycolysis. Because glycolysis is linked to mitochondrial metabolism, such an increase in glycolysis rate might provide the energy required for entrance of quiescent HSCs into the cell cycle and activation of the mitochondrial network. Increased mitochondrial activity is essential for actively dividing HSCs and their commitment into each type of lineage-differentiated hematopoietic cells. In fact, compromised mitochondrial function and perturbation of metabolic pathways result in impaired differentiation and leukemia.
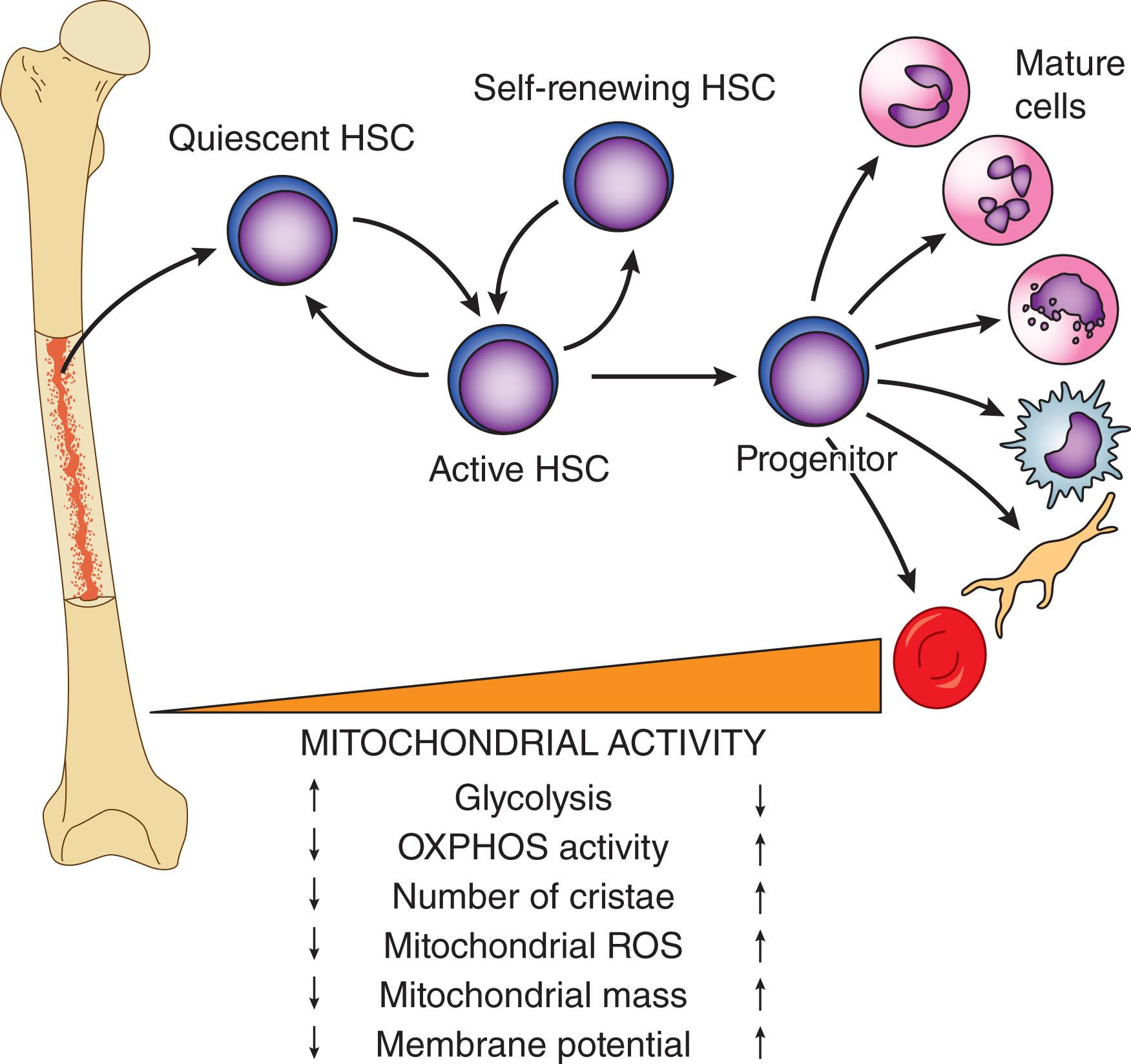
Metabolic cues and mitochondrial content and activity vary drastically within different stages of hematopoiesis. LT HSCs are characterized by low mitochondrial mass, mitochondrial membrane potential (MMP), and reactive oxygen species (ROS) as opposed to committed progenitors and differentiated mature cells. The increase in the mitochondrial activity upon cell commitment allows the differentiating cells to meet their altered high metabolic and bioenergetic demands. Such metabolic rewiring is accompanied by profound alterations in the mitochondrial ultrastructure and dynamics, both of which determine HSC fates. Indeed, mitochondrial dynamics, morphology, and metabolism reciprocally influence each other to maintain the balance between self-renewal and proper differentiation of HSCs. Impairment of mitochondrial metabolism affects mitochondrial dynamics and morphology, resulting in loss of HSC quiescence and self-renewal potential. Conversely, aberrations in mitochondrial dynamics compromise the capacity of HSCs to differentiate into lymphoid cells.
