Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
At birth, infants become colonized with trillions of commensal microbes that play important roles in health and disease. The majority of these microbes reside in the gastrointestinal tract, reaching densities of 10 12 bacteria per gram of intestinal content in the colon. Complex microbial communities are also found on all human surfaces, including the skin, oropharynx, vagina, and lung. These microbiomes are highly abundant—bacteria represent an astounding 50% to 90% of the cells within or on our bodies and are accompanied by less well-defined but significant numbers of viruses, fungi, and archaea. Overall, the human microbiome contains thousands of species spanning the microbial phylogenetic tree. Because the majority of these microbes are anaerobic and are not routinely recovered using standard microbial culture techniques, the biological impacts of the human microbiome have historically been underappreciated. However, recent technological advances in high-throughput DNA sequencing provided powerful tools to define and study these microbial communities. The microbiome is now understood to be a complex ecosystem in which there is tremendous cross talk between microbial species and between microbes and their host. This communication and interdependence between potential pathogens and the host is likely especially important in severely immunocompromised hosts, such as transplant recipients and oncology patients.
So how does one understand and study the microbiome and its importance to human health? A first step is to catalog the microbes present in a particular anatomic location. Currently, the most widely used culture-independent approach to identify bacterial and fungal microbes is to amplify and sequence the variable regions of the highly conserved 16S ribosomal RNA (rRNA) gene from bacteria or 18S rRNA gene from fungi. These sequences can then be assigned to specific bacteria or fungi and used to determine the abundances of specific microbes within a sample. This approach is inexpensive and high-throughput but has the disadvantage of identifying only bacterial or fungal members of the community. A second approach, referred to as “shotgun” metagenomics, sequences all of the DNA present in a sample. This approach is more expensive and involves substantially more sophisticated analyses to define the members of the microbial community. However, shotgun sequencing provides information on a broader range of microbes, including viruses and archaea, and yields more detailed information on gene content, which can be used to infer the functional capacity of the microbial community.
Taxonomically, microbes are classified by kingdom, phylum, class, order, family, genus, and species. The gut microbiome consists of bacteria primarily from five phyla ( Table 10.1 ). The neonatal gut microbiome has low diversity (relatively few bacterial species) and is typically composed of bacteria from the genera Bifidobacterium and Lactobacillus and facultative anaerobes from the family Enterobacteriaceae. During the first 3 years of life, the gut microbiome is fluid and undergoes substantial shifts in composition with the introduction of solid foods, with strict anaerobes from the orders Clostridiales and Bacteroidales replacing the neonatal microbiome. Alteration of this early life gut microbiome can disrupt immune system development and function. This is most evident in germ-free animals that lack commensal microbes and have myriad health consequences, including abnormal development and function of the immune system and increased susceptibility to infections and autoimmunity. Human epidemiologic studies also reported associations between early life microbiome perturbations (e.g., cesarean delivery, antibiotic exposures) and the later development of asthma, atopy, and autoimmune disorders. Taken together, these studies suggest that microbial exposures are important for educating the developing immune system, with a lack of host-microbe interactions predisposing to immune dysregulation. This concept, often referred to as the “hygiene hypothesis,” was first proposed to explain the geographic distribution of cases of seasonal allergic rhinitis, but has since been extended to other areas of medicine, including hematopoietic stem cell transplantation, oncology, and solid organ transplantation (SOT). ,
| Phyla | Class | Order | Family | Genus |
|---|---|---|---|---|
| Actinobacteria | Actinobacteria | Actinomycetales Bifidobacteriales |
Corynebacteriaceae Bifidobacteriaceae |
Corynebacterium Bifidobacterium a |
| Bacteroidetes | Bacteroidia | Bacteroidales b | Bacteroidaceae Prevotellaceae Rikenellaceae |
Bacteroides Prevotella Alistipes |
| Firmicutes | Clostridia | Clostridiales b | Clostridiaceae Eubacteriaceae Lachnospiraceae Ruminococcaceae |
Clostridium Faecalibacterium Eubacterium Blautia Ruminococcus |
| Negativicutes Bacilli |
Veillonellales Lactobacillales |
Veillonellaceae Enterococcaceae Lactobacillaceae Streptococcaceae |
Dialister Enterococcus c Lactobacillus a Streptococcus c |
|
| Proteobacteria | Gamma proteobacteria | Enterobacteriales | Enterobacteriaceae a | Enterobacter c Escherichia c Klebsiella c |
| Verrucomicrobia b | Verrucomicrobiae | Verrucomicrobiales | Akkermansiaceae | Akkermansia |
a Bacteria that predominate in infants.
b Bacteria that predominate in children and adults.
c Endogenous bacteria that commonly cause invasive infection.
