Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
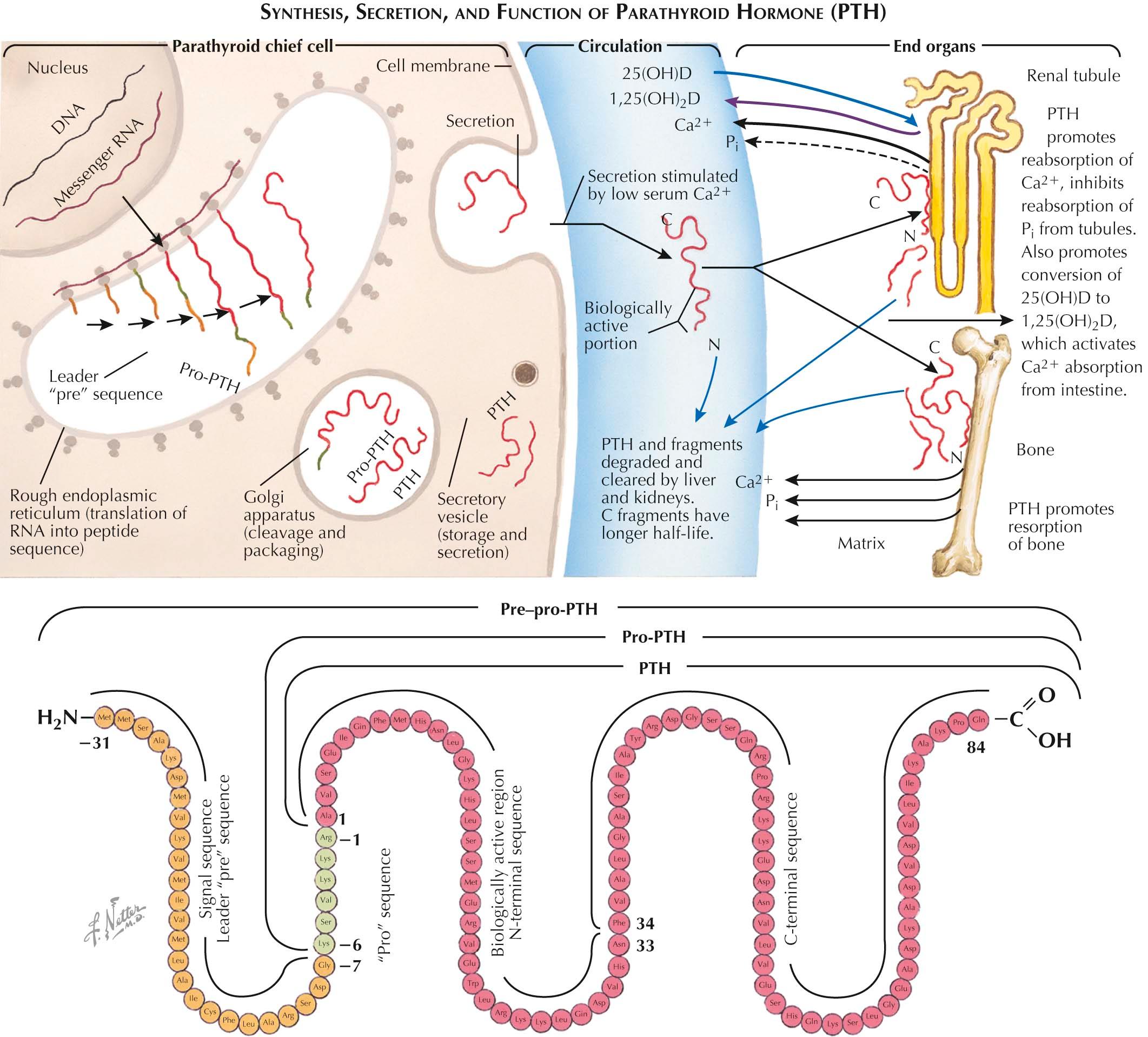
The parathyroid gland regulates the calcium in the extracellular fluid by sensing small changes in calcium levels and rapidly modifying the secretion of parathyroid hormone (PTH). With a fall in the calcium level, PTH secretion is increased and, in turn, leads to increased calcium concentration and suppressed PTH secretion, thus completing a feedback loop. PTH raises the calcium level by promoting the entry of calcium from bone, renal tubule, and intestine. In bone, PTH increases resorption of formed bone, which contains calcium and phosphate. In the kidney, PTH enhances renal tubular reabsorption of calcium (which decreases urinary calcium excretion) and decreases tubular reabsorption of phosphate (which eliminates the phosphate released from bone). PTH indirectly increases calcium absorption from the gut by increasing the synthesis of the active form of 1,25-dihydroxyvitamin D, or 1,25(OH) 2 D, from its precursor 25-hydroxyvitamin D, or 25(OH)D. This metabolite of vitamin D mediates the gut effect of PTH by directly activating calcium absorption in the small intestine.
Compared with other secretory systems, the parathyroid cell contains minimal amounts of hormone stored in the granules. Consequently, regulation of secretion occurs at the gene level and by cell proliferation. PTH is initially synthesized as pre–pro-PTH, a large precursor peptide of 115 amino acids. During translation from its messenger ribonucleic acid (mRNA), the initial “pre” sequence directs the growing peptide across the membrane of the endoplasmic reticulum and is then cleaved before synthesis of the entire hormone is completed. The 90-amino-acid pro-PTH is then transported through membrane channels to the Golgi apparatus where the hexapeptide extension of pro-PTH is removed. PTH is stored in the secretory vesicles until secretion occurs. Once secreted it is metabolized to inactive products composed of fragments from the carboxyl (C)-terminal end. Modern-day assays for PTH recognize the intact molecule and do not measure the inactive C-terminal fragments.
The regulation of PTH secretion is more complex than previously recognized. Its secretion is controlled not only by serum calcium and phosphorus but also by the active form of 1,25-dihydroxyvitamin D (1,25-DHVD) and by fibroblastic growth factor-23 [FGF23] and its membrane receptor Klotho.
Serum calcium and phosphorus have a post-transcriptional effect on gene PTH expression. Hypocalcemia increases mRNA levels and PTH secretion. Chronic low-calcium states also induce cell proliferation. Increased serum phosphorus has opposite effects. It decreases secretion of PTH via a regulatory step at the mRNA level where specific proteins stabilize or destabilize the concentration of PTH mRNA.
FGF23 is a regulatory protein of phosphorus. It is a skeletal protein that acts on the kidney to decrease synthesis of 1,25-DHVD and increase phosphorus excretion through binding to a renal membrane protein called Klotho. The parathyroid cell also has this Klotho receptor protein. The binding of FGF23 to parathyroid cell Klotho receptor causes an activation of specific intracellular kinases that suppress PTH gene expression and subsequent secretion of the hormone.
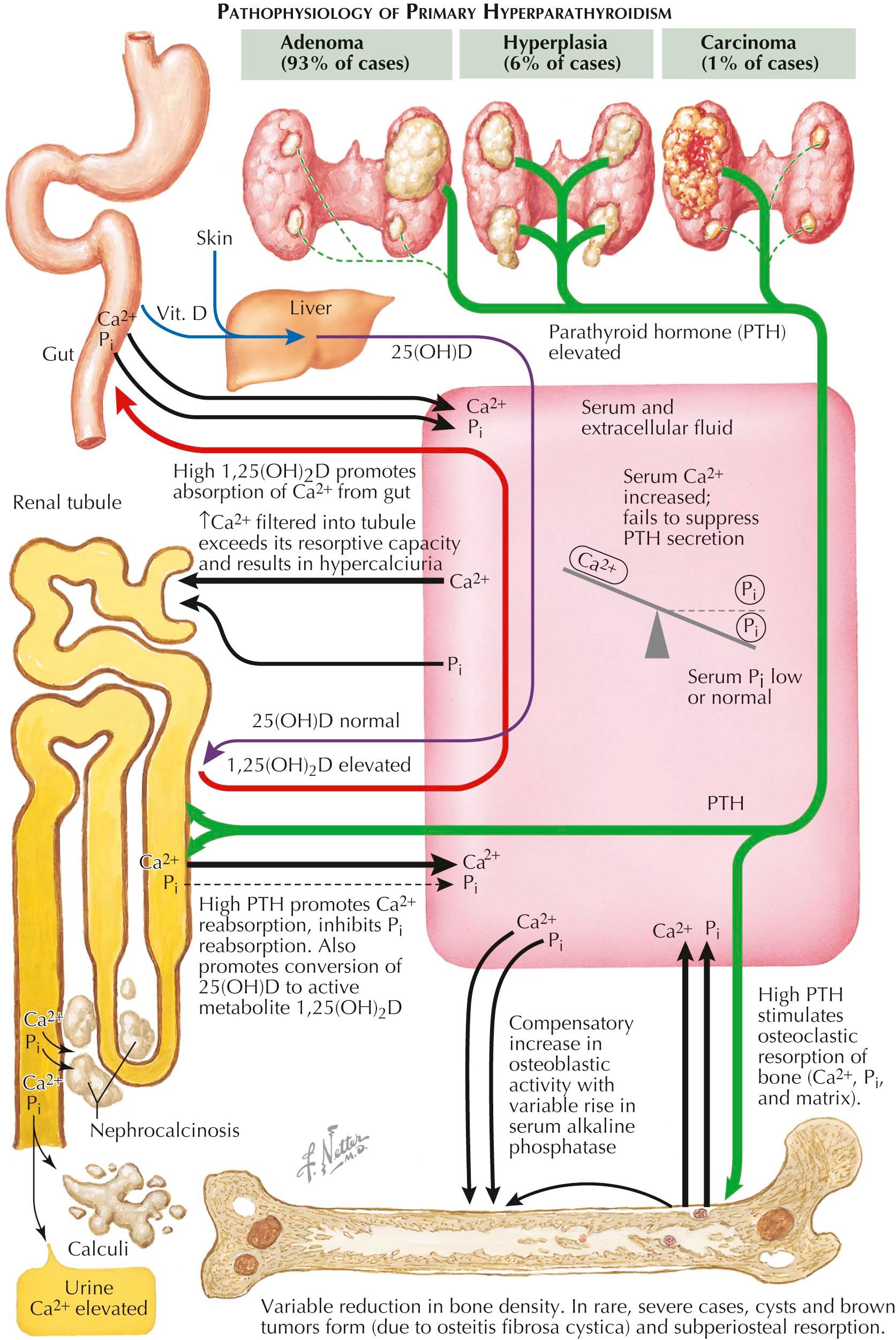
Primary hyperparathyroidism is caused by excessive production of parathyroid hormone (PTH) by enlarged parathyroid glands (see Plate 3-2 ). In 85% of cases, only a single parathyroid gland is enlarged (adenoma); in 15% of cases, all four glands are enlarged (hyperplasia). These two pathologic types of hyperparathyroidism have identical clinical manifestations and can only be distinguished at surgery, although nuclear imaging may sometimes identify hyperplasia of all the glands. This technique is a localizing procedure and not a diagnostic test for hyperparathyroidism. Parathyroid carcinoma occurs in less than 1% of patients, and severe hypercalcemia is often the initial symptom. Histologic diagnosis is helpful. Clinically this problem is suggested when recurring parathyroid “adenomas” develop after repeated removal of an adenoma.
Hyperparathyroidism causes hypercalcemia as a result of increased resorption of bone, reabsorption of calcium in the renal tubule, and absorption of calcium from the gut. PTH also decreases reabsorption of phosphate in the renal tubule, and in moderate-to-severe hyperparathyroidism the serum phosphate level is reduced; in the mild form of the disease, the serum phosphate level is often normal. Another form of the disease, although less often appreciated, is the entity called normocalcemic or eucalcemic hyperparathyroidism.
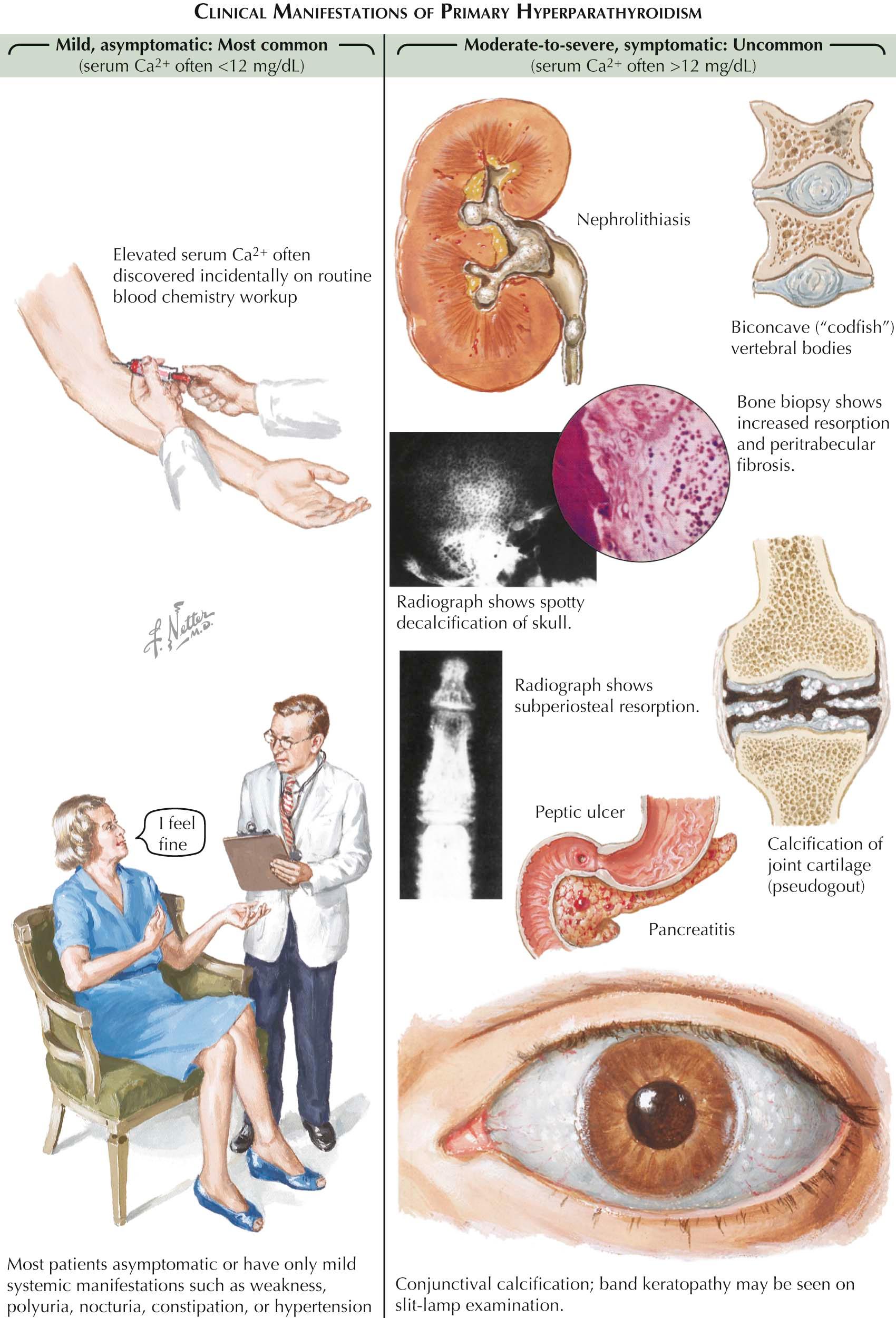
Hyperparathyroidism is a chronic indolent disorder that slowly increases serum calcium levels over many years and may be evident for decades before it produces significant clinical problems. It was considered a rare disorder until 2 decades ago, but routine screening of serum by automated techniques greatly increased recognition of increased serum calcium in many patients and the suspicion for this disorder. Most patients have mild hypercalcemia (serum calcium level < 12 mg/dL) and have few or no symptoms. The most common clinical complaint in newly diagnosed hyperparathyroidism is no complaint at all. Those patients with clinical manifestations may show a spectrum of problems such as fatigue, lethargy, constipation, nocturia, abdominal discomfort, changes in mental status of minor degree (e.g., poor concentration, forgetfulness, depression) osteoporosis, bone pain, fractures, renal colic and stones, unexplained anemia, and weight loss. With severe hypercalcemia (>12 mg/dL), confusion and coma may supervene along with anorexia, nausea, vomiting, and dehydration. It is a clinical observation that older patients tolerate high serum calcium levels poorly and may manifest these later problems more often than younger patients. In a small number of patients there is clinical or radiographic evidence of hyperparathyroid bone disease. Typical findings include serum calcium levels greater than 12 mg/dL, serum PTH levels several times higher than normal, high serum alkaline phosphatase, and diffuse bone pain.