Beyond OXPHOS and ATP generation, mitochondria drive HSC fate decisions by regulating biosynthesis and the epigenome. Mitochondrial metabolites generated by OXPHOS and TCA cycles control DNA methylation, chromatin remodeling, and posttranslational modifications of various proteins, including histones and other epigenetic factors. In turn, such changes shape the epigenetic landscape and affect gene repertoires, ultimately compromising HSC function and cell fates.
Mitochondria are signaling organelles that govern HSC fate decisions by also regulating calcium homeostasis and pathways involved in inflammation and cell death. In fact, each of these pathways is differently impacted by various levels of mitochondrial ROS.
Although mitochondria are the major source of ROS generation, they are also the main targets of ROS. Mitochondrial DNA and the mitochondrial proteome (mitochondrial and nuclear encoded proteins) are particularly vulnerable to ROS-mediated damages. This is due in part to their close proximity to the ETC, which is the primary site of ROS generation. A subtle elevation in ROS levels, in part, triggers HSC commitment and differentiation. Alternatively, excessive ROS levels impair HSC multilineage differentiation and induce uncontrolled proliferation and sustained cumulative damages, leading to HSC exhaustion, loss of self-renewal potential, and cell death.
Unlike other cellular compartments, mitochondria are poorly equipped with quality control elements such as chaperones and proteases, which monitor proper protein folding and eliminate misfolded and oxidative damaged proteins. Accumulation of such damaged proteins further impacts the ETC activity and electron leakage from ETC, leading ultimately to increased ROS levels. The increased ROS levels, in turn, can trigger vicious cycles of mitochondrial damage and sustained energetic catastrophe. To reduce oxidative and metabolic stress that can be detrimental for HSC fates and integrity, HSCs use complex regulatory pathways and defense mechanisms that are summarized in Fig. 10.4 .
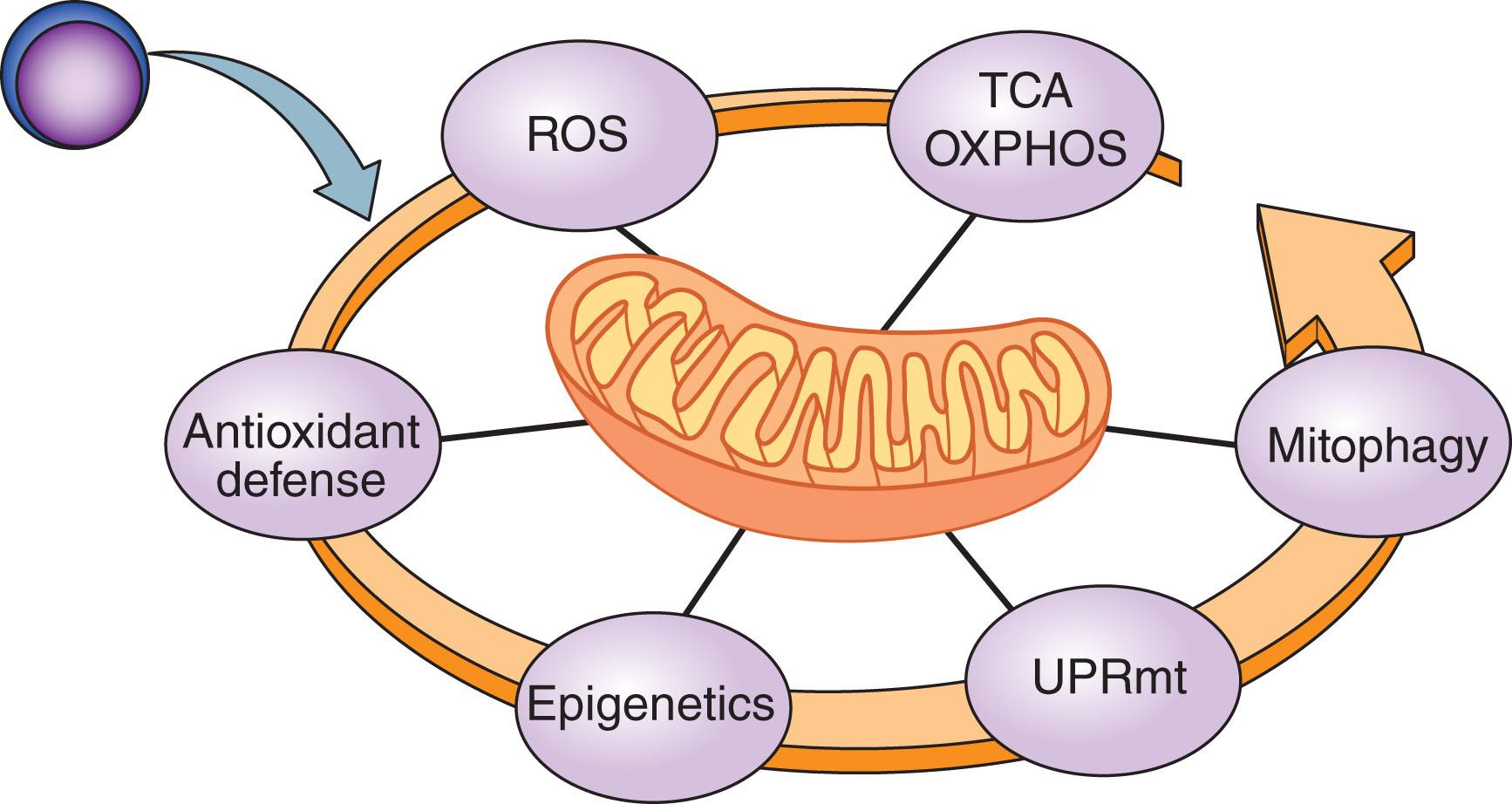
In the following sections we will discuss each of these topics and review the latest advances in our understanding of the multiple mechanisms by which the mitochondrial network controls stemness and differentiation of HSCs during normal hematopoiesis, leukemic transformation, and regeneration.
