Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
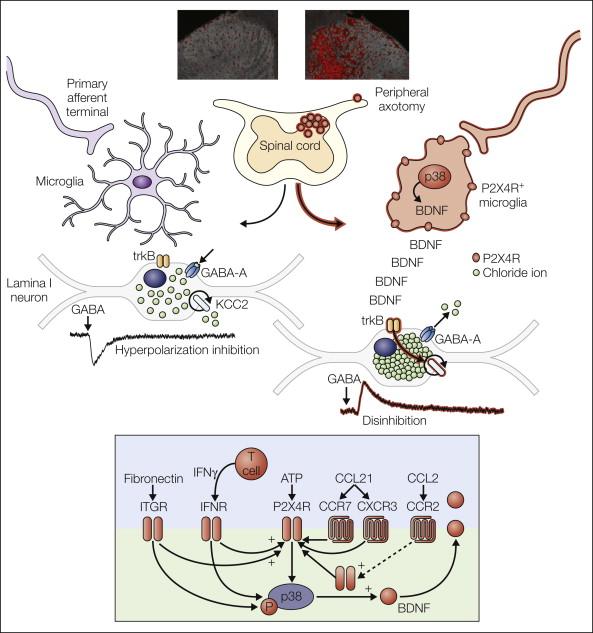
Neuron–glial interactions are increasingly recognized as being key for physiological and pathological processes in the central nervous system. Microglia in the spinal dorsal horn respond to injury to peripheral nerves by adopting a specific response state characterized by up-regulation of the purinergic receptor P2X 4 . In this P2X4R + state, microglia release brain-derived neurotrophic factor, which disinhibits neurons in the spinal nociceptive processing network. The transformation in processing caused by signaling of P2X4R + microglia to nociceptive transmission neurons may account for the main symptoms of neuropathic pain in humans.
The world of pain research and therapy owes a great deal to the little-known German anesthetist and surgeon Carl Ludwig Schleich (1859–1922) for two specific contributions. First, Schleich was a pioneer of regional anesthesia and refined the technique considerably by introducing a new, safer method of infiltration anesthesia, as detailed in his book Schmerzlose Operationen ( Painless Operations ) ( ). However, within that book are contained his theories of brain function, which make for remarkable reading. In what would be an extremely prescient proposal, Schleich rejected the accepted neural network concept of the time being championed by Sigmund and suggested an active role for glial cells. It was while listening to a piano recital that he was struck with inspiration and announced “glia as a damper pedal, an apparatus for switching registers … an inhibition regulator” ( ). Schleich postulated that glial cells control neuronal excitation in the brain, a theory now widely held and of intense research interest throughout neuroscience. Schleich lived in less enlightened times; both he and his theory were ignored, and he suffered the final ignominy of being described as though his own brain were “turning into glue” ( )—a reference to the Greek word for glue being the etymological root of glia.
Study of the nervous system has been a story of controversy since the first revelations of the inner structure of this “black box” were revealed, pioneered by discovery of the reazione nera or “black reaction,” the revolutionary tissue-staining technique of Camillo Golgi in 1873. Golgi’s silver staining revealed a new world of cellular structures, and it was immediately clear that other structures, distinct from neurons, were present in the tissue samples. These non-neuronal structures had no place in the prevalent theory of the day and were put to rest by one of the foremost physiologists of the time, Rudolf Virchow, who had previously dismissed these cells as Nervenkitt, or “nerve glue” ( ). The glue cells were deemed to not contribute to the “physiological explanation of mental phenomena” ( ) and were subsequently ignored.
Though recognized as structural elements in their own right, this was the time of the advent of the “neuron doctrine.” This was established by the great Spanish histologist and founder of modern neuroanatomy Santiago Ramon y Cajal, who posited the nervous system as being made up of discrete individual cells. It was in opposition to Golgi himself who had developed his reticular theory proposing that every neuron in the entire nervous system is physically linked with its neighbors (ironically, a system that has more resonance with the structure of astrocytes than neurons). Cajal’s improvement of Golgi’s pioneering staining techniques showed clear differentiation of neurons from neuroglia (now known as astrocytes and the source of Schleich’s fascination), but it also revealed a further population of cells that he termed the “third element” ( ). It was to be the work of Cajal’s student Pio del Rio Hortega to unravel the mystery of this third element ( ). By further refining the metallic impregnation techniques of Cajal, he was able to successfully stain this cell population and in 1919 identify and define it as two distinct populations that he named microglia and oligodendroglia ( ). This was an extremely controversial claim at the time, and debate raged between Rio Hortega and Cajal about the nature of the third element and stimulated a surge of research on the function of these enigmatic cells. However, this interest soon dwindled, and by the mid-20th century, microglia were once again the neglected cell population in the central nervous system (CNS) ( ). Interest was sparked anew toward the end of the century with the realization that microglia are the resident macrophage population and therefore the immune effectors of the CNS, and in more recent years the role of microglia in particular in CNS function and malfunction has been revisited ( ). Given their immune role, microglia represent the first line of defense against damage to the CNS, and with the understanding that peripheral neuropathy is manifested as a pathological state of the CNS ( ), there is intense interest in their role in pain pathophysiology.