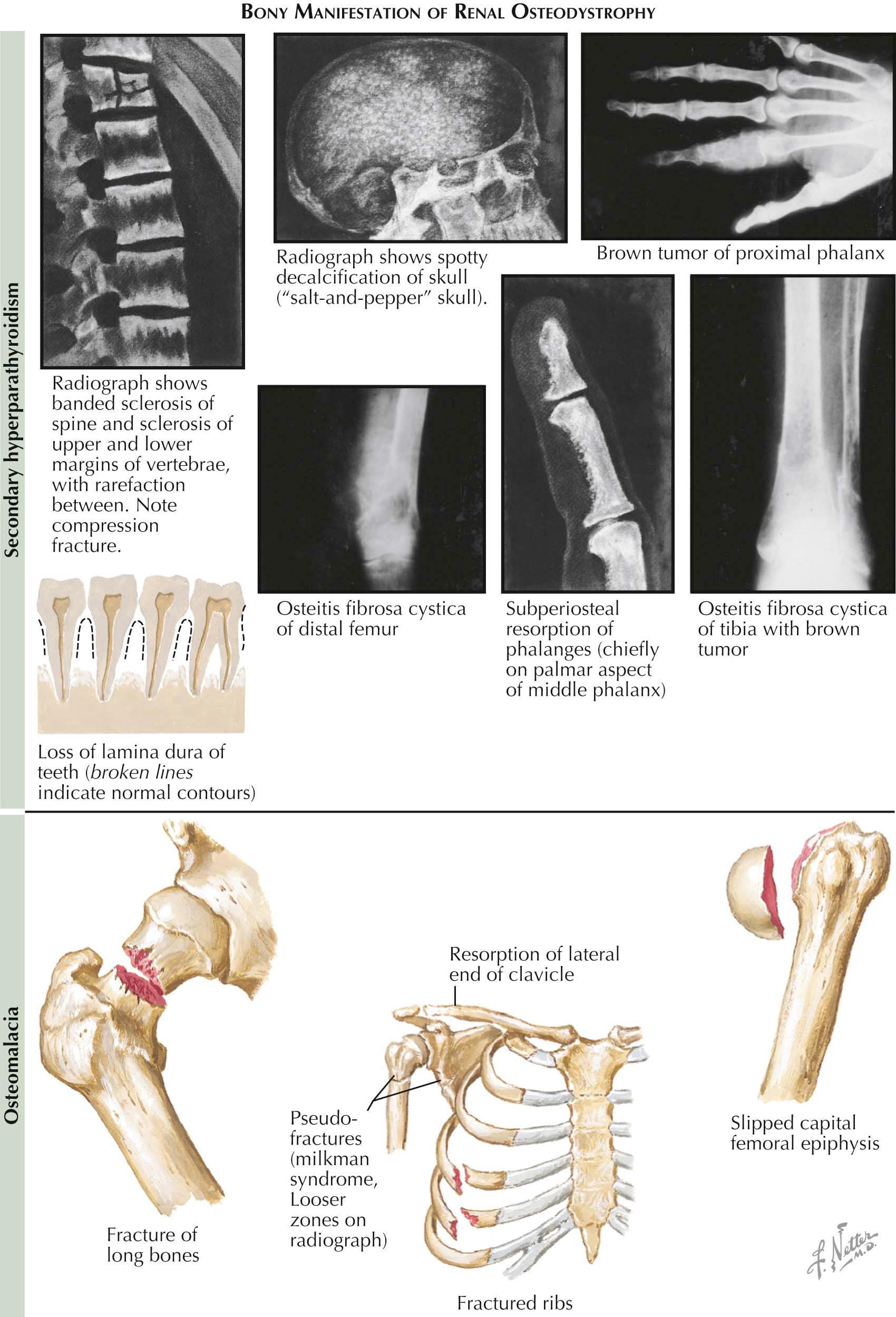
In patients with severe hyperparathyroidism and bone disease, radiographs may show subperiosteal bone resorption (highly specific to hyperparathyroidism) around the phalanges and distal ends of the clavicles and diffuse decalcification of the skull (salt-and-pepper skull) that resembles multiple myeloma. Bone cysts (also called brown tumors), if present, are often the sites of pathologic fractures. With bone loss in the spine, the intervertebral discs herniate into the vertebral bodies, creating a “codfish” appearance on radiographs. Even if there is no radiographic evidence of bone disease, excessive, PTH-mediated bone resorption may increase the risk of osteoporosis, a situation of particular concern in postmenopausal women who display the greatest incidence of this problem and already are at high risk for primary osteoporosis. This problem is often diagnosed as osteoporosis from a low bone density test. Histologically, the bone changes are more complex than simple osteoporosis. PTH activates osteoclastic resorption of bone, which can lead to significant bone loss. There is also a compensatory increase in osteoblastic bone formation; however, resorption ultimately exceeds formation, leading to bone loss. Osteoblasts release alkaline phosphatase, and serum levels may be elevated in patients with significant bone involvement. In severe cases of hyperparathyroidism, cystic areas of skeletal erosions may appear along with areas of fibrous tissue in adjacent bone marrow (osteitis fibrosa cystica).
Nephrolithiasis (urinary calculi) occurs in approximately 10% of patients with hyperparathyroidism (see Plate 3-3 ). Nephrocalcinosis is rarer and is typically seen in severe hyperparathyroidism with bone involvement. PTH increases calcium reabsorption in the renal tubule, the increased renal filtration produced by elevated serum calcium levels does not compensate, and the result is hypercalciuria. In some patients, calcium precipitates in the renal tubule and forms urinary calculi, common manifestations of kidney stones.
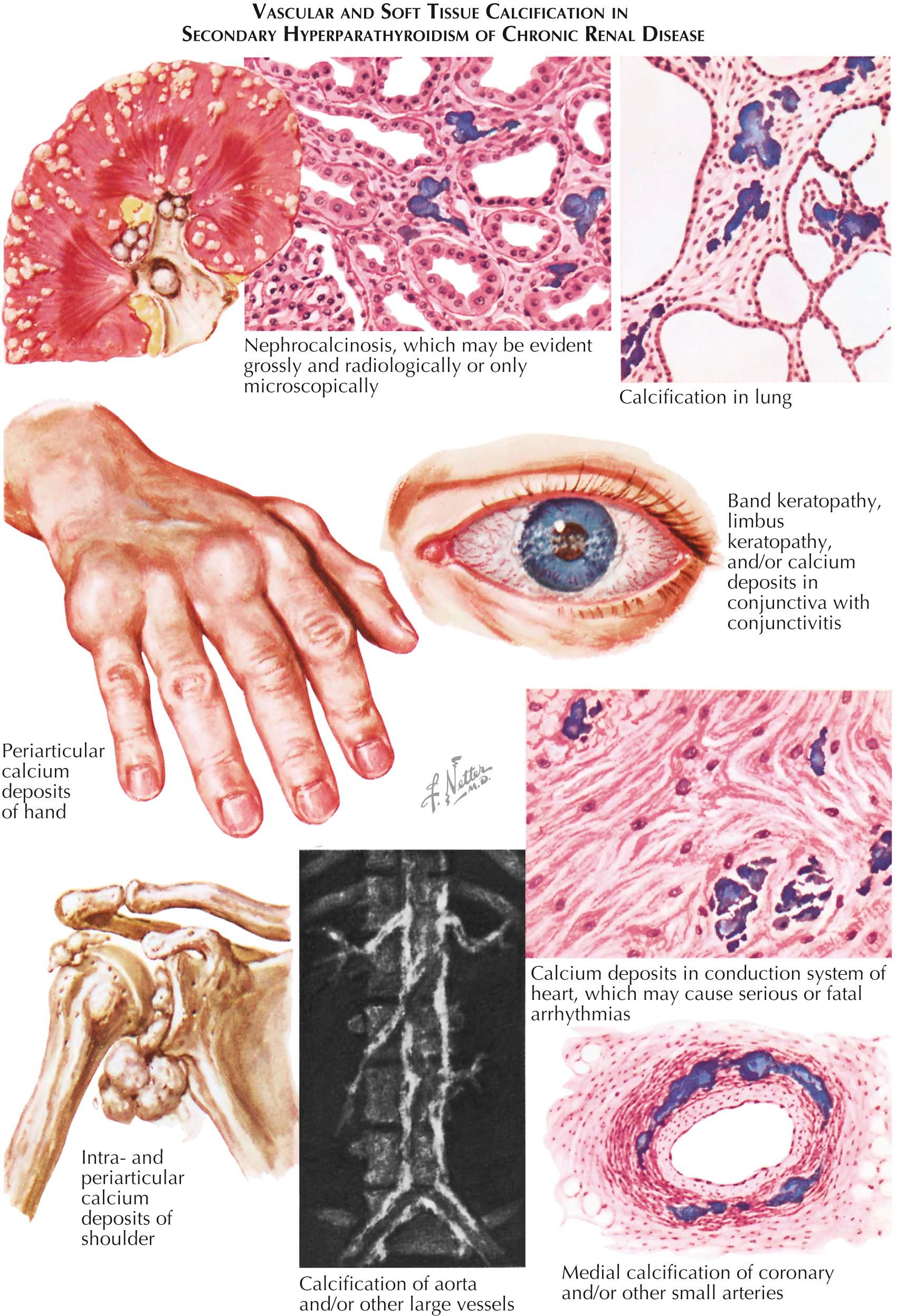
In severe hypercalcemia, precipitates can occur in the renal interstitium and incite an inflammatory reaction (nephrocalcinosis). By the time interstitial calcium deposits are visible on radiography, renal function is already considerably reduced. Hypercalcemia also decreases the capacity to concentrate urine, which frequently leads to polyuria and dehydration (if compensatory fluid intake does not compensate for this fluid loss). Pancreatitis and peptic ulcers, although rare, may occur with hyperparathyroidism. Gastrin-secreting tumors associated with hyperparathyroidism may cause ulcers in patients with multiple endocrine neoplasia (MEN) syndromes. Prolonged or severe hypercalcemia often results in calcium deposits in the medial and lateral edges of the cornea (band keratopathy).
Surgical excision of the single, enlarged parathyroid gland is the treatment of choice for patients with an adenoma. Treatment of hyperplasia is subtotal parathyroidectomy, or removal of all but one or one-half gland. However, excision of too little tissue leads to persistent hypercalcemia and removal of too much tissue causes hypoparathyroidism. Asymptomatic disease is a conundrum in clinical practice. When to intervene surgically is the topic of many guidelines published through the years.
Three familial autosomal dominant syndromes of hyperparathyroidism have been identified among patients with parathyroid hyperplasia. In two of these syndromes, MEN I and II, neoplasms occur in other endocrine glands. MEN I is characterized by pituitary adenomas and islet cell tumors in the pancreas, whereas MEN II is characterized by a high incidence of pheochromocytoma and medullary carcinoma of the thyroid. Patients with familial hypocalciuric hypercalcemia, the third and rarest syndrome, exhibit lifelong asymptomatic hypercalcemia that persists even after subtotal parathyroidectomy. This syndrome is distinguished from typical hyperparathyroidism by an absence of hypercalciuria and a high familial incidence of asymptomatic hypercalcemia without other endocrinopathies. This disorder is due to abnormalities in the calcium-sensing receptors in the kidney that fail to excrete appropriate amounts of calcium, thereby raising the serum level.
Cancer of the parathyroid glands can rarely be cured by surgical excision because it has metastasized by the time it is detected. Patients with parathyroid carcinoma usually die of uncontrollable hypercalcemia.
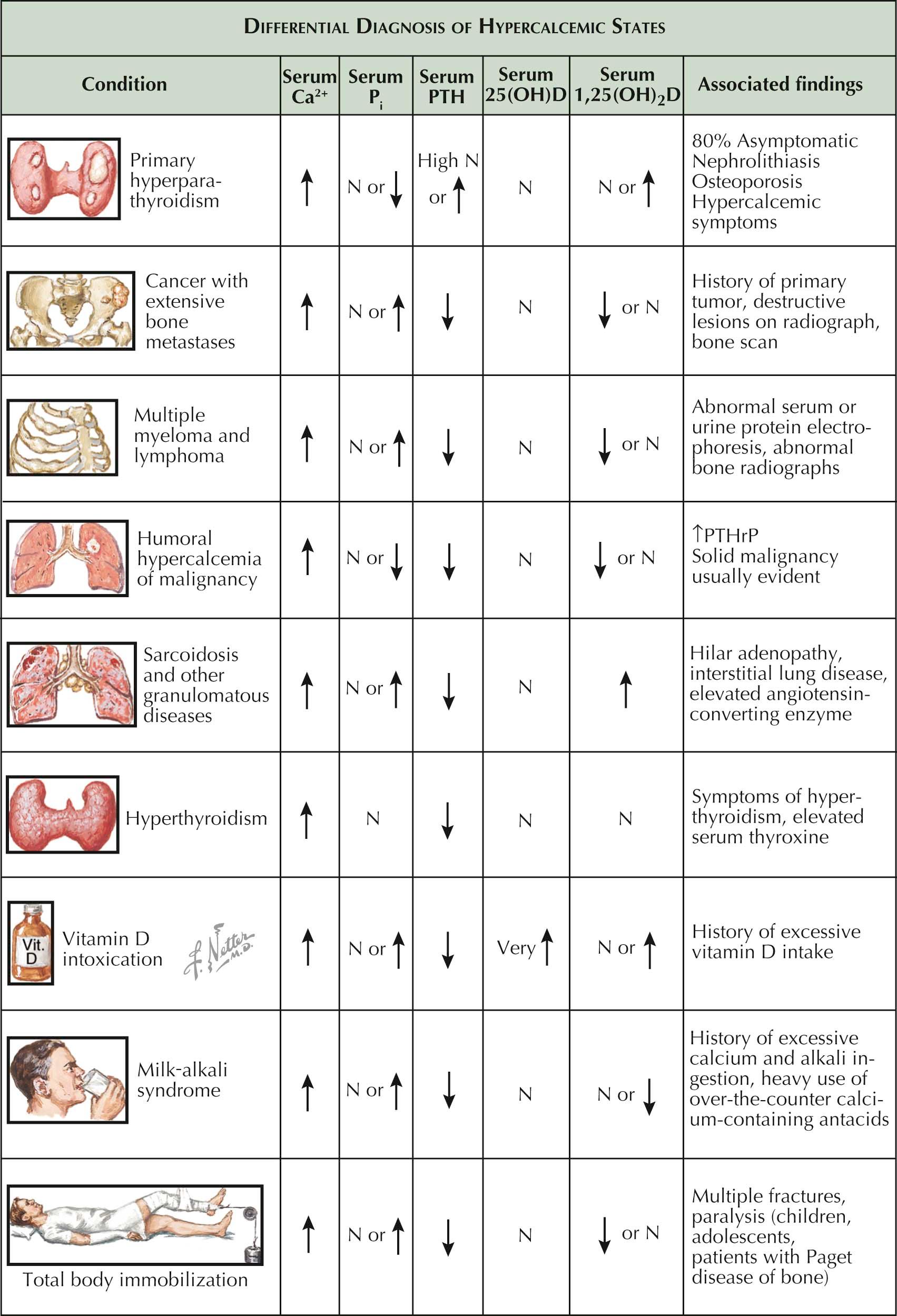
The cause of hypercalcemia can often be determined on the basis of the patient's history and a careful physical examination, because most of the disorders that give rise to hypercalcemia (aside from primary hyperparathyroidism) are clinically apparent by the time the condition occurs. Tests that measure serum levels of parathyroid hormone (PTH), inorganic phosphate (P i ), and vitamin D metabolites aid in the diagnosis, but the clinical history of hypercalcemia should not be discounted as a diagnostic aid. Hyperparathyroid disease is a chronic, slowly developing disorder, and results from old laboratory tests show the existence of hypercalcemia for many years.
The serum level of PTH distinguishes primary hyperparathyroidism from other disorders causing hypercalcemia, because it is the only hypercalcemic disorder resulting from excessive production of PTH. Serum phosphate levels are inversely proportional to PTH levels because PTH promotes the urinary excretion of phosphate. Although hypophosphatemia suggests hyperparathyroidism, it may also be present in patients with cancer, because certain tumors secrete phosphaturic factors. In addition, anorexia alone will lower serum phosphorus, thereby confounding its use as a diagnostic tool for parathyroid disease. High levels of 25-hydroxyvitamin D, or 25(OH)D (also known as calcidiol) suggest an excessive intake of vitamin D. A very high concentration of this usually inactive compound can result in hypercalcemia. A high concentration of 1,25-dihy-droxyvitamin D, or 1,25(OH) 2 D (also known as calcitriol) occurs only in hyperparathyroidism, granulomatous diseases, and, rarely, malignant disease.
Primary hyperparathyroidism is the most common cause of hypercalcemia; often, the hypercalcemia is the only clinical manifestation (see Plates 3-2 and 3-3 ). In patients with mild-to-moderate hyperparathyroidism, radiographs usually do not reveal bone disease and the serum phosphate level is often normal. Also, serum 1,25(OH) 2 D levels are elevated in less than 50% of patients. Therefore, the most specific and reliable tool in the diagnosis of hyperparathyroidism is the measurement of serum PTH level.
Malignancies cause hypercalcemia by increasing bone resorption either locally through skeletal metastases (e.g., breast cancer and multiple myeloma) or by secreting hormonal factors that stimulate resorption (e.g., lung and renal cell cancer). Hypercalcemia is usually a late manifestation of hypercalcemia due to a nonparathyroid mechanism and usually occurs only after the tumor is apparent. The presence of such a tumor is therefore an important clue to the etiology of hypercalcemia. Hypercalcemia is rarely the first manifestation of occult cancer but is more typically seen in patients with widespread end-stage disease.
Sarcoidosis and, rarely, other granulomatous diseases such as tuberculosis and histoplasmosis also lead to hypercalcemia, because of an increase in the synthesis of 1,25(OH) 2 D. Hyperparathyroidism is the other disease in which the level of 1,25(OH) 2 D is elevated, but the PTH level is also elevated. In sarcoidosis, by contrast, the PTH level is normal or reduced and the level of the angiotensin-converting enzyme (ACE) activity is often elevated. Although this finding is not specific to sarcoidosis, the absence of high ACE activity levels makes the diagnosis very unlikely. Certain malignancies such as lymphomas cause hypercalcemia also by increasing the synthesis of 1,25(OH) 2 D. Fortunately these are rare events.