HSCs have a low protein synthesis rate and a limited folding capacity in comparison with committed progenitors and differentiated cells. To achieve rapid and effective blood cell replacement with various hematopoietic progenitor and differentiated cells, HSCs face a burst in mitochondrial biogenesis. This burst is accompanied by an increased need for protein synthesis. Due to the poor folding capacity, the increase in protein synthesis can exceed and overwhelm the capacity of the protein quality control system, leading to mitochondrial proteostasis stress, increased oncogenic transformation, and impaired HSC regenerative potential. To cope with the mitochondrial proteostatic stress, mitochondria signal the stress to the nucleus. Such events establish the mitochondria-to-nucleus signaling crosstalk, which result in the activation of the mitochondrial unfolded protein response (UPRmt). The UPRmt acts by coordinating the function of several pathways, all of which culminate in induction of proteases and chaperones that are required for proper protein folding and their complex assembly. Thus UPRmt monitors transcriptional programs and critical pathways that ensure overall mitochondrial fitness and integrity. A recent study identified a novel arm of UPRmt in HSCs, which is regulated by the coordinated interplay of Sirtuin (SIRT7), a histone deacetylase, and nuclear respiratory factor 1 (NRF1), a master regulator of mitochondrial biogenesis. In response to bioenergetic stress, HSCs activate SIRT7, which represses the activity of NRF1. In turn, this suppression reduces mitochondrial translation and biogenesis and alleviates stress. Remarkably, SIRT7 expression, which is downregulated with aging, is associated with reduced HSC regenerative capacity. In fact, in aged HSCs, mitochondrial protein folding stress is also linked to activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome. Repression of the NLRP3 inflammatory pathway by activated SIRT2, a cytoplasmic deacetylase, regulates the functional deterioration of aged HSCs. Failure to engage UPRmt can irreversibly damage HSC cellular and metabolic fitness, leading to increased ROS generation, uncontrolled HSC proliferation and differentiation, and loss of HSC integrity and cell death.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here