Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The incidence of melanoma continues to rise rapidly worldwide, but mortality rates are declining. Each year in the United States, nearly 100,000 new cases of invasive melanoma are detected, and approximately 7000 patients die from melanoma. Melanoma is the leading cause of death from cutaneous malignant disease, and it accounts for 1 to 2% of all cancer deaths in the United States.
The rising incidence is attributed to increasing sun exposure, especially early in life, as well as to more aggressive diagnosis and even over-diagnosis. Melanoma affects all age groups, with a median age at diagnosis of 63 years. Melanoma is largely a disease of Whites, with a very low incidence in Black Americans, Asians, and Hispanics.
Risk factors for melanoma include family history of melanoma, prior melanoma or nonmelanoma skin cancer, inherited genetic susceptibility, and sun exposure. Artificial exposure to UV radiation by indoor tanning beds is likewise a risk factor for melanoma. Individuals who have blond or red hair, blue eye color, and freckles have a tendency to burn rather than to tan and have higher rates of melanoma. The pattern of sun exposure may also be important; intermittent intense exposure, rather than chronic sun exposure, may carry a higher risk for melanoma.