Hyperthyroidism, or thyrotoxicosis, rarely causes mild hypercalcemia. The diagnosis is based on clinical and laboratory evidence of hyperthyroidism and restoration of a normal serum calcium level with antithyroid therapy.
The milk-alkali syndrome is typically caused by excessive intake of both milk and absorbable alkalis such as sodium bicarbonate and calcium carbonate. Because absorbable alkalis are not often used as antacids, this syndrome is rare; however, ingestion of large amounts of calcium (usually more than 4 g/day) can produce the syndrome. The diagnosis is made by a careful history and by observing the patient's response to withdrawal of calcium.
Immobilization due to fractures or paralysis increases calcium release from bone resulting in hypercalcemia and hypercalciuria is common. Hypercalcemia, although uncommon, may develop when the rate of bone turnover is high, as in childhood or adolescence, and in extensive Paget disease.
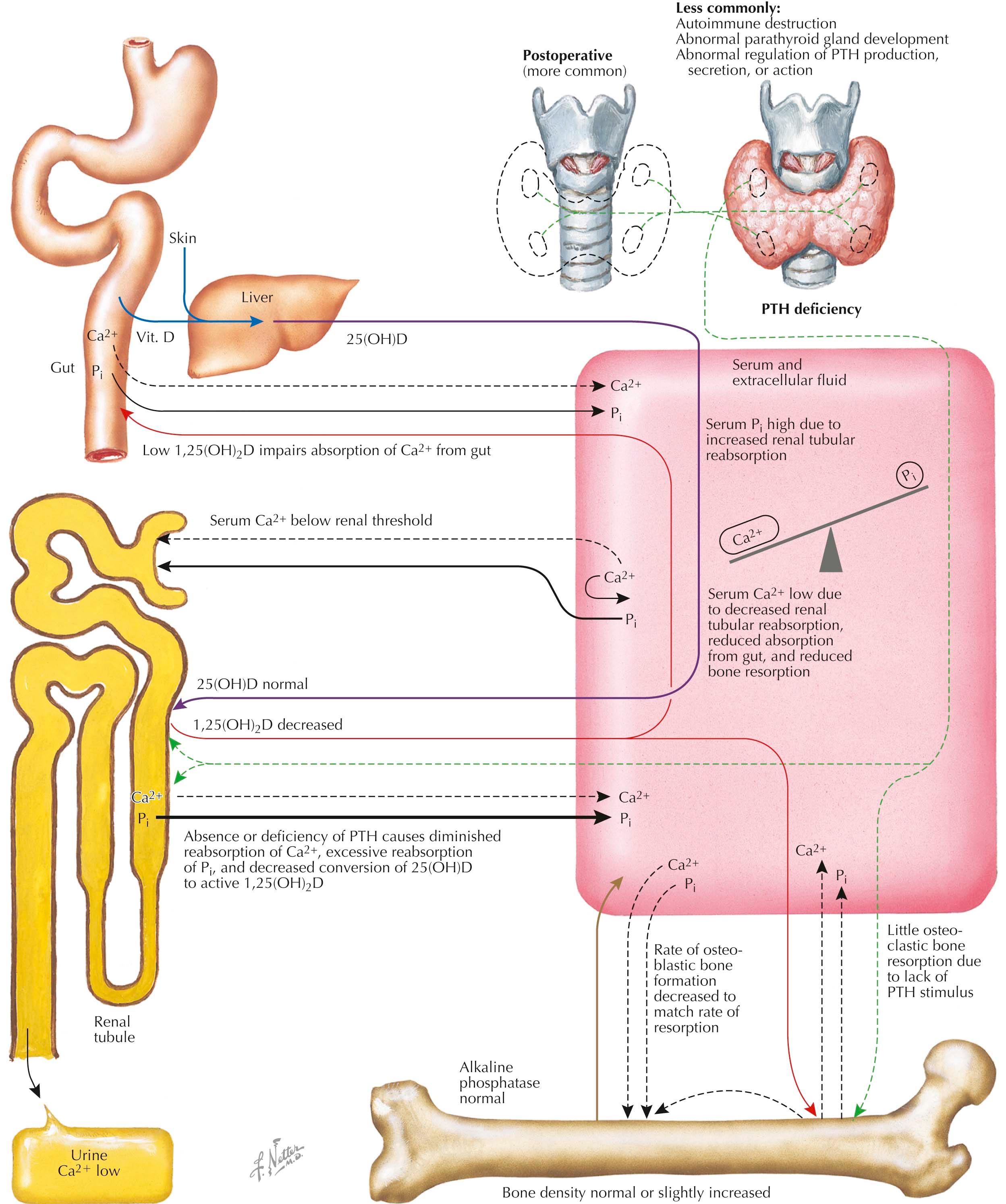
The most common cause of hypoparathyroidism is the inadvertent removal or destruction of the parathyroid glands during thyroid or parathyroid surgery. Idiopathic hypoparathyroidism is less common and usually begins in childhood; its occurrence may be sporadic or familial. Current evidence suggests that, in many cases, the mechanism is autoimmune, and serum levels of parathyroid hormone (PTH) are low because the parathyroid glands have been destroyed by antibodies or are stimulated by antibodies that bind to the calcium-sensing receptor. Autoimmune hypoparathyroidism can occur as an isolated defect or as part of the type 1 polyendocrinopathy syndrome that affects endocrine glands as well as other tissues. In some patients with the type 1 polyendocrinopathy, a defect in cellular immunity leads to chronic mucocutaneous candidiasis (see Plate 3-6 ), with later development of hypoparathyroidism and Addison disease. Additional hormonal deficiencies may also arise due to destruction of other endocrine glands, including diabetes mellitus, primary hypothyroidism, and primary hypogonadism. In addition, many patients will also develop features of an ectodermal dystrophy with vitiligo and nail and dental defects. Most patients with the type 1 polyendocrinopathy syndrome have loss of function mutations in both copies of the AIRE gene, which encodes an autoimmune regulatory protein. Hypoparathyroidism can also be congenital and may occur as an isolated defect or as part of a complex syndrome, with the DiGeorge sequence representing the most common cause. DiGeorge sequence is a developmental field defect that affects the third and fourth branchial pouches and can arise from intrauterine exposure of the fetus to maternal alcoholism, retinoic acid, or poorly controlled diabetes mellitus. More commonly, the DiGeorge sequence is due to large deletions on chromosome 22q11 that result in the loss of multiple contiguous genes and that represent the most common chromosome deletion syndrome in humans. DiGeorge sequence is associated with facial, cardiac, thymic, and parathyroid defects that apparently occur due to the loss of a transcription factor (TBX1) that is required for normal development of these embryonic tissues.
Deletions within two nonoverlapping regions of 10p can lead to a phenotype similar to DiGeorge sequence, namely, the hypoparathyroidism, sensorineural deafness, and renal dysplasia (HDR) syndrome. Unlike patients with the DiGeorge sequence, individuals with HDR do not exhibit cardiac, palatal, or immunologic abnormalities. The HDR disorder is due to haploinsufficiency of the GATA binding protein-3 (GATA3) gene, which is located within a 200-kb critical HDR deletion region on 10p14-10pter and encodes a C-terminal zinc-finger protein essential for DNA binding.
The hypoparathyroidism, retardation, dysmorphism (HRD) syndrome, also known as the Sanjad-Sakati (SS) syndrome, is a rare form of autosomal recessive hypoparathyroidism associated with other developmental anomalies. In addition to parathyroid dysgenesis, affected patients have severe growth and mental retardation, microcephaly, microphthalmia, small hands and feet, and abnormal teeth. This disorder is seen almost exclusively in individuals of Arab descent. The Kenny-Caffey syndrome is an allelic disorder that is characterized by hypoparathyroidism, dwarfism, medullary stenosis of the long bones, and eye abnormalities. Both disorders are due to mutations in the tubulin-specific chaperone E (TBCE) gene on chromosome 1q42-43.
Hypoparathyroidism may also occur as an isolated genetic condition. The leading cause of autosomal dominant hypoparathyroidism is an activating mutation of the CASR gene encoding the calcium-sensing receptor. Parathyroid glands are present but secrete little PTH because the activated calcium-sensing receptor behaves as if the extracellular calcium concentration is elevated. Activation of the same receptor in the distal renal tubule decreases calcium reabsorption, so urinary excretion of calcium is inappropriately high. By contrast, autosomal dominant and recessive mutations in the GCM2 gene at 6p23-24 lead to parathyroid gland aplasia or dysplasia and cause severe isolated hypoparathyroidism in neonates.
Transient hypocalcemia and hypoparathyroidism are common in the neonatal period, presumably because of the underactivity and immaturity of the parathyroid glands and/or renal tubules. Despite hypocalcemia, serum PTH levels are low or inappropriately normal. Maternal hypercalcemia (as seen in hyperparathyroidism) may further suppress the fetal parathyroid gland and produce tetany in the neonate. In patients with alcoholism or malabsorption syndromes, hypomagnesemia leads to functional impairment of the parathyroid glands and hypocalcemia. In these patients, magnesium replacement increases serum levels of both parathyroid hormone (PTH) and calcium.
The biochemical hallmarks of hypoparathyroidism are a low serum calcium level and a high serum phosphate level, which result from a lack of PTH. Hypocalcemia occurs because less calcium is absorbed from the gut and resorbed from the skeleton and more calcium is cleared by the kidney. Absorption of calcium from the gut is reduced because synthesis of 1,25-dihydroxyvi-tamin D, or 1,25(OH) 2 D, which enhances absorption, is decreased in the absence of PTH. Because both PTH and 1,25(OH) 2 D are critical activators of osteoclastic bone resorption, low levels of both hormones lead to decreased mobilization of calcium from skeletal stores. Because the renal tubular reabsorption of calcium is responsive to PTH, the threshold for calcium excretion is reduced in subjects with hypoparathyroidism, and when the serum calcium level is therapeutically raised to normal, urinary excretion of calcium is inappropriately elevated. However, if the condition is not treated, the serum calcium concentration is usually below the renal threshold and urinary excretion of calcium is therefore low. Hyperphosphatemia in hypoparathyroidism occurs because phosphate reabsorption by the renal tubule increases when PTH levels are low.
Although acute reduction of PTH diminishes bone resorption, when PTH levels are chronically low, the rate of bone formation falls to match the rate of bone resorption. In chronic hypoparathyroidism, the net results are a reduced rate of bone turnover and a normal or slightly increased bone mass.
Most patients with idiopathic hypoparathyroidism exhibit severe hypocalcemia (serum calcium level < 7 mg/dL), but symptoms are mild when hypoparathyroidism has been chronic. On the other hand, patients with postsurgical hypoparathyroidism have variable hypocalcemia but typically have more severe clinical symptoms. Patients with the mildest form may have latent hypoparathyroidism; they can maintain normal serum calcium and phosphate levels, although physiologic or pathologic stresses, such as pregnancy or diarrhea, may cause the onset of hypocalcemia. Patients with postsurgical hypoparathyroidism often manifest moderate-to-severe hypocalcemia that is challenging to manage.
Hypoparathyroidism is treated by a combination of oral calcium supplements and vitamin D analogs; in mild cases, calcium supplementation alone is sufficient. Calcium supplements serve several important roles. First, they ensure a constant daily intake of calcium and thereby reduce day to day fluctuations in dietary calcium due to differences in food consumption. Second, they provide a ready source of gastrointestinal calcium and thereby reduce mobilization of skeletal calcium. And third, oral calcium supplements reduce absorption of dietary (and secreted) phosphorus from the intestine and thereby help maintain a normal serum phosphorus concentration. In most patients, calcium absorption from the intestine is too low, necessitating some form of supplemental vitamin D (in addition to calcium salts) to enhance absorption. Because the activation of vitamin D is impaired in these patients, large amounts of the parent compounds ergocalciferol (vitamin D 2 ) or cholecalciferol (vitamin D 3 ) are required (50,000 to 100,000 IU/day). In contrast, 1,25(OH) 2 D (calcitriol) and dihydrotachysterol can be used at much lower doses because they do not require PTH-dependent activation by the 1α-hydroxylase enzyme in the kidney. Because the renal threshold for calcium is lower in patients with hypoparathyroidism, the total serum calcium concentration should be maintained in the low-to-normal range (8 to 9 mg/dL) to avoid hypercalciuria and nephrolithiasis.
Although the manifestations of acute hypocalcemia as seen in hypoparathyroidism can be quite dramatic, the clinical signs and symptoms of chronic hypocalcemia are subtle and may be easily overlooked. For this reason, chronic hypocalcemia is often diagnosed incidentally during investigation of nonspecific symptoms, often neurocognitive, which include lassitude, irritability, depression, or even psychosis. These symptoms are due primarily to the hypocalcemia. There may also be evidence of increased neuromuscular excitability, with signs and symptoms ranging from paresthesias, described as “pins and needles” sensations around the mouth and in the hands and feet, to tetany with muscle cramps and spasms, laryngeal stridor, apnea in neonates, and seizures. The Chvostek or Trousseau sign may be elicited even in patients with asymptomatic hypocalcemia.
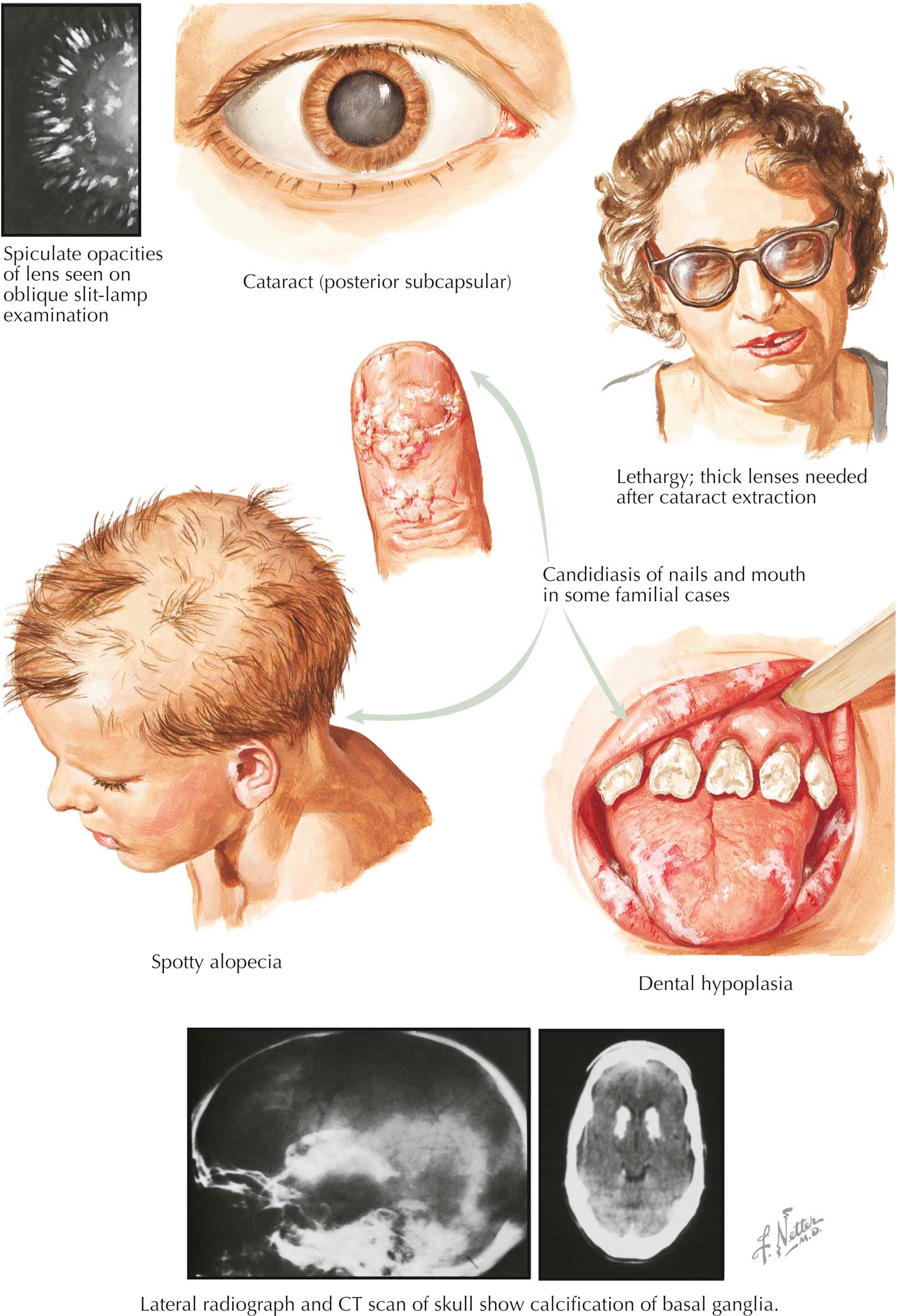
Despite the hypocalcemia, soft tissue calcifications may develop in patients with hypoparathyroidism when elevated serum phosphorus levels lead to an elevated (i.e., >70 mg 2 /dL 2 ) calcium × phosphorus solubility product. A patient with poorly controlled, long-standing hypoparathyroidism may develop calcifications in the lens (cataracts) that opacify the lens and can impair vision. Calcifications may also develop in the basal ganglia and, if they are extensive, cause a movement disorder with features of Parkinson disease. These calcifications can be seen on standard radiographs of the skull or on computed tomography, which is a more sensitive technique.