Commensal microbes also prevent infection by providing a barrier to colonization and overgrowth by more virulent bacteria. This concept—referred to as “colonization resistance”—was originally described in 1954 when it was noted that mice treated with the antibiotic streptomycin were susceptible to nontyphoidal Salmonella infection at a dose 10,000-fold lower than the typical minimal infectious dose. The mechanisms that account for colonization resistance are becoming increasingly well understood ( Fig. 10.1 ). It is now recognized that commensal gut bacteria can inhibit pathogen colonization through competition for carbohydrates and other micronutrients, secretion of antimicrobial substances (e.g., peptides, short-chain fatty acids), and through interactions with the host immune system. The classic example of an infection that results from a loss of colonization resistance of the gut microbiome is Clostridium difficile colitis. A loss of gut microbial diversity and anaerobic commensal bacteria, most frequently after the administration of antibiotics, predisposes to C. difficile colonization and infection. Restoration of gut microbial diversity through fecal microbiome transplantation is increasingly being used as a treatment for recurrent C. difficile infection and is widely regarded as the most successful microbiome therapeutic in modern medicine.
Several concepts are important to understanding the impact of the microbiome on human health and disease. First, anatomic location is a critical determinant of the microbiome. For example, the skin microbiome is markedly different from the gut microbiome and, within the gut, the microbiome of the small intestine differs substantially from that of the large intestine. This illustrates the importance of local environmental factors, such as nutrient availability, temperature, and pH, in regulating human microbiome composition. Second, the microbiome performs specific functions, such as digesting dietary fiber, secreting metabolites, and modifying bile acids, and microbiomes with varied composition can provide similar functions. In turn, composition cannot be used to accurately predict microbiome function; microbiomes with similar compositions may perform different functions and have different effects on their host. Third, microbiome alterations observed in the setting of a disease may implicate the microbiome as an important driver of that disease state, or alternatively, may reflect the effect of the disease on the microbiome. Longitudinal studies that include characterization of the microbiome before disease onset are generally needed to establish or refute a causal relationship between the microbiome and a disease state. This is an important distinction to make because it has implications for the potential of microbiome therapeutics to prevent or ameliorate the disease.
In general, microbiomes associated with disease states have lower diversity and high abundances of one or a few potentially pathogenic microbes. This microbiome state is referred to as “dysbiosis” and occurs when the microbiome has a negative impact on the host. A dysbiotic microbiome has disproportionately lost beneficial microbes while microbes that are potentially detrimental to the host have expanded to dominate the microbial community. Such a microbiome may harm the host through loss of microbial metabolic functions that benefit the host. For example, antibiotics that deplete fiber-fermenting anaerobes deprive the host of short-chain fatty acids, which nourish enterocytes and help maintain gut barrier function. Lower abundances of these anaerobes can lead to disruption of the gut mucosal barrier and translocation of intestinal microbes, which can result in bacteremia and sepsis.
In this chapter, we discuss three emerging themes that relate the microbiome to children undergoing hematopoietic stem cell transplantation (HSCT), treatment for cancer, or SOT ( Fig. 10.2 ). First, the microbiome influences risk for infection. Although many infections in immunocompromised patients originate from endogenous microbes, a healthy microbiome also prevents colonization, overgrowth, and invasion by exogenous pathogens. Second, the microbiome influences immune system function in children at risk for graft-versus-host disease (GVHD) and allograft rejection. Third, the microbiome has the potential to be a powerful tool to predict and prevent infections and other complications in immunocompromised children.
HSCT is associated with substantial alterations of the gut microbiome. Although the gut of healthy individuals typically contains a diverse microbial community composed of approximately 1000 bacterial species, the gut microbiome of patients early after HSCT is frequently far less diverse and is often dominated by a single bacterial species. These shifts in gut microbial diversity and composition occur rapidly—often over the course of days—and are associated with exposure to antibiotics, chemotherapy-induced gut mucosal injury, and dietary changes. Marked increases in the relative abundances of Enterococcus species and Proteobacteria occur frequently after HSCT and are associated with receipt of antibiotics. These shifts are offset by losses of key commensal gut bacteria, including microbes from the genera Faecalibacterium and Ruminococcus . Changes in nutritional intake and, in particular, a lack of enteral intake early after HSCT also contribute to alterations in the gut microbiome. Enteral feeding via a nasogastric tube has been associated with lower GVHD risk and infectious mortality compared with parenteral nutrition in observational studies of allogeneic HSCT recipients. A randomized controlled trial (the NEPHA study) is currently underway to compare these nutritional approaches.
Several studies suggest that the gut microbiome may be a useful biomarker for the prediction of outcomes after allogeneic HSCT. Most of this research has been conducted in adults and has focused on gut microbial diversity. The diversity of the gut microbiome at the time of engraftment is strongly associated with mortality after allogeneic HSCT. In a study of 80 adults, patients with lower diversity of the gut microbiome had markedly worse survival 3 years after allogeneic HSCT (36% vs. 67%). Gut microbial diversity was most strongly associated with mortality from GVHD and infections, suggesting the importance of the gut microbiome to the pathophysiology of these conditions. The gut microbiome also was associated with the risk of relapse among patients undergoing HSCT as treatment for malignancy. In a study of 541 adult patients with hematologic malignancies, the presence of Eubacterium limosum in the gut microbiome predicted a lower risk of relapse at 2 years after allogeneic HSCT.