Microglia are now known to derive from a distinct macrophage population that comes from embryonic myeloid progenitors in the yolk sac ( ), and they invade and populate the CNS through the pial membranes ( ). Microglia have been likened to the electricians of the CNS; they exist outside the neuronal circuit and are able to delve in and modulate the electrical activity within ( ). Unlike the reticular-like syncytial system of astrocytes throughout the CNS, microglia are not physically connected but reside within their own adjacent, non-overlapping microdomains within the brain and spinal cord ( Fig. 4-1 ). Such a spatially restricted system allows the microglial response to react in an anatomically precise fashion after pathology or damage. Under physiological conditions, quiescent microglia are not “resting” but are in a state of motility and surveillance, with cellular processes continuously scanning their microenvironment ( ).
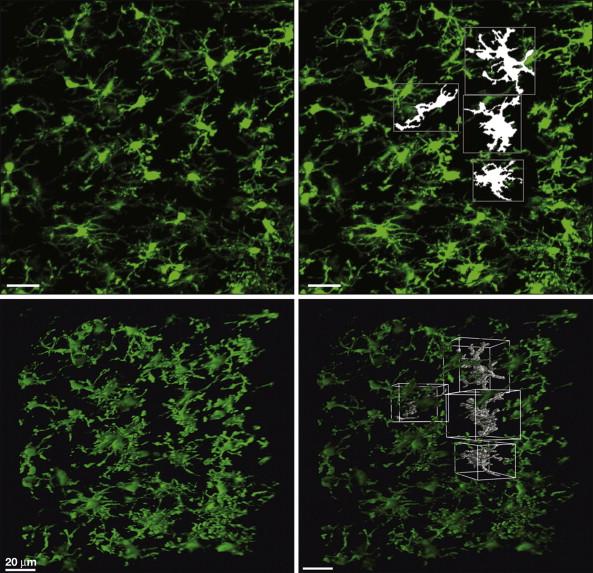
How this grid-like network of microglia that extends throughout the CNS interacts and modulates the underlying cellular circuitry of the CNS is of considerable interest as a fundamental cellular mediator underlying the pathophysiology of neuropathic pain. The extensive toolbox that microglia possess in terms of cytokines, chemokines, neurotrophins, and neurotransmitters has made this population of glial cells a rich seam of research, and a wealth of knowledge now exists and also remains to be discovered.
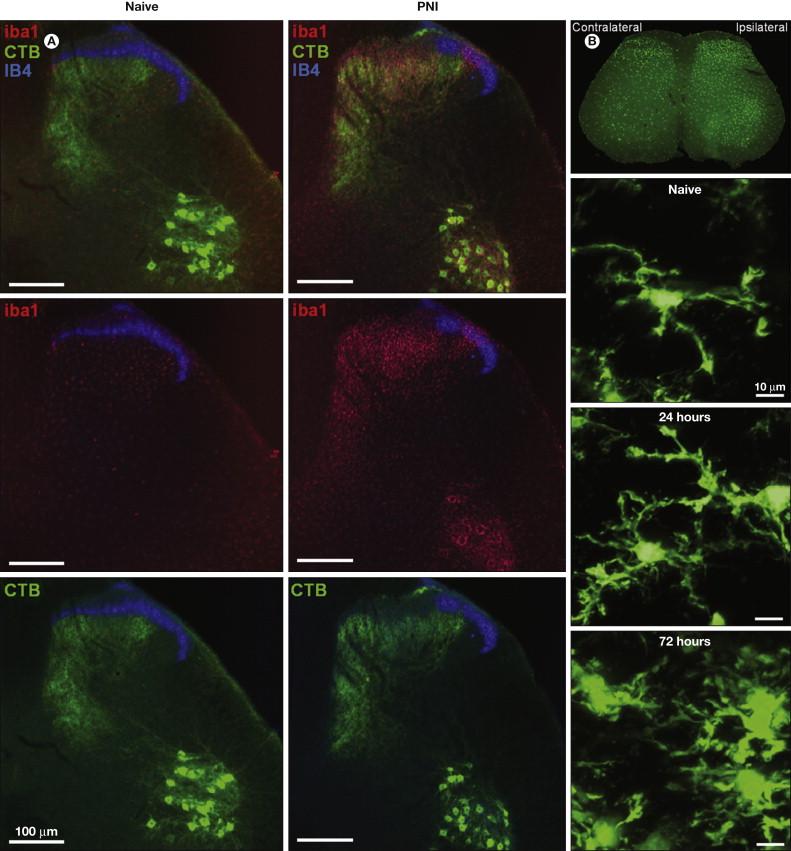
Considerable progress has been made since microglia were heralded as “sensors of pathology” ( ), but it is important to reject the inflexible notion of microglial “activation” as a causative factor underlying peripheral nerve injury (PNI)-induced pain behavior. The classic morphological and proliferative responses of microglia within the spinal cord following PNI ( Fig. 4-2 ) are a signifier of microglia reactivity but do not necessarily constitute a “pro-pain” phenotype. A common misconception is that resolving these microglial signatures following nerve damage will resolve the behavioral changes. That being said, microglia in the spinal dorsal horn do respond to and are critical mediators of the pathobiology of PNI; all existing neuropathic pain models now envisage some requisite degree of spinal microglial response ( ) ( Fig. 4-3 ). However, such microglial changes are less evident in inflammatory and chemotoxic models of pain ( ), and the role of microglia in the pain states that result from these insults remains to be elucidated ( ). As stated above, microglia exist throughout the neuraxis, including at the level of primary afferents, spinal nociceptive circuitry, and projections to the brain. For a definitive causal role of microglia in pain to be stated, tests of both sufficiency and necessity must be proven.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here