The condition of the teeth provides a clue to the patient's age at onset of the disease. Dental hypoplasia with poor dental root formation indicates that the disease occurred before age 6. If onset was during childhood, there is crumbling of the teeth because of poor enamel structure. An increased density of the lamina dura can also be seen on dental radiographs.
In patients with hypoparathyroidism, the skeleton is usually not demineralized; in most cases, bone density is normal or slightly increased.
Hypoparathyroidism can occur as part of a familial tendency to the development of autoimmune destruction of multiple endocrine glands. The type 1 polyendocrinopathy syndrome that produces hypoparathyroidism is due to mutations in the AIRE gene that encodes an immune regulatory protein (see earlier), and affected patients often manifest a defect in cell-mediated immunity and an absence of delayed cutaneous hypersensitivity reactions to Candida. Affected patients may have chronic Candida infections of the skin, especially the hands, toes, and nails, as well as infections of the oral mucosa and vagina, but systemic candidiasis is not a feature of this syndrome. Occasionally, these lesions respond to long-term antifungal therapy. Affected patients present initially with chronic fungal infections with subsequent development of hypoparathyroidism and Addison disease. Individuals have an increased incidence of autoimmune primary hypothyroidism, diabetes mellitus, and primary hypogonadism. Alopecia, vitiligo, hepatitis, and pernicious anemia also occur with increased frequency.
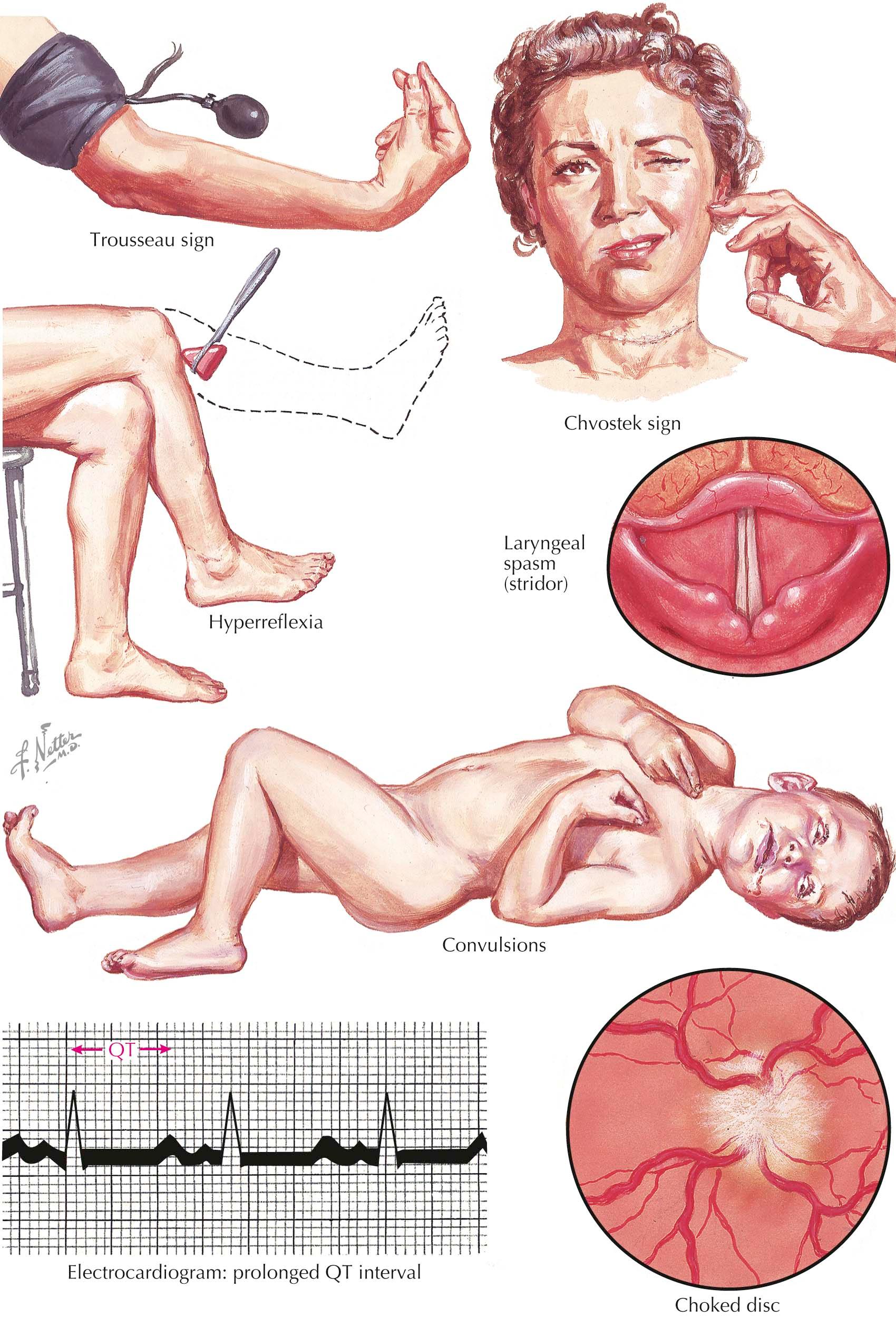
The symptoms of hypocalcemia emphasize the vital role of calcium in neuromuscular function. Hypocalcemia increases neuromuscular excitability, which can lead to tetany. The most severe form of tetany is characterized by tonic contractions of the muscles of the forearm and hand and, less commonly, by laryngospasm and seizures ranging from classic types (generalized and focal) to brief absence seizures. Often it is not clear if hypocalcemia is the direct cause of seizures or if it lowers the seizure threshold in a patient with a predisposition for epilepsy.
More typically, patients with hypocalcemia may be asymptomatic or experience milder symptoms such as muscle cramps and paresthesias. The paresthesias, described as “pins and needles” sensations in the hands, feet, and around the mouth, are episodic and often occur at times of stress, vomiting, or hyperventilation. This can be explained by the fact that metabolic or respiratory alkalosis increases the binding of serum calcium to albumin and decreases the concentration of free ionized calcium that interacts with cells.
Symptoms of hypocalcemia are also more likely to occur when the serum calcium level has fallen abruptly; chronic hypocalcemia, in contrast, can be asymptomatic with very low levels of serum calcium. Asymptomatic hypocalcemia must be differentiated from the low total serum calcium concentration (with a normal ionized calcium level) that occurs with hypoalbuminemia. The corrected total serum calcium concentration can be calculated by measuring the serum albumin level and adding 0.8 mg/dL to the total serum calcium level for each 1 g/dL reduction in the serum albumin level from 4 g/dL (corrected calcium = 0.8 × [4.0 − patient's albumin] + serum calcium).
Tetany can be elicited in patients with no overt signs of hypocalcemia by inducing the Chvostek and Trousseau signs. The Chvostek sign is produced by tapping the facial nerves at the angle of the jaw, which causes contracture of the ipsilateral facial muscles. The Trousseau sign is elicited by applying a blood pressure cuff to the upper arm and inflating it to just above the systolic blood pressure for 3 minutes. The resulting carpopedal spasm, with contractions of the fingers and inability to open the hand, is a result of increased neuromuscular irritability caused by hypocalcemia and aggravated by ischemia. This may be painful for the patient if sustained for too long but is believed to be a more specific marker of hypocalcemia than the Chvostek sign.
Other nonspecific signs and symptoms of hypocalcemia are lethargy, psychomotor depression, and impaired cognitive function with poor school performance in children. Hypocalcemia also decreases the contractility of the heart muscle, which can provoke or aggravate congestive heart failure in patients with heart disease. In these patients, heart failure can improve with administration of calcium. Hypocalcemia also leads to prolongation of the rate-corrected QT interval on an electrocardiogram. An unusual ocular manifestation of chronic hypocalcemia is papilledema, caused by increased pressure of the cerebrospinal fluid, which improves with reversal of hypocalcemia.

In pseudohypoparathyroidism (PHP) type 1, tissues such as the kidney fail to respond to the action of parathyroid hormone (PTH) owing to an inability to generate the second messenger cyclic adenosine monophosphate (cyclic AMP). Signs, symptoms, and laboratory findings are those of hypoparathyroidism, with the exception of an elevated PTH level in response to hypocalcemia (see Plates 3-5 to 3-7 ). Both hypocalcemia and hyperphosphatemia are present, but the parathyroid glands are enlarged. PHP type 1 is divided into two major forms, type 1a and type 1b. Patients with PHP type 1a also have a characteristic physical appearance known as Albright hereditary osteodystrophy (AHO), which includes round face, subcutaneous ossifications, short stature, and brachydactyly, particularly shortening of the fourth and fifth metacarpals and metatarsals. Associated findings are mild primary hypothyroidism, growth hormone deficiency, and, occasionally, primary hypogonadism. Patients with PHP type 1b generally have only PTH resistance, although some patients have mild hypothyroidism and brachydactyly.
PTH activates its target cells by increasing the activity of adenylyl cyclase, thereby increasing cellular levels of cyclic AMP. Cyclic AMP activates a cascade of proteins that produces the physiologic effect. Patients with PHP type 1 have heterozygous mutations of the maternally derived allele that reduce expression or function of the α-subunit of the heterotrimeric G protein Gs, which couples receptors for hormones such as PTH, thyroid-stimulating hormone (TSH), and others to activation of adenylyl cyclase. Patients with PHP type 1a have mutations in the GNAS gene that directly affect production of Gα s , whereas patients with PHP type 1b have mutations in or near GNAS that disrupt genomic imprinting and thereby reduce synthesis of Gα s . Although PTH binds to the cell, it fails to elicit an effect because the lack of functional Gα s “uncouples” the receptor from adenylyl cyclase. Hence, there is no production of its second messenger, cyclic AMP, and the biochemical abnormalities of hypoparathyroidism develop. Hypocalcemia occurs because of decreased calcium absorption from the intestine due to low PTH-mediated synthesis of 1,25(OH) 2 D. The serum phosphate level is high because the proximal renal tubule is resistant to PTH, and as a result the tubular reabsorption of phosphate is very high. In contrast to true hypoparathyroidism, the serum PTH level is elevated in response to hypocalcemia.
Because Gα s couples receptors for many different hormones to adenylyl cyclase, patients with PHP type 1a have impaired responsiveness not only to PTH but also to other hormones such as TSH, growth hormone–releasing hormone (GHRH), and gonadotropins and develop obesity. By contrast, the defect in PHP type 1b tends to be less severe, and PTH resistance is the principal manifestation of the disorder. Bone is variably responsive to PTH; in most patients bone density is increased, although in some cases, osteitis fibrosa cystica occurs as a result of high PTH levels. As in hypoparathyroidism, the hypocalcemia ranges from latent to severe. Treatment of pseudohypoparathyroidism is the same as that for hypoparathyroidism, but patients rarely develop hypercalciuria because the distal renal tubule remains responsive to PTH.
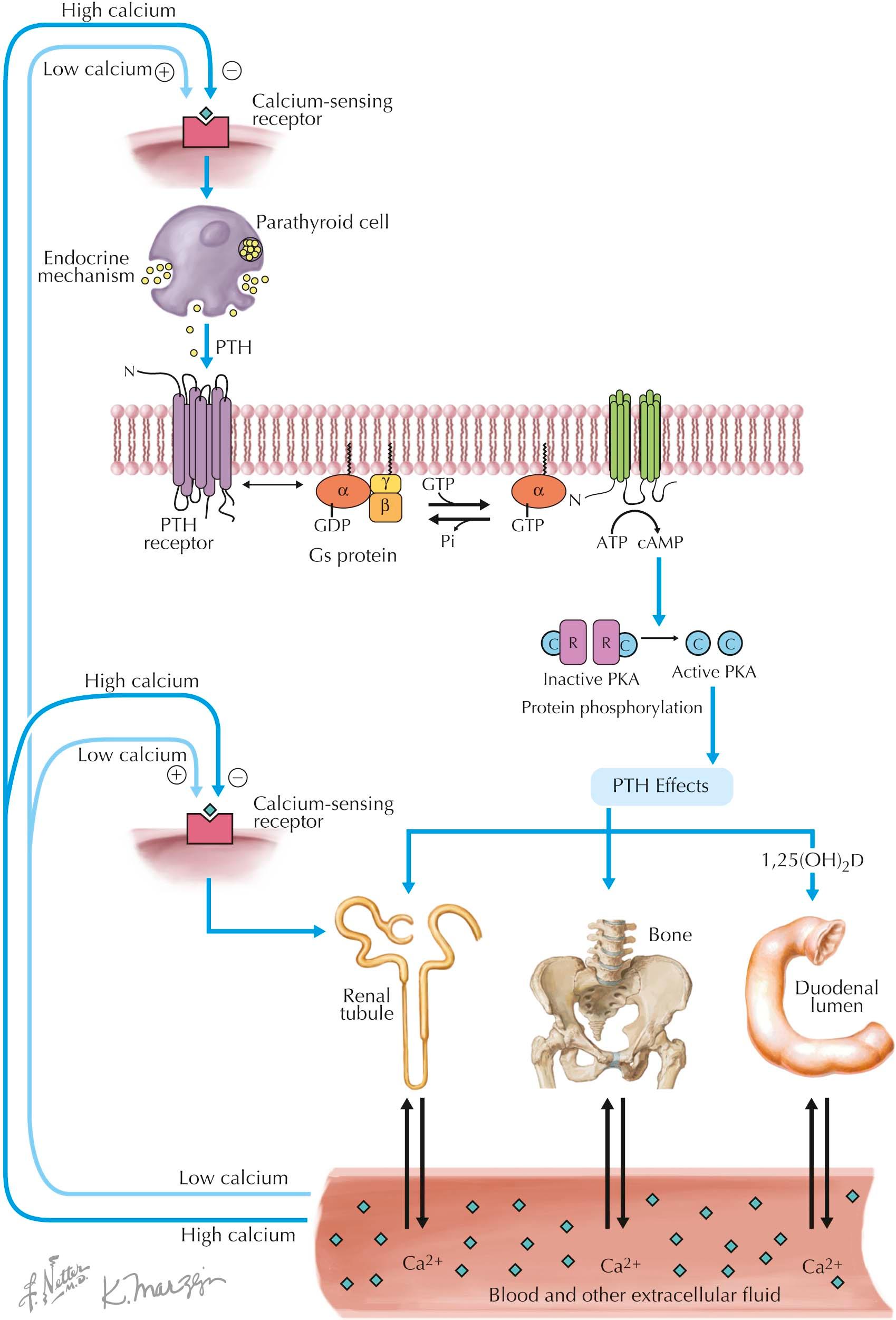
Parathyroid hormone (PTH) has two target organs: kidney and bone. In the kidney, PTH enhances the reabsorption of calcium in the distal tubule and decreases the reabsorption of phosphate in the proximal tubule. It also increases the synthesis of 1,25-dihydroxyvitamin D, or 1,25(OH) 2 D (calcitriol), the active form of vitamin D, from its precursor 25-hydroxyvitamin D, or 25(OH)D (calcidiol). Increased secretion of 1,25(OH) 2 D leads to increased intestinal calcium and phosphate absorption. In bone, PTH stimulates the release of minerals from hydroxyapatite. Initially, there is a rapid activation of existing osteoclasts, the large multinucleated bone cells that resorb bone. These cells resorb mineralized bone and release calcium, phosphate, and fragments of bone proteins into the circulation. After this initial phase, new osteoclasts are also recruited. There is also a compensatory increase in bone formation by osteoblasts (the process of bone remodeling is highly coordinated); however, the net effect is bone resorption.
PTH produces its effects on target cells by stimulating the synthesis of second messengers, most notably cyclic adenosine monophosphate (cyclic AMP) by the enzyme adenylate cyclase (see Plate 3-9 ). This intracellular second messenger activates protein kinase A, which catalyzes the phosphorylation of several cellular proteins and thereby modifies their activity.
Although all of the targets of phosphorylation have not been identified, they likely include proteins involved in the transport of calcium and phosphate, as well as proteins that regulate gene transcription. To activate adenylyl cyclase, PTH binds to specific heptahelical receptor molecules on the surface of the cell. The first segment of PTH, containing 34 of the 84 amino acids in the hormone, is the only component required for binding and activating receptor molecules; the function, if any, of the remainder of the peptide is unknown (see Plate 3-1 ).
Adenylate cyclase is a separate molecule on the inner surface of the cell membrane (see Plate 3-9 ). However, for adenylate cyclase to convert adenosine triphosphate (ATP) to cyclic AMP, it must interact with Gα s , which is activated by the PTH-receptor complex. Gα s is one component of the heterotrimeric G protein, Gs, and can bind and hydrolyze guanosine triphosphate (GTP). In the “off” state guanosine diphosphate (GDP) is bound to Gα s , which enhances its affinity for the βγ subunit. Ligand-bound receptors interact with the heterotrimeric Gs and promote release of GDP, thereby allowing GTP to bind to Gα s . Gα s -GTP dissociates from the βγ subunit and in the “on” state is then able to stimulate adenylyl cyclase. After a very brief period of time the GTP is hydrolyzed to GDP, and the Gα s -GDP molecule then reassociates with βγ to end a cycle of hormone activation. As noted earlier, expression or function of Gα s is reduced in PHP type 1, which impairs receptor activation of adenylyl cyclase. Cyclic AMP is rapidly degraded by intracellular phosphodiesterase enzymes, although some of it also leaks out of the cell. In the kidney, cyclic AMP produced under the influence of PTH leaks out of proximal renal tubule cells and is excreted in the urine.