Despite recent advances in histocompatibility matching and donor selection, GVHD remains a leading cause of morbidity and mortality among children after allogeneic HSCT. Our current understanding of the pathogenesis of GVHD suggests that chemotherapy-induced damage to the gut mucosa results in translocation of microorganisms or their products—most notably lipopolysaccharide—triggering an innate immune response that ultimately leads to activation of alloreactive donor T lymphocytes ( Fig. 10.3 ). Research conducted in the 1970s and 1980s established the importance of the gut microbiome to the pathophysiology of GVHD. In these studies, germ-free mice developed GVHD at a far lower rate after allogeneic HSCT than conventionally raised mice. , These findings spurred numerous efforts to prevent GVHD in patients undergoing allogeneic HSCT through suppression or decontamination of the microbiome. A variety of approaches were attempted, including “sterile” diets, laminar airflow isolation, skin cleansing protocols, and gut decontamination through the administration of high-doses of nonabsorbable oral antibiotics. Unfortunately, these strategies were not consistently effective for GVHD prevention in clinical studies and were thus not widely implemented.
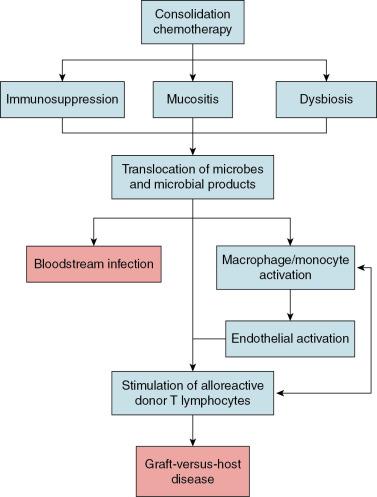
The recent development of high-throughput sequencing technologies led to renewed interest in understanding how the gut microbiome influences the risk of GVHD. Although the diversity of the gut microbiome is a strong predictor of the risk of GVHD, the relative abundances of specific microorganisms also appear to be important. In particular, higher relative abundances of Enterococcus have been observed in patients with GVHD, consistent with studies demonstrating that enterococci can impair gut mucosal integrity and stimulate activation of the innate immune system. Other studies suggest that specific gut anaerobes may be protective for GVHD. In particular, higher relative abundances of certain bacteria from the order Clostridiales (e.g., Blautia ) appear to be associated with a lower risk of GVHD. Limited data from clinical studies also suggest that anaerobic bacteria may be protective from the onset of GVHD. In a single-center retrospective study of adult allogeneic HSCT recipients, the use of antibiotics with an anaerobic spectrum of activity during the peritransplant period was associated with higher GVHD mortality. Although the associations identified in these studies are noteworthy, our understanding of the mechanisms by which the gut microbiome influences GVHD risk remains limited. Further research is needed to better delineate the complex interactions that exist between the gut microbiome and the host immune system to inform strategies to prevent GVHD through manipulation of the gut microbiome.
There has been much interest in investigating whether the gut microbiome modifies the risk of infections in HSCT recipients. Most studies conducted to date focused on bloodstream infections, particularly those caused by enteric bacteria. Overgrowth of the gut microbiome by Enterococcus or Proteobacteria is associated with a higher risk of bloodstream infection caused by these organisms among adults after allogeneic HSCT. Moreover, antibiotics appear to be an important precipitating factor for overgrowth of the gut microbiome by these bacteria by decreasing colonization resistance. The administration of metronidazole increases the risk of enterococcal domination, consistent with reports that gut anaerobes provide a barrier to colonization and overgrowth by enterococci, whereas fluoroquinolones reduce the incidence of domination by Proteobacteria. Interestingly, and somewhat unexpectedly, gut bacteria also appear to influence the risk of viral infections, including those arising outside the gastrointestinal tract, which could result from the extensive cross talk that occurs between the gut microbiome and the host immune system. Although our understanding of these complex and bidirectional interactions is limited, the gut microbiome appears to play an important role in establishing and maintaining virus-specific memory T-lymphocyte responses. This finding mirrors those of studies of viral infections in germ-free or antibiotic-treated mice in which the presence of commensal microbes alters susceptibility to some viral infections. In clinical studies, the gut microbiome’s influence on viral infections has been most clearly shown for respiratory viruses. Several studies demonstrated associations between antibiotic exposures or the composition of the gut microbiome and the risk and severity of respiratory virus infections among adults after allogeneic HSCT. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here