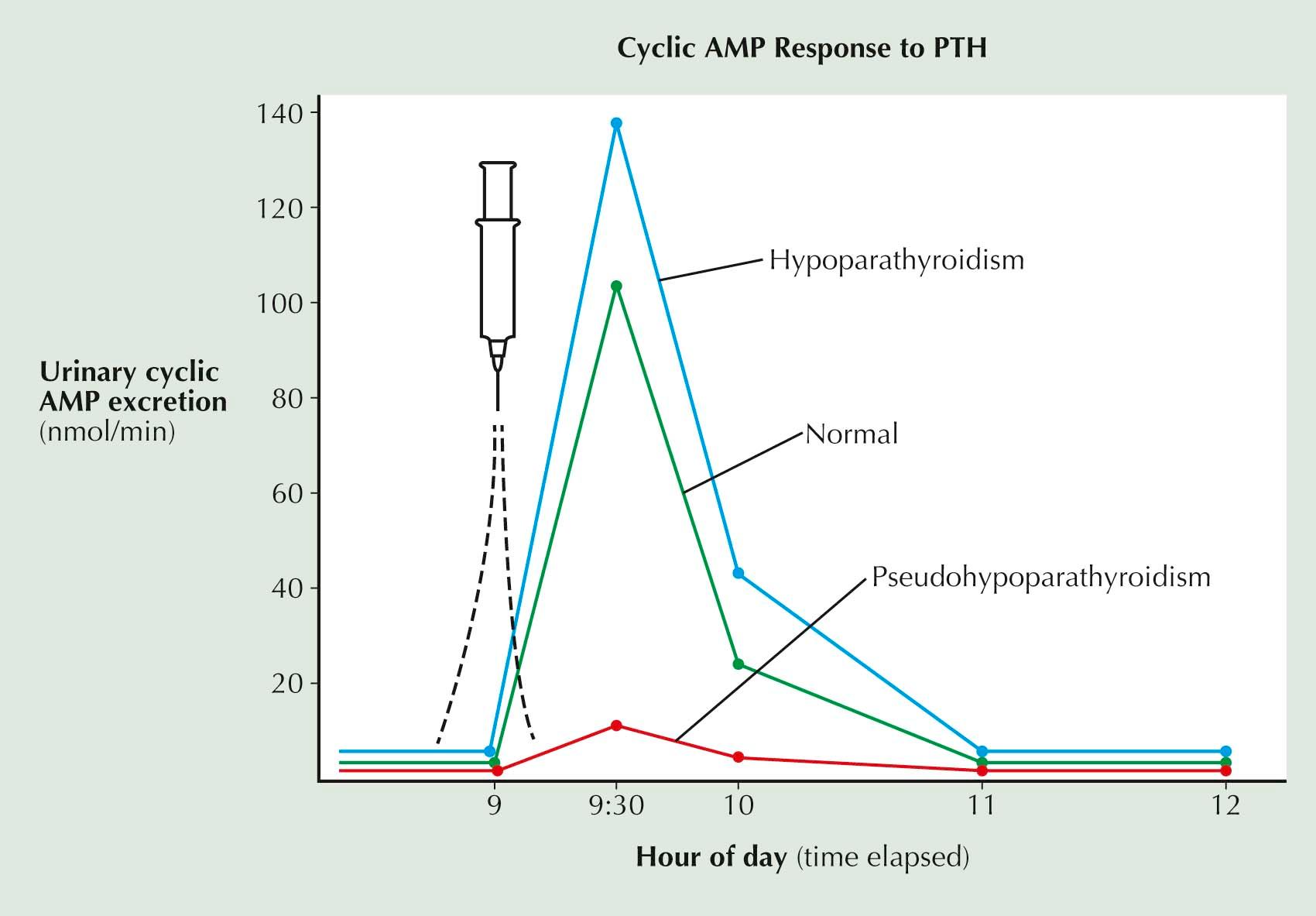
Normally, about half of the cyclic AMP in urine is derived from the renal action of PTH; the other half comes from circulating cyclic AMP, which is filtered through the glomerulus. Measurement of cyclic AMP excreted in the urine can be used as an index of the level of circulating PTH (see Plate 3-10 ); excretion of cyclic AMP is increased in hyperparathyroidism and decreased in hypoparathyroidism.
In PHP type 1, cyclic AMP is not synthesized in response to PTH, because of a deficiency of the coupling protein Gα s . As a result, little cyclic AMP is produced in target tissues in response to PTH, and functional hypoparathyroidism develops (see Plate 3-5 ). This defect can be demonstrated in patients by measuring the level of cyclic AMP in urine after an injection of PTH. In normal persons and in patients with hypoparathyroidism, the rise in the excretion of cyclic AMP in urine is rapid and marked; in patients with PHP type 1, it is blunted or absent (see Plate 3-10 ).
The gene encoding Gα s , GNAS, is a very complex transcriptional unit that derives considerable plasticity through use of alternative first exons, alternative splicing of downstream exons, antisense transcripts, and reciprocal imprinting. Four alternative exons, NESP55, XLαs, exon A/B, and exon 1, splice onto exons 2 to 13 of GNAS. Transcripts originating from alternative exons A/B and XLαs are expressed exclusively from the paternal allele. Exon A/B transcripts are probably nontranslated. By contrast, the XLαs protein shares C-terminal sequences with Gα s and functions in G-protein–coupled signal transduction. Transcripts starting with exon 1 encode Gα s and are expressed from both the maternal and paternal alleles in most tissues. Loss of one functional GNAS allele (i.e., haploinsufficiency) does not cause hormone resistance in these tissues, because 50% of normal Gα s activity is sufficient to ensure normal transmembrane signal transduction. By contrast, suppression of the paternal allele occurs in cells such as renal proximal tubule cells, thyroid follicular cells, pituitary somatotrophs, and the paraventricular nucleus of the hypothalamus. Hence, mutations of the maternal GNAS allele results in expression of little if any Gα s protein in these imprinted tissues and is associated with hormone resistance. Thus, variable hormone resistance, from tissue to tissue between patients, reflects an unusual set of requirements that specifies that the tissue must exhibit imprinting of Gα s transcripts and the mutation must be on the maternal GNAS allele. By contrast, when the same GNAS mutation is carried on the paternal allele hormone, responsiveness is normal. Subjects with paternally inherited GNAS mutations have phenotypical features of AHO without hormonal resistance, a condition termed pseudopseudohypoparathyroidism (pseudoPHP). Subjects with pseudoPHP have a normal urinary cyclic AMP response to PTH, which distinguishes them from occasional patients with PHP type 1a who maintain normal serum calcium levels without treatment. It is not unusual to find extended families in which some members will have only AHO (pseudoPHP) whereas others will have hormone resistance as well (PHP type 1a), based on the parental origin of the identical GNAS mutation.
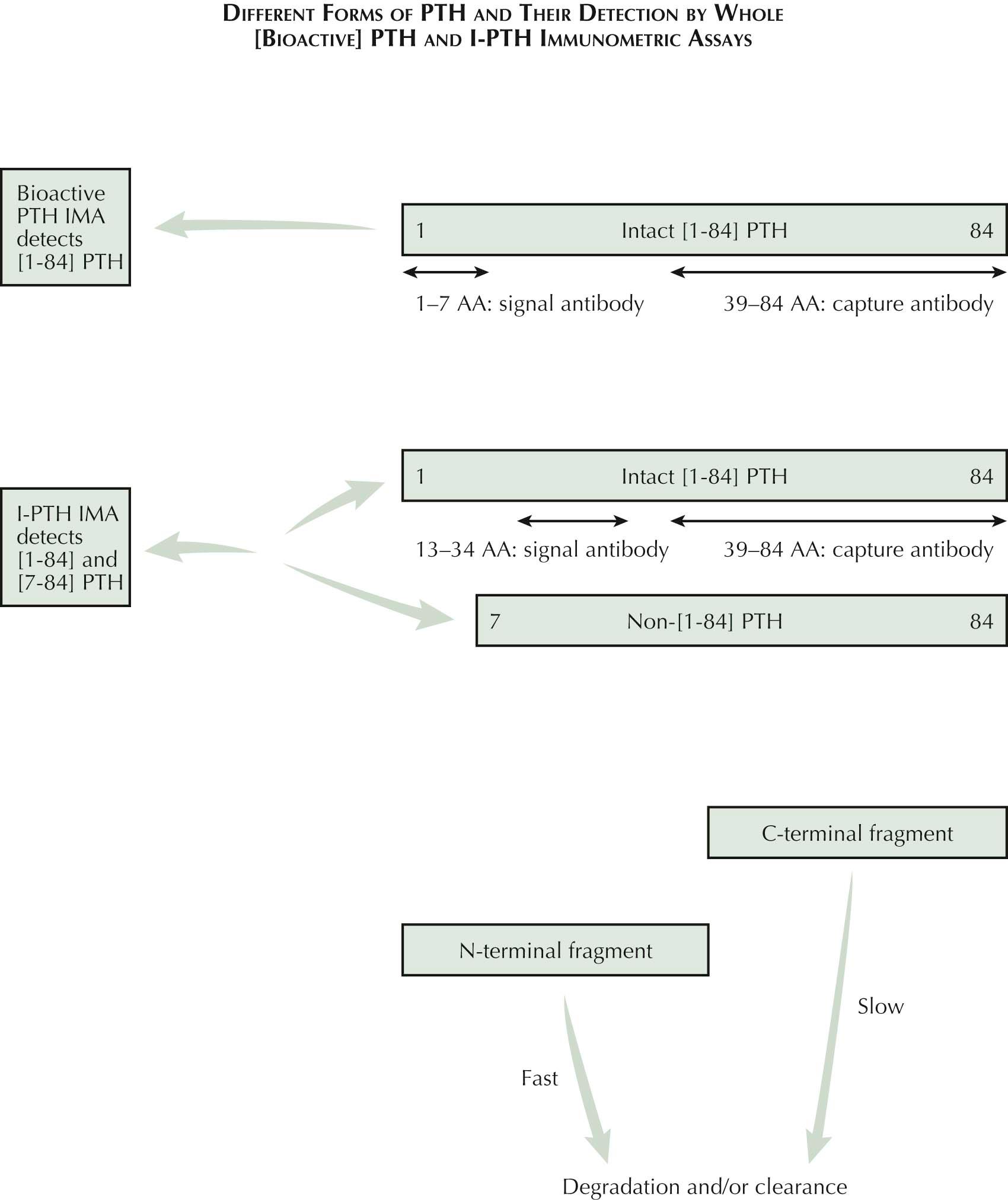
The major circulating form of parathyroid hormone (PTH) in serum is an 84 amino acid [1-84] polypeptide. This form acts on type 1 PTH receptor via interaction with the first 34 amino acids. After secretion in the circulation, PTH is cleaved into C-terminal and amino (N)-terminal fragments. The N-terminal fragments contain the first 33/34 amino acids and are biologically active, just like the whole molecule, have a short half-life, and disappear rapidly from the circulation. The C-terminal fragments contain the remaining 34 to 84 amino acids (see Plate 3-11 ). In contrast, the half-life of the C-terminal is several times longer than that of either the N-terminal or the intact hormone; this fragment is therefore the most abundant form of PTH in serum. Because the C-terminal fragment is cleared by the kidney, renal insufficiency further causes this fragment to accumulate in the circulation.
Measurement of biologically active intact PTH is essential for accurate clinical assessment. Today most laboratories use commercially available U.S. Food and Drug Administration (FDA)–approved two sites or sandwich immunometric assays (IMAs) on different automated immunoassay platforms. These assays are designed to capture the intact molecule by using well-characterized N-terminal and C-terminal specific monoclonal or polyclonal antibodies, one used as a capture antibody bound to solid support and the other tagged with nonisotopic ligand and used as a signal antibody. These assays improved detection of intact PTH by decreasing the detection of PTH fragments and are called intact PTH assays (I-PTH). Measurement of I-PTH provided better clinical correlation than older N-terminal or C-terminal radioimmunoassays. Most I-PTH IMAs have low detection limit and high reproducibility, facilitating the diagnosis of hypoparathyroidism and differentiating primary hyperparathyroidism from hypercalcemia of malignancy.
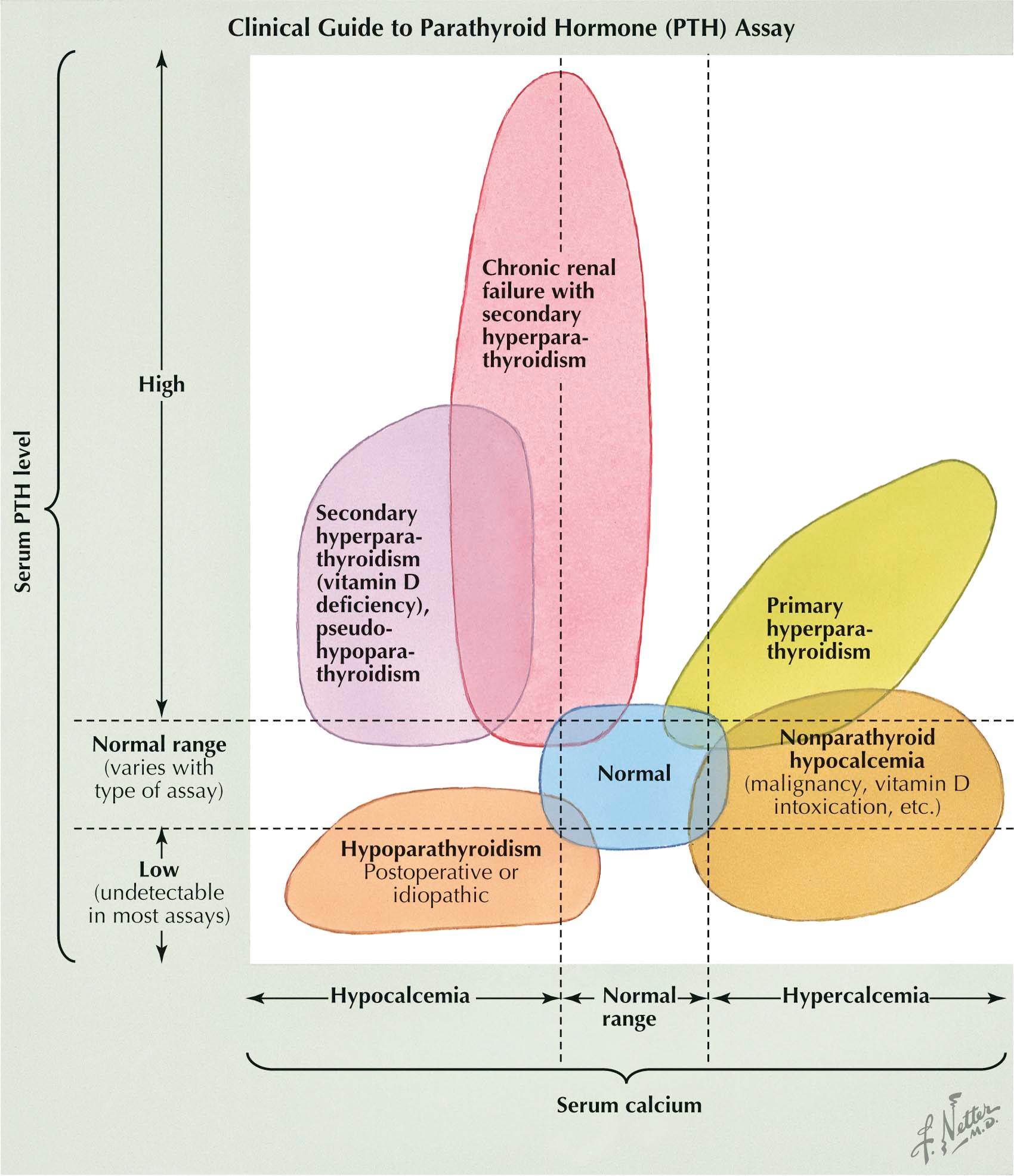
If primary hyperparathyroidism is the cause of hypercalcemia, the serum PTH level is usually increased; in general, the elevation is proportional to the degree of hypercalcemia. Primary hyperparathyroidism is unlikely to be the cause if the serum calcium level is substantially increased and the PTH level is in the low to normal range. The PTH assay can also determine whether hypocalcemia is due to hypoparathyroidism or due to a nonparathyroid mechanism such as vitamin D deficiency. In patients with hypoparathyroidism, the serum PTH level is inappropriately low or even normal, despite the presence of hypocalcemia. In hypocalcemia due to nonparathyroid mechanisms, PTH secretion is stimulated (secondary hyperparathyroidism) and thus serum levels are high. In pseudohypoparathyroidism, PTH levels are high but hypocalcemia develops because of resistance to the effects of PTH (see Plate 3-12 ).
Although these I-PTH assays provide accurate measurement of PTH secretion and show excellent diagnostic sensitivity for primary hyperparathyroidism and of hypercalcemia of malignancy, their performance for management of secondary hyperparathyroidism in patients with renal insufficiency has been questioned owing to the presence of higher levels of a non-[1-84]PTH molecular form. This molecular form of PTH was later identified as an N-terminally truncated segment, the [7-84]PTH peptide. This molecular form accumulates in renal failure and is detected by I-PTH assays (see Plate 3-11 ). This accounts for the larger portion of I-PTH in patients with renal failure than in normal subjects and contributes to the major proportion of nonsuppressible fraction of I-PTH. This [7-84]PTH fragment is capable of binding to PTH receptor but has no biologic activity. Therefore it competes with I-PTH for receptor binding and serves as a PTH antagonist. In renal failure, a twofold increase in I-PTH is accompanied with about a sevenfold increase in [7-84]PTH fragment, leading to overestimation of I-PTH levels and of PTH-associated osseous abnormalities in uremia. After realizing this shortcoming of the I-PTH IMA assays, the efforts were made to develop the next generation of assays that employ the detection antibody that has specificity for the first four amino acids in the PTH molecule (see Plate 3-11 ). These assays are called “whole/total PTH assay” as well as “bioactive PTH assays.” The specificity of these assays was confirmed by their inability to detect synthetic PTH fragments lacking one or more N-terminal amino acids. Although there is excellent correlation between these two assays in normal individuals and in patients with primary hyperparathyroidism, PTH concentrations are 40% to 50% lower in patients with end-stage renal disease by “bioactive/whole PTH” assays than those obtained using the I-PTH IMA. In patients with end-stage renal disease, bioactive/whole-PTH may provide more accurate assessment of need for vitamin D and/or calcium treatment.
Primary hyperparathyroidism is often characterized as a multiglandular disease, requiring the surgical exploration and identification of all glands on both sides. One of the new applications of the I-PTH assay is the rapid measurement of intraoperative PTH. This has been suggested as a cost-effective way of predicting the necessity of other gland exploration and further neck dissection after the removal of an adenoma. PTH levels before and during surgery are measured in a timely manner by simply reducing the incubation time of I-PTH assays and hence called rapid PTH. PTH has a short half-life, and normal PTH secretion does not recover immediately after surgery. This provides a window of time shortly after surgical removal of parathyroid adenoma (5 to 10 minutes) to monitor the decreasing levels of PTH in blood. Rapid PTH assays can accurately predict the postoperative outcome if a 50% or more drop in PTH is present and are useful in allowing selective unilateral exploration during parathyroid surgery. Therefore, the biochemical intraoperative monitoring with rapid PTH in a timely manner provides a valuable guidance to the endocrine surgeons when directing selective unilateral exploration or if there is a need to explore other parathyroid lobes during surgery.
In summary, the advent of totally automated immunometric I-PTH assays has led to improved accurate clinical discrimination of parathyroid disorders and has provided a tool to measure PTH levels in real time during surgical procedures. Also, development of bioactive I-PTH assay provides more accurate assessment of secondary hyperparathyroidism in patients with end-stage renal disease and their need for vitamin D and/or calcium treatment.

Nutritional rickets, a metabolic bone disease characterized by impaired mineralization of osteoid (matrix), is reported infrequently because the basic pathophysiology is due to severe (<10 ng/mL) vitamin D deficiency, which is far less common now than in the past, since dairy products were fortified with vitamin D in the 1940s. Osteomalacia (adult rickets) has multiple causes in addition to severe vitamin D deficiency. Renal osteodystrophy compromises a group of metabolic bone diseases accompanying chronic kidney disease (CKD) and are defined by quantitative bone histomorphometry and may be associated with increase in fracture risk. Although a large number of etiologic factors may contribute to these disorders, the basic defect is a deficiency at the tissue level of calcium or phosphate, or both, which impairs the normal mineralization and growth of the skeleton in the child (rickets) or leads to impaired mineralization of osteoid in the adult (osteomalacia).
The causes of rachitic and osteomalacic syndromes are numerous and include a variety of genetic errors, nutritional abnormalities, metabolic disorders, and chronic renal diseases. Quite independent of cause, the clinical manifestations of the disorders may be remarkably similar, making it difficult for the physician to solve the often tangled puzzle of causation and introduce the appropriate treatment. However, recent discoveries relating to the disease mechanisms and the introduction of newer hormonal and drug treatments are contributing to better management and may lead to a cure.
Besides being interesting, the history of rickets and osteomalacia is important for the classification of the disorders. Both diseases were known in antiquity, but one of the clearest descriptions appeared in a 17th-century Latin text by Glisson. Investigations by Schmorl in the late 19th century established the role of sunlight in the prevention of the disease, and dietary factors were identified in the first part of the 20th century. Despite this knowledge, nutritional rickets remained a common occurrence and many children in working-class families in the temperate zones exhibited the characteristic symptoms of short stature, rib cage deformities, and bowed extremities.
Vitamin D was discovered in the 1920s, and the use of the sterol as a food supplement made nutritional rickets rare in all but the most economically disadvantaged communities. However, within a short time, additional cases were reported, which appeared to be resistant to even massive doses of vitamin D. Scientists such as Albright, Butler, Fanconi, and others, studying the defects that led to this metabolic disorder, identified the mechanisms of calcium transport in the gut, kidneys, and bone cells, as well as the roles played by exogenous factors (the polar metabolites of vitamin D) and endogenous factors (parathyroid hormone [PTH] and phosphate). The identification and synthesis of 1,25-dihydroxyvitamin D, or 1,25(OH) 2 D, a better understanding of the handling of calcium and phosphate by the kidneys, and the discovery of the osteocyte-derived phosphaturic peptide FGF-23 (fibroblast growth factor 23) have defined mechanisms behind various forms of osteomalacia and renal bone disease.
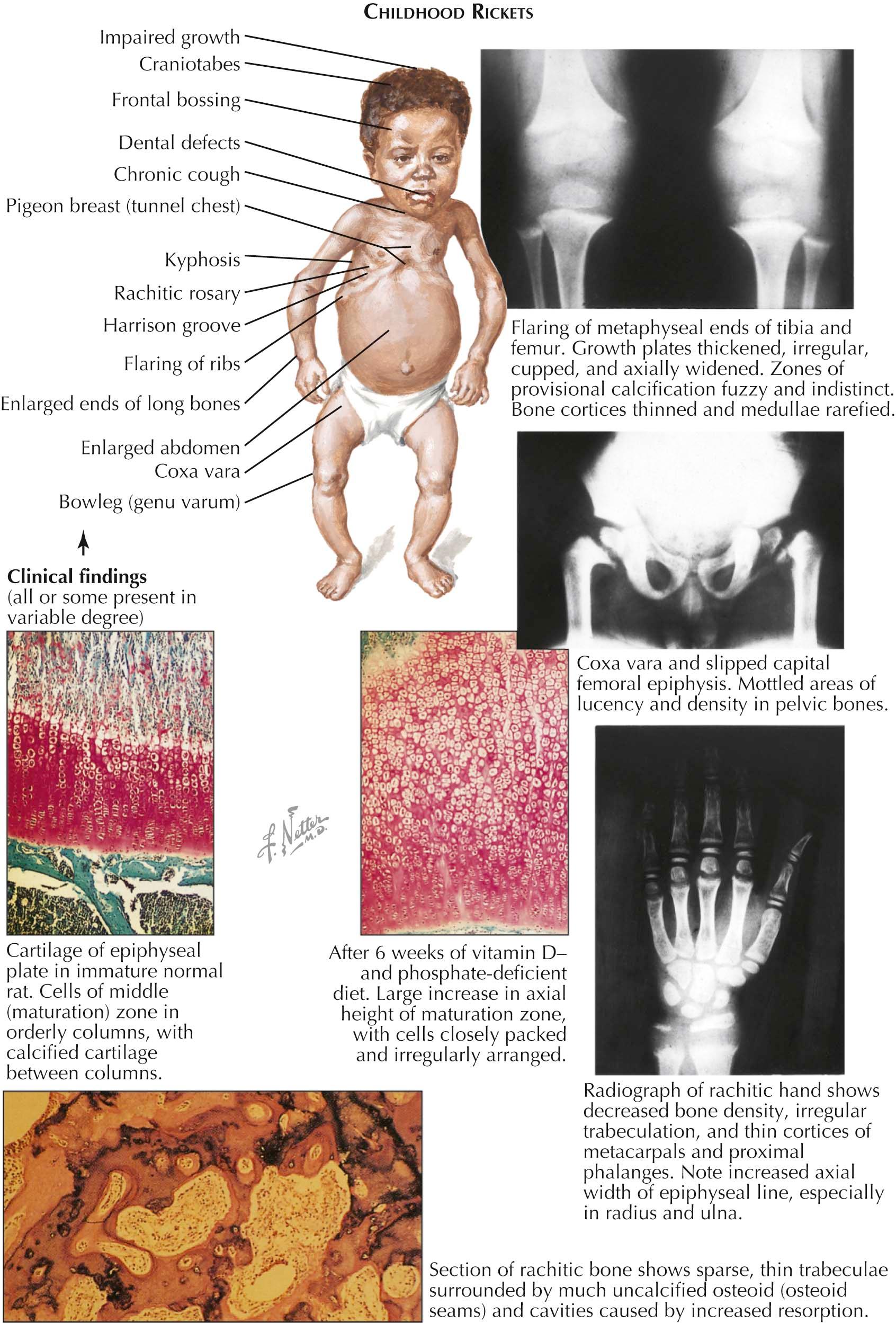
Clinical Manifestations. In severe childhood rickets (see Plate 3-13 ), growth is impaired and height is generally below the third quartile. However, unless there is concurrent severe nutritional disturbance, weight is usually normal. Affected children are apathetic and irritable and frequently remain immobile, sitting in a Buddha-like position. The head displays a number of abnormalities, including softening and deformity of the skull (craniotabes), prominence of the frontal bones (frontal bossing), and caries and enamel defects. Examination of the thorax may reveal flaring and deformity of the ribs, funnel chest (pectus excavatum) or pigeon breast, an indentation at the insertion of the diaphragm into the lower ribs (Harrison's groove), and nodules at the costochondral junctions (rachitic rosary). Frequent manifestations are respiratory infections and a chronic cough.
Children with rickets may also have a gentle thoracic kyphosis (rachitic cat back) and a rachitic potbelly, which, together with the bowed extremities and apathetic facies, emphasize their Buddha-like appearance. Examination of the extremities also uncovers abnormalities such as symmetric enlargement of the ends of the long bones (most prominent at the elbows and wrists), bowleg (genu varum), and, less frequently, knock-knee (genu valgum). Fractures occur frequently.
Histologic Features. In patients with rickets, the histologic appearance of the epiphyseal plate is pathognomonic. Comparison of normal and rachitic epiphyseal plates in rats shows a greatly increased axial height of the epiphyseal plate (sometimes as much as 20 times), principally because of the increased number of cells in the maturation zone; the cells have lost their columnar organization and occur in profligate profusion. Both the zone of provisional calcification of the cartilage and the primary spongiosa of the metaphysis have irregular contours and lack calcific mineral deposition.
Although changes in bone structure are no less pronounced in osteomalacia, they are not specific to that disorder because similar changes may occur in several other metabolic bone disorders (most notably hyperparathyroidism and fibrous dysplasia). The cortices are thin, and the trabeculae are small and irregularly shaped, with evidence of osteoclastic resorption of bone (a mild-to-moderate secondary hyperparathyroidism is characteristic of most rachitic syndromes). The most characteristic histologic feature, however, is the presence of a wide zone of unmineralized bone, or osteoid seam, which surrounds the mineralized trabeculae. In the section shown in Plate 3-13 , the mineralized bone appears dark and the osteoid seams are pink.
Radiographic Findings. Radiographic findings reflect the histologic changes: thinned cortices and rarefied medullary bone, with indistinct and fuzzy trabecular markings. However, the radiographic hallmarks are the enormously increased axial height of the epiphyseal plate and the poor definition or absence of the zone of provisional calcification, which is normally seen as a dense, white line separating the growth plate, or physis, from the metaphysis. Often noted are cupping and flaring of the ends of the long bones, usually because of a softening of the epiphyseal-metaphyseal region. Slipped capital femoral epiphysis at the widened and severely weakened plate is an occasional finding, particularly in patients with renal osteodystrophy (see Plate 3-22 ).
Unlike osteomalacia, rickets is a disease of growth. If growth slows, either for natural reasons or because the patient becomes ill with other manifestations of the disease, a phenomenon known as the paradox of rickets may occur; that is, the characteristic epiphyseal changes seen on radiography appear to improve. The radiograph of the hand in Plate 3-13 illustrates this paradox. The hand shows evidence of advanced rachitic changes in the rapidly growing distal radius and ulna, less severe manifestations in the metacarpals, even milder signs in the slowly growing proximal phalanges, and virtually no signs at all in the least active physeal regions of the middle phalanges.
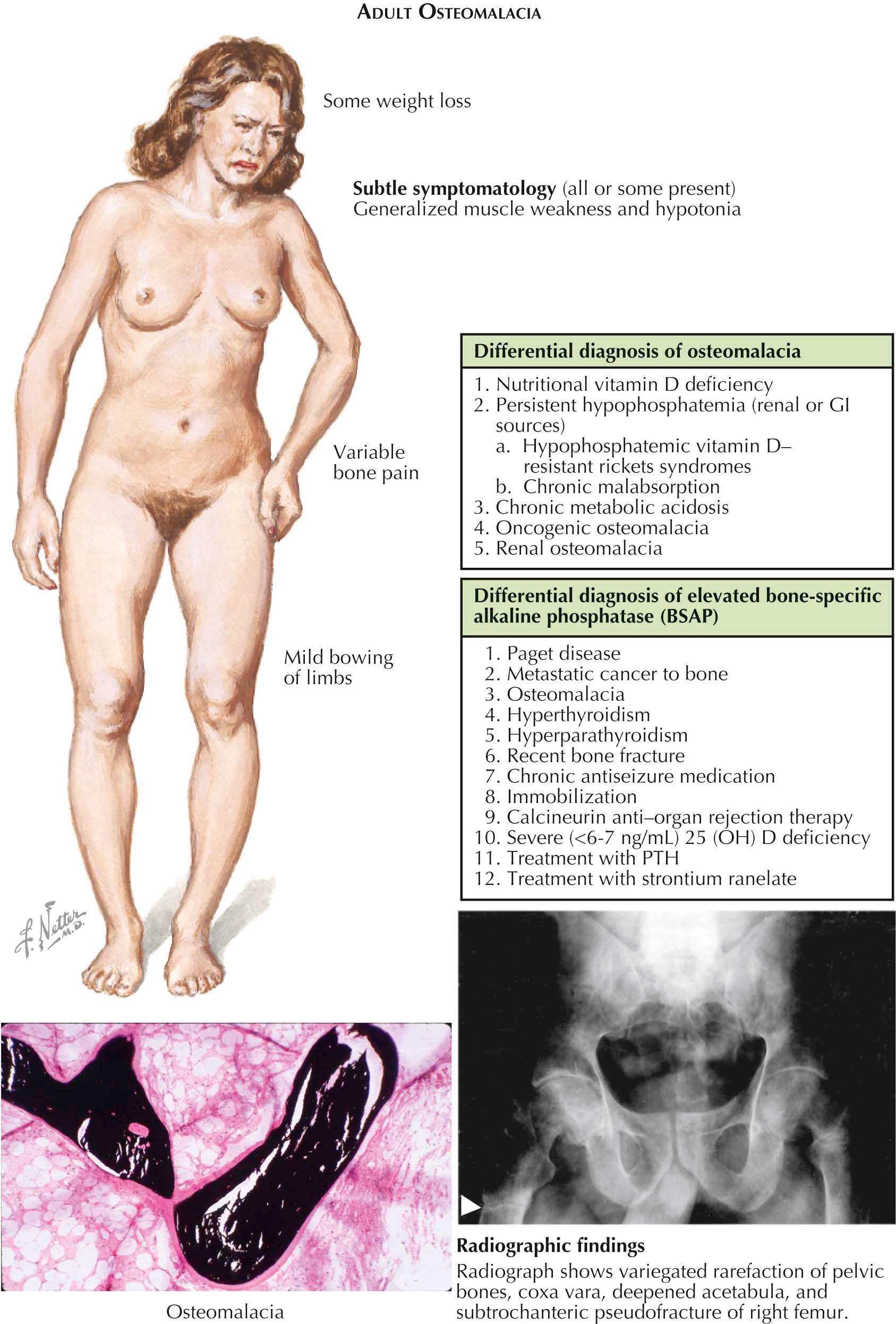
Clinical Manifestations. The diagnosis of adult osteomalacia (see Plate 3-14 ) may be difficult to establish because the changes may be considerably more subtle than those seen in childhood rickets. In early stages, the patients may be asymptomatic and the changes are biochemical—the most sensitive being an elevated serum total alkaline phosphatase. If the total alkaline phosphatase is elevated, then a bone-specific alkaline phosphatase (BSAP) should be obtained. If the BSAP is elevated, then there is a differential diagnosis of elevated BSAP (see Plate 3-14 ). By exclusion, osteomalacia can be strongly suspected but the gold standard is quantitative bone histomorphometry. A bone biopsy is diagnostic, and there are very specific histomorphometric criteria for the diagnosis of osteomalacia (see Plate 3-14 ). Once a specific diagnosis is established, then adult osteomalacia has a very narrow group of causes (see Plate 3-14 ). By biochemical testing, the etiology can be determined; and, by correcting the biochemical abnormalities and keeping them corrected, the symptoms of osteomalacia can be eliminated and the histomorphometry normalized.
Patients with adult and advanced osteomalacia may complain of generalized weakness, especially proximal muscle weakness, bone pain, easy fatigability, and malaise. The physical findings are minimal: tenderness of bony prominences or, in more serious cases, muscle weakness that is severe enough to cause an abductor-lurch type of gait (the gluteal, or Trendelenburg, gait). In long-standing cases, a bone deformity such as bowleg, coxa vara, or kyphosis may be common.
Radiographic signs are equally subtle, showing for the most part only a diffuse osteopenia, similar to that seen in other metabolic bone diseases such as postmenopausal or senile osteoporosis, hyperparathyroidism, hyperthyroidism, and diffuse skeletal metastatic tumors such as those seen in multiple myeloma. One distinctive feature seen in more advanced osteomalacia, present in about 25% of cases, is virtually pathognomonic of osteomalacia. Focal collections of osteoid produce localized, narrow, ribbon-like zones of decreased density in the cortices. These zones are almost always symmetric and are located at right angles to the long axes of the bones. On radiography, they resemble partial fractures. These usually painless pseudofractures—called Looser's zones, Umbauzonen, or milkman syndrome—are usually seen on the concave sides of a long bone, the medial side of the femoral neck, the ischial and pubic rami, the clavicle, the ribs, and the axillary border of the scapula. They may serve as stress risers, thus leading to a true fracture (particularly in the femoral neck or in the pubis).
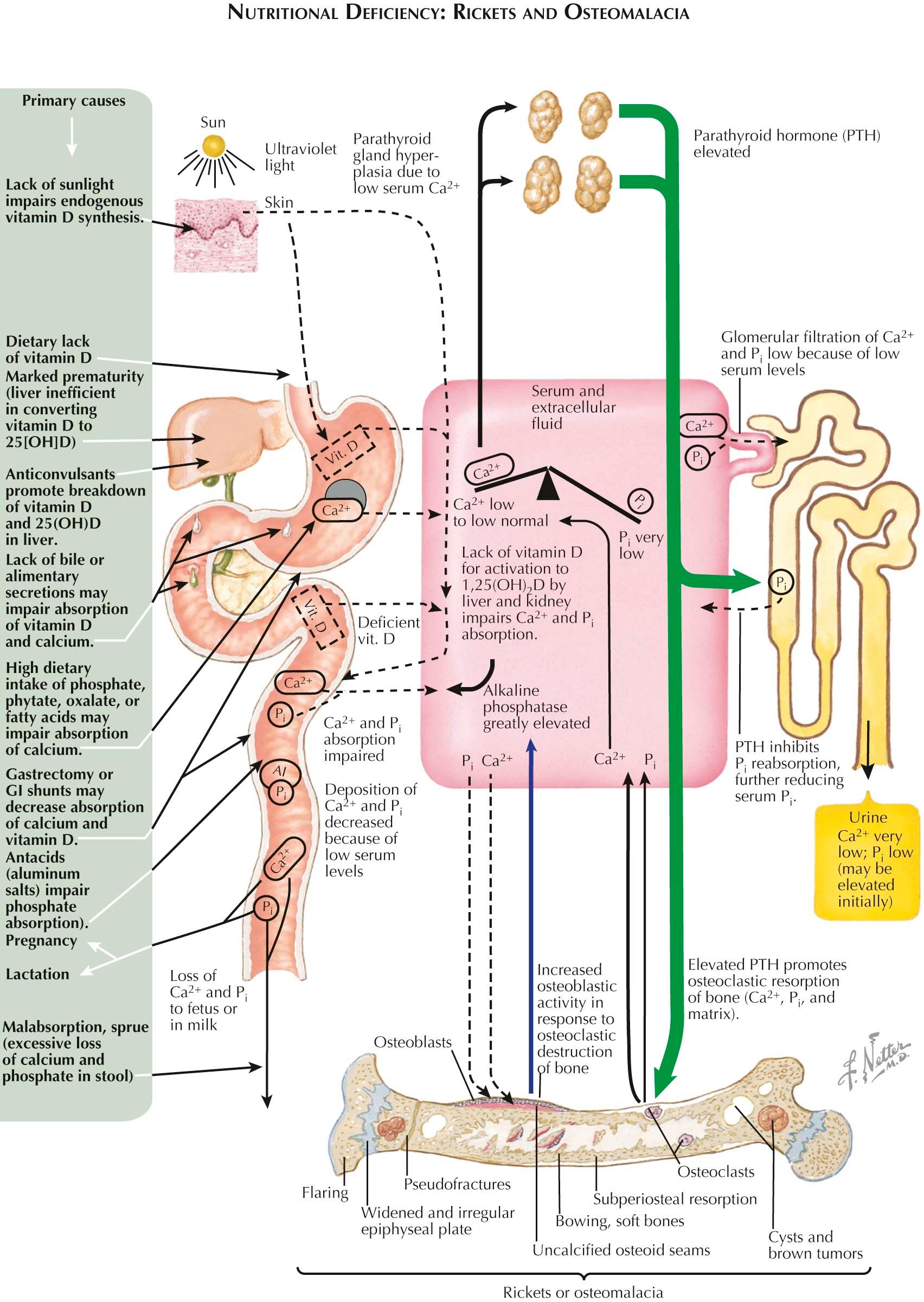
The classic and most clearly understood cause of nutritional-deficiency rickets and osteomalacia (see Plate 3-15 ) is a severe chronic deficiency of vitamin D. Deficiency of this fat-soluble sterol vitamin can be dietary, inadequate exposure to sunlight, or malabsorption. Malabsorption can be due to a variety of gastrointestinal conditions, including asymptomatic celiac disease. Because fat-soluble vitamins need to be bound to bile salts to be absorbed in the terminal ileum, deficiency of bile salts may also lead to low vitamin D levels. Although histomorphometric changes of defective mineralization can be seen with 25(OH)D levels less than 10 ng/mL, clinical osteomalacia is usually not seen unless the serum vitamin D levels are even lower and consistently low for months before recognizable fractures or muscle weakness is seen.
Although the Institute of Medicine (IOM) nutritional dietary recommendation for the U.S. population is 800 IU/day of vitamin D, serum levels are often lower than adequate for many clinical outcomes at this intake. The widespread availability of reliable measurements of serum 25(OH)D levels has made the management of adequate vitamin D replacement a standard of care. Many professional societies recommend that the serum 25(OH)D level be kept between 30 and 50 ng/mL.
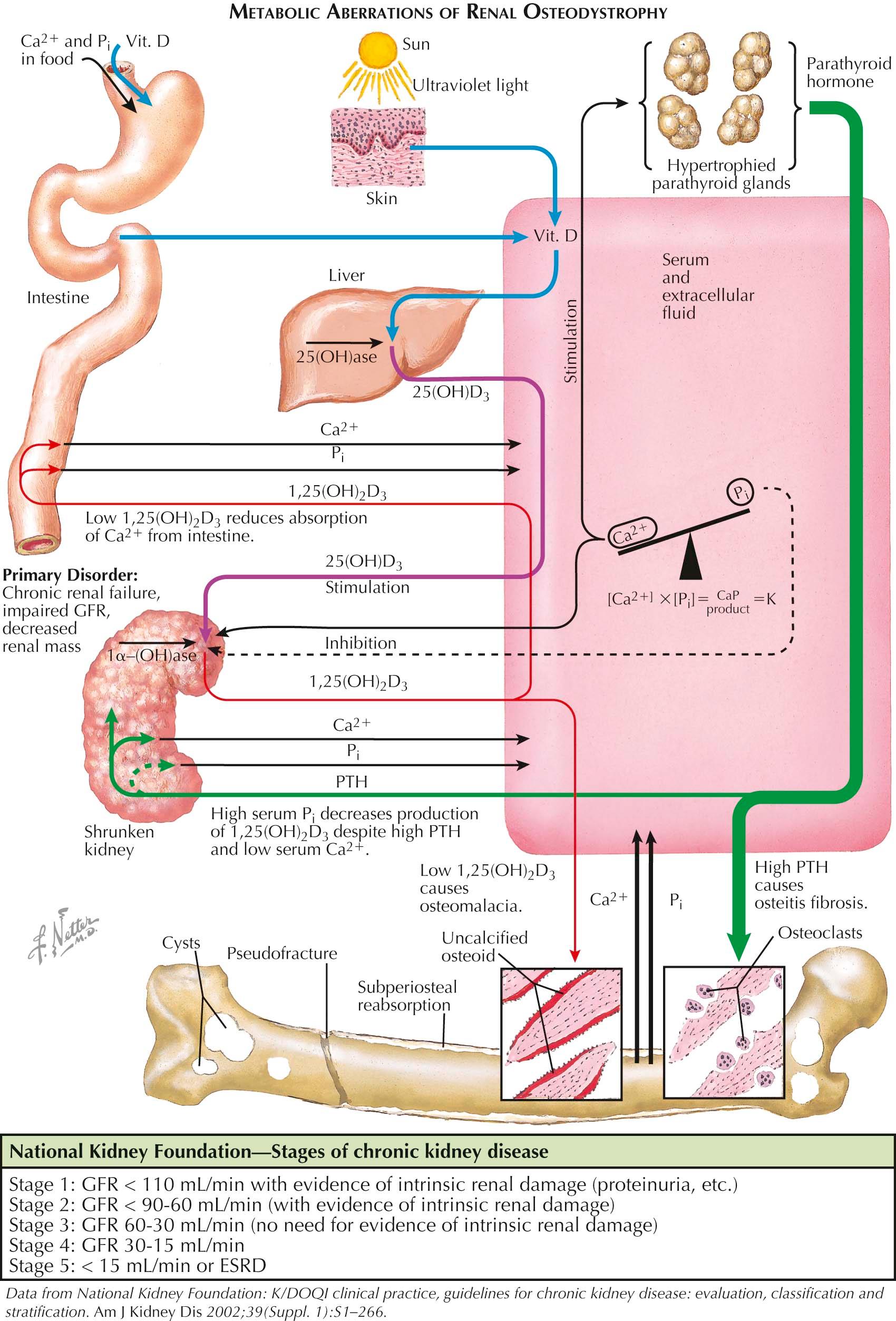
Vitamin D has receptors throughout many tissues and acts as both an endocrine as well as an autocrine/paracrine hormone. The endocrine properties have direct effects on bone and muscle tissue whereas the autocrine/paracrine pathways are involved in the immunologic system. 25(OH)D is converted into additional active metabolite 1,25(OH) 2 D in renal tubules and monocyte cell lines. The synthesis of 1,25(OH) 2 D is tightly regulated by serum PTH, calcium, and phosphorus such that it requires very high or very low 25(OH)D to change to serum 1,25(OH) 2 D. This regulation is important because either high or low 1,25(OH) 2 D can lead to either hypercalcemia or hypocalcemia. In syndromes of reduced 1,25(OH) 2 D production, such as hypophosphatemic vitamin D–resistant rickets, oncogenic osteomalacia, or severe CKD, a secondary hyperparathyroidism develops, in part owing to hypocalcemia and in part owing to the direct effect(s) of 1,25(OH) 2 D to regulate PTH synthesis. The biochemical abnormalities shown in Plate 3-15 lead to a syndrome that manifests all the histologic and radiographic findings of rickets and/or osteomalacia, as well as those of secondary hyperparathyroidism. In addition, the discovery of FGF-23 has led to a greater understanding of the interactions between the parathyroid gland, kidney, and bone. FGF-23, synthesized by the osteocyte, is a major regulator of renal phosphate reabsorption as well as PTH synthesis. Preliminary data suggest that FGF-23 also has effects on osteoid mineralization. FGF-23 increases early in CKD, much earlier than PTH, and is the peptide responsible for the phosphaturia and inhibition of 1,25(OH) 2 D levels in the syndrome of oncogenic osteomalacia. The information presented in Plates 3-5 and 3-12 puts into perspective the links between bone/kidney/parathyroid glands that share common pathways in the pathophysiology of many, if not most, of the clinical conditions associated with osteomalacia.
As shown in the left half of Plate 3-15 , other defects or conditions may result in a rachitic or an osteomalacic syndrome. For example, in a premature infant, the immature liver cannot adequately convert vitamin D to 25(OH)D. Chronic use of anticonvulsant medications may lead to a deficiency of 25(OH)D by interfering with the microsomal enzyme systems in the liver. Some nutritional disorders interfering with calcium absorption that may also lead to a similar syndrome are excessive dietary ingestion of phytate (in certain coarse cereals), oxalate (in spinach), citrate or phosphate, and an increased intake of aluminum salts (usually in the form of antacids) that can cause a phosphate deficiency. Any condition in which the gut wall is damaged (e.g., tuberculosis, celiac syndromes, sarcoidosis, presence of surgical shunts) or in which rapid transit of gastrointestinal contents occurs (e.g., biliary disease, postgastrectomy syndromes) may also cause a rachitic or an osteomalacic syndrome due to a deficiency of either calcium or vitamin D, or both.
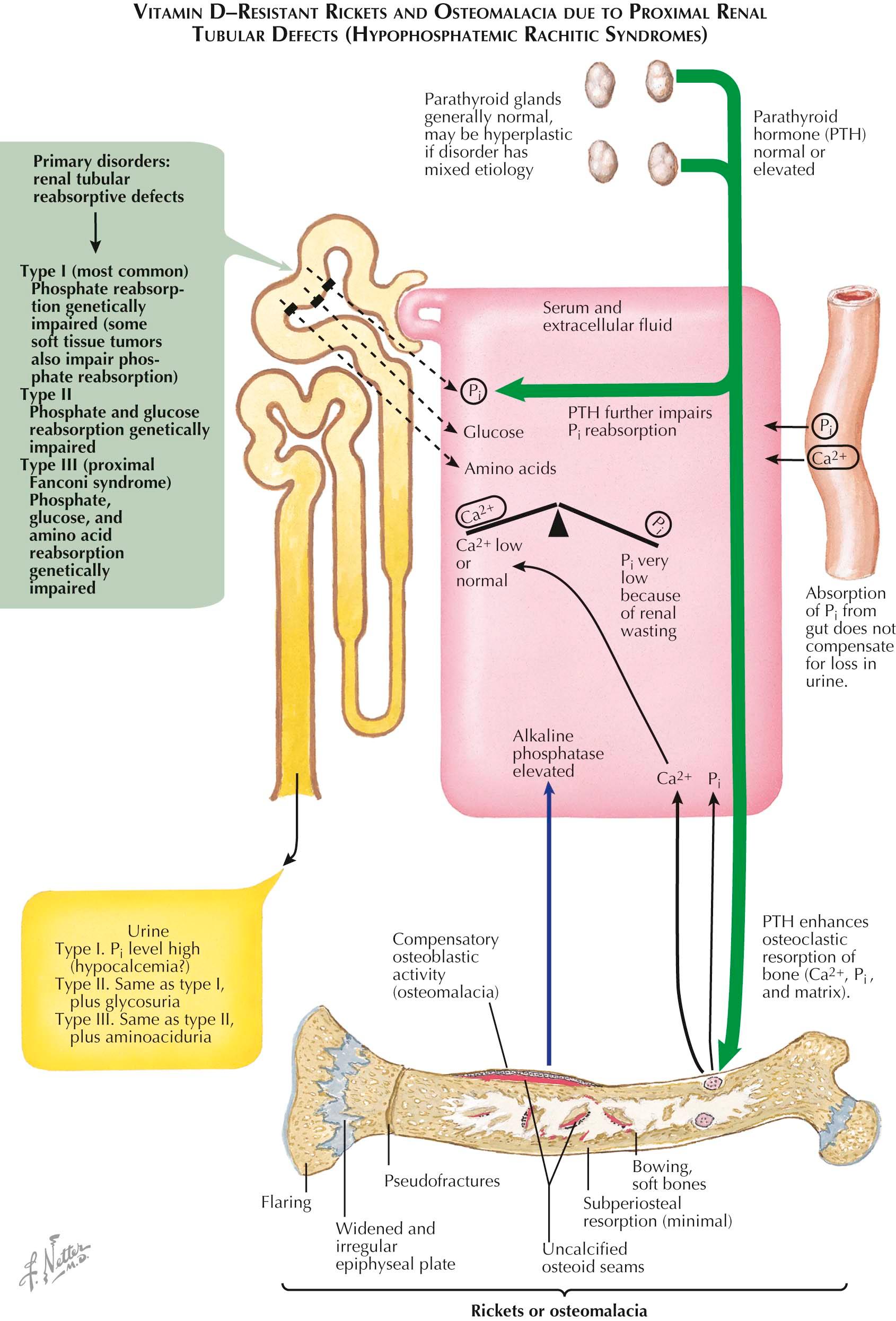
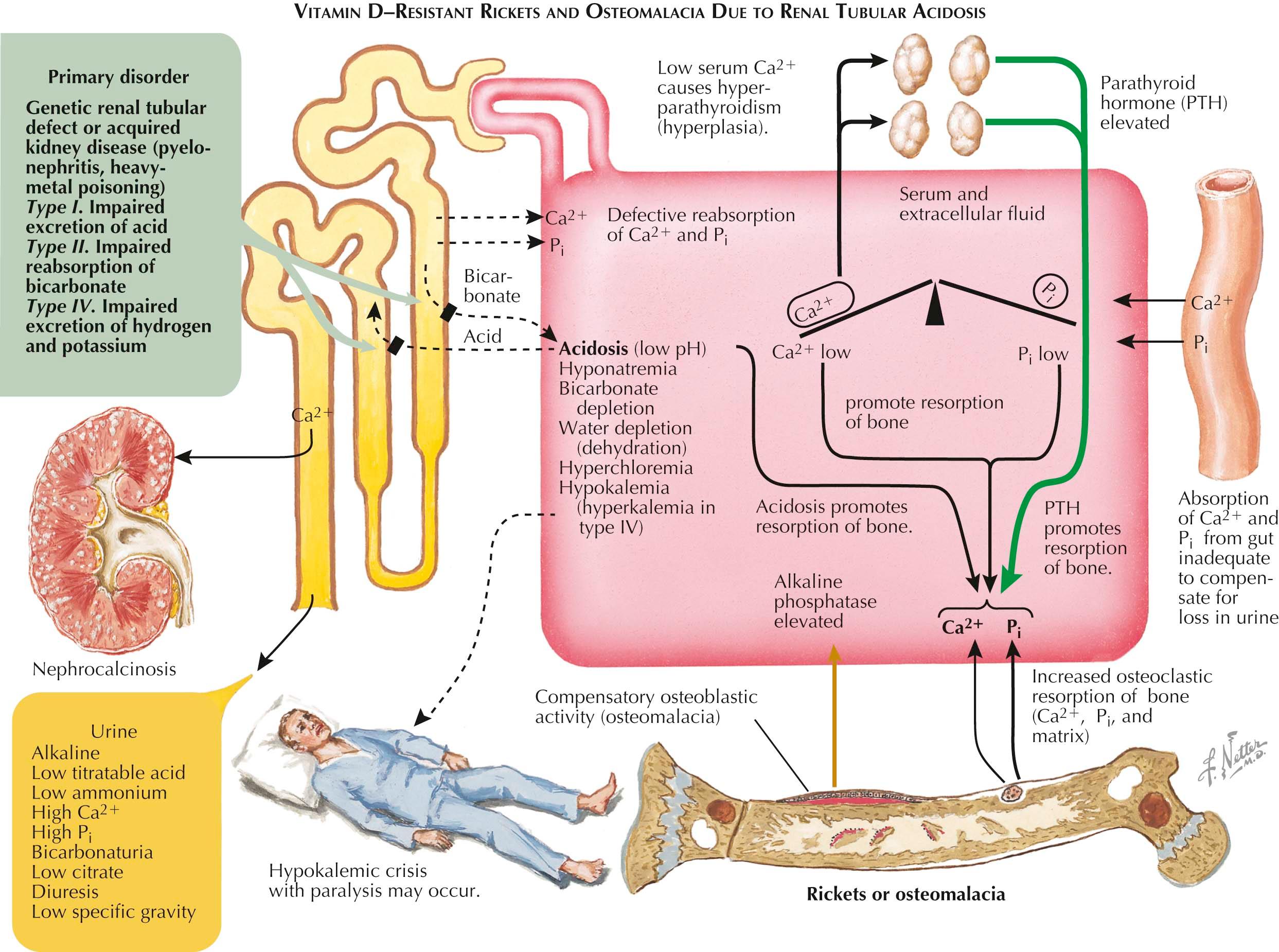
In countries with vitamin D supplementation, genetic or acquired rachitic and osteomalacic syndromes (see Plate 3-16 ) that are resistant to high therapeutic doses of vitamin D are now more common than those associated with vitamin D deficiencies. Almost all of these syndromes are renal in origin and are associated with a narrow or broad reabsorptive defect in the renal tubule that leads to hypophosphatemia (thus, they are also known as hypophosphatemic vitamin D–resistant rickets, or phosphate diabetes).
The most common of these disorders is type I, a sex-linked dominant genetic disorder in which the renal tubule does not reabsorb phosphate; as a result, the disease produces all the features of rickets or osteomalacia, as seen on histologic and radiographic examinations. In the purest form of the disorder there is no abnormality of calcium or vitamin D metabolism and results of serum analysis and urinalysis demonstrate profound hypophosphatemia, marked lowering of the percent tubular reabsorption of phosphate (%TRP), and an increased serum alkaline phosphatase level. Many of the syndromes are impure, however, and either show evidence of a defect in vitamin D metabolism (see Plate 3-18 ) or some degree of loss of calcium as fixed base (see Plates 3-17 and 3-19 ). Under these circumstances, the serum calcium concentration may be low or low to normal and the PTH level may be increased, which aggravates the already severely phosphate-depleted state.
Three phosphate-wasting lesions in the proximal tubule have been identified. Type I, first described by Albright, is seen most frequently. It is transmitted as a sex-linked dominant trait and the reabsorptive defect is confined to phosphate only. In type II, the defect is broader and involves both phosphate and glucose. In type III, the proximal Fanconi syndrome, the reabsorptive defect is for phosphate, glucose, and various amino acids.
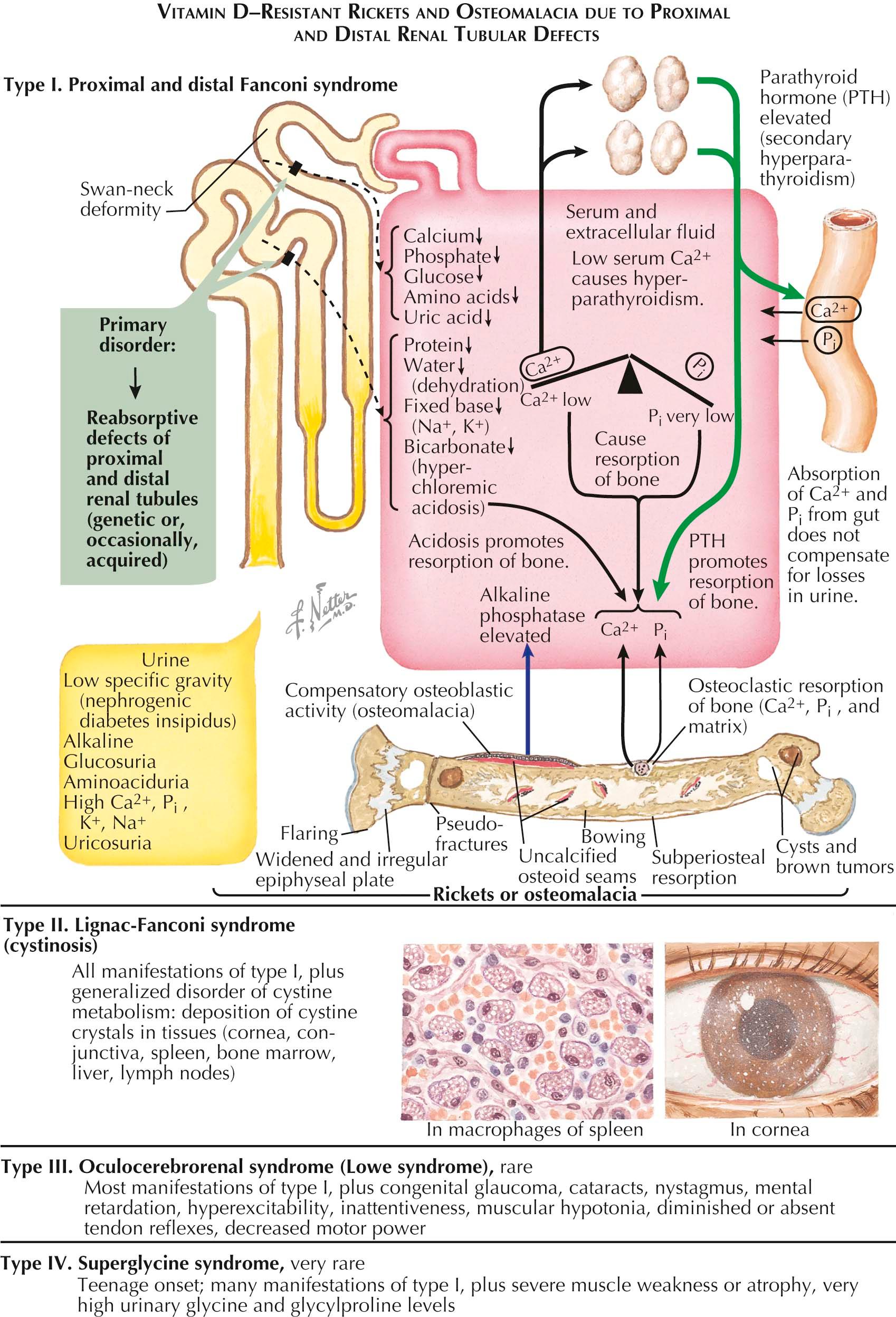
In another group of vitamin D–resistant rachitic and osteomalacic syndromes, the range of renal tubular defects is considerably broader and may interfere more severely with normal metabolism (see Plate 3-17 ). Reabsorption of phosphate, glucose, and amino acids in the proximal tubule is impaired, and, in addition, the functions of the distal tubule are significantly altered. Consequently, in patients with type I syndrome, known as the proximal and distal Fanconi syndrome, or the Debré-de Toni-Fanconi syndrome, the kidney's ability to reabsorb water, bicarbonate, proteins, and fixed base is to some degree impaired. This represents a severe challenge to the patient, particularly the newborn, and requires major replacement therapy.
Typically, the patient is a very ill child, often dehydrated and hypoproteinemic, with rachitic changes in the bones. The rachitic and osteomalacic patterns in these patients result from the failure of tubular reabsorption of phosphate coupled with the loss of fixed base, including calcium. This unfortunate combination results in both rachitic changes and a mild-to-moderate secondary hyperparathyroidism (which worsens the bone lesions and intensifies the hypophosphatemia). These conditions have little relationship to vitamin D; in fact, treatment with even high doses of the sterol vitamin has little effect.
Biochemical changes in type I disease include hypocalcemia, hypophosphatemia, increased serum alkaline phosphatase level, and normal serum levels of 25(OH)D and 1,25(OH) 2 D. The patient is likely to have signs of renal tubular acidosis (hyperchloremia, hyponatremia, and hypokalemia in association with an alkaline urine). Urinalysis reveals a low fixed specific gravity and the presence of excessive concentrations of metabolites, including calcium, sodium, and potassium ions; phosphate (as a result of a greatly lowered %TRP); uric acid; amino acids; and proteins.
Three other less common types of the severe form of vitamin D–resistant rickets and osteomalacia are often included under the proximal and distal Fanconi syndrome. (1) Type II, Lignac-Fanconi syndrome, is almost identical to type I but has an additional defect in the metabolism of cystine. This defect leads to the deposition of crystals of the amino acid in the viscera, bone marrow, and eyes (the diagnosis may be made by slit-lamp examination). As a result of the deposits, cirrhosis of the liver and renal failure frequently supervene by puberty. (2) Type III disease is known as oculocerebrorenal, or Lowe, syndrome. Patients with this condition have many of the manifestations of type I disease but may also have a broad range of ocular and neurologic abnormalities, which include congenital glaucoma, nystagmus, mental retardation, muscular hypotonia, and weakness. (3) Type IV, the superglycine syndrome, is rare. It is less severe than the other types and usually has a later onset. Presenting symptoms are profound motor weakness and very high urinary concentrations of glycine and glycylproline.
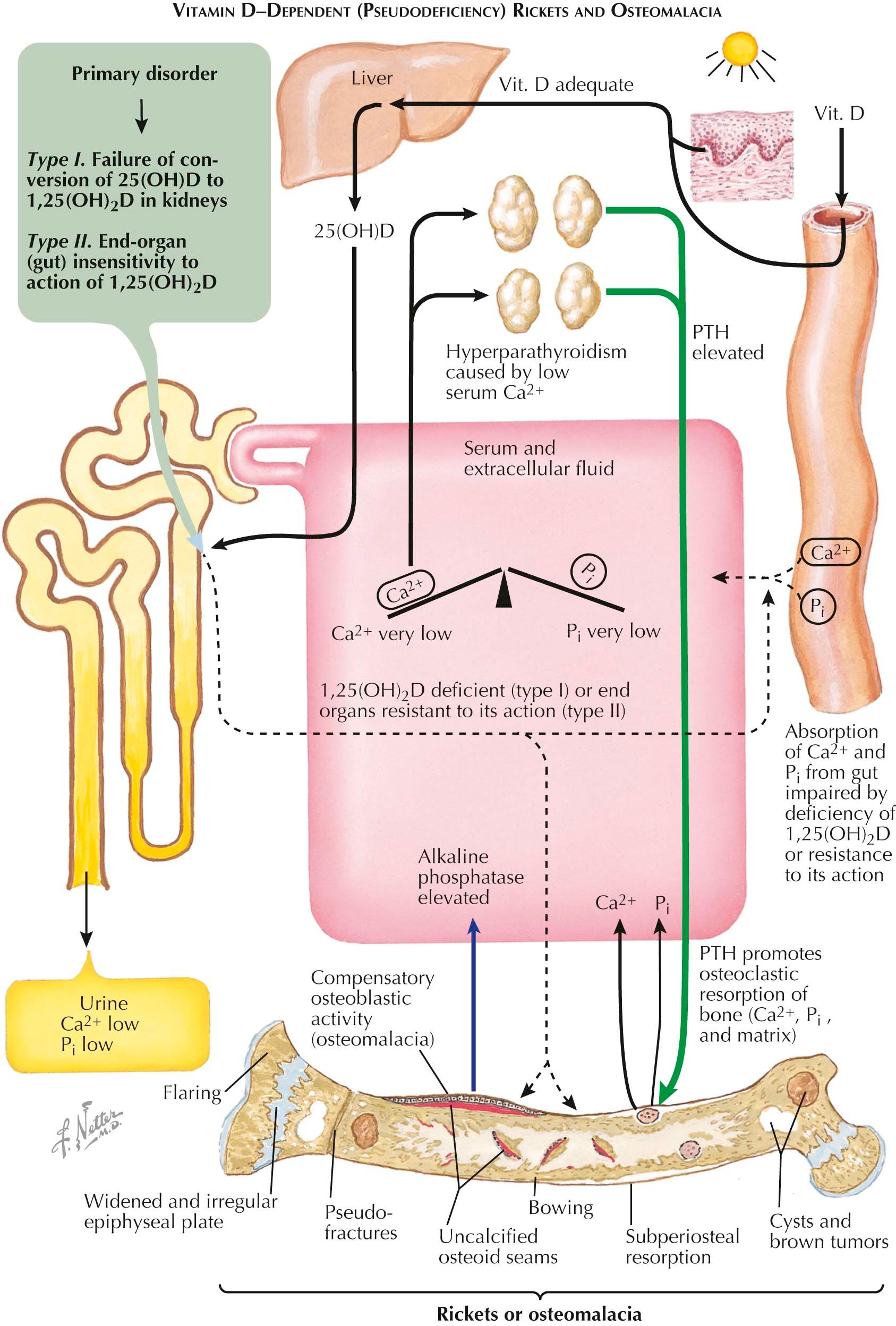
This major category of vitamin D–dependent rickets is characterized by abnormalities of vitamin D metabolism (see Plate 3-18 ). In this group of syndromes, the error is also almost always inherited but involves either a failure of conversion of 25(OH)D to the potent 1,25(OH) 2 D or a relative end-organ insensitivity of the gut (and, presumably under certain circumstances, the renal cell as well) to the patient's autogenous 1,25(OH) 2 D. In both of these circumstances, orally administered vitamin D in standard or therapeutic doses does not help to increase reabsorption of calcium by the renal tubule, and hypocalcemia and rachitic manifestations develop. In response to the lowered serum calcium level, the parathyroid glands elaborate PTH, which further depletes the skeleton's calcium reserves and produces hyperphosphaturia and hypophosphatemia.
All of the findings and chemical abnormalities are similar to those seen in the classic nutritional deficiency syndrome, with the exception of the concentrations of the polar metabolites of vitamin D. In the first form of the disorder—failure of conversion of 25(OH)D to 1,25(OH) 2 D in the kidney—serum levels of 25(OH)D may be very high (hence, the term pseudo ) while levels of 1,25(OH) 2 D may be low. In the latter form of the disorder—end-organ insensitivity to the action of 1,25(OH) 2 D—the serum levels of both 25(OH)D and 1,25(OH) 2 D are usually normal or high. Both forms are often successfully treated with administration of 1,25(OH) 2 D.
A specific disorder that fits into the so-called pseudo-deficiency is oncogenic osteomalacia. This syndrome is due to mesenchymal tumors that develop in adults for unknown cause. These tumors are often very small, subcutaneous, and, more often than not, benign. However, these tumors secrete FGF-23 and induce renal phosphate wasting and hypophosphatemia and inhibit renal production of 1,25(OH) 2 D. Hence, the classical biochemical triad is hypophosphatemia, normal 25(OH)D, and low 1,25(OH) 2 D. Serum levels of FGF-23 are available clinically and can help confirm the diagnosis. Although patients with oncogenic osteomalacia can have symptomatic improvement with oral phosphorus and 1,25(OH) 2 D replacement, the cure is finding the mesenchymal tumor responsible for producing FGF-23 and having it removed. The latter is often a diagnostic challenge because these tumors are small and may be located anywhere in the human body. The radioisotope, octreotide, is taken up by these tumors such that periodic scans, often with positron emission tomographic accentuation, are necessary to find these tumors. Removal of the tumor is a cure. Total tumor removal will be accompanied by normalization of the BSAP, serum phosphorus, 1,25(OH) 2 D, and FGF-23 levels and the patient will never again need oral phosphorus or 1,25(OH) 2 D replacement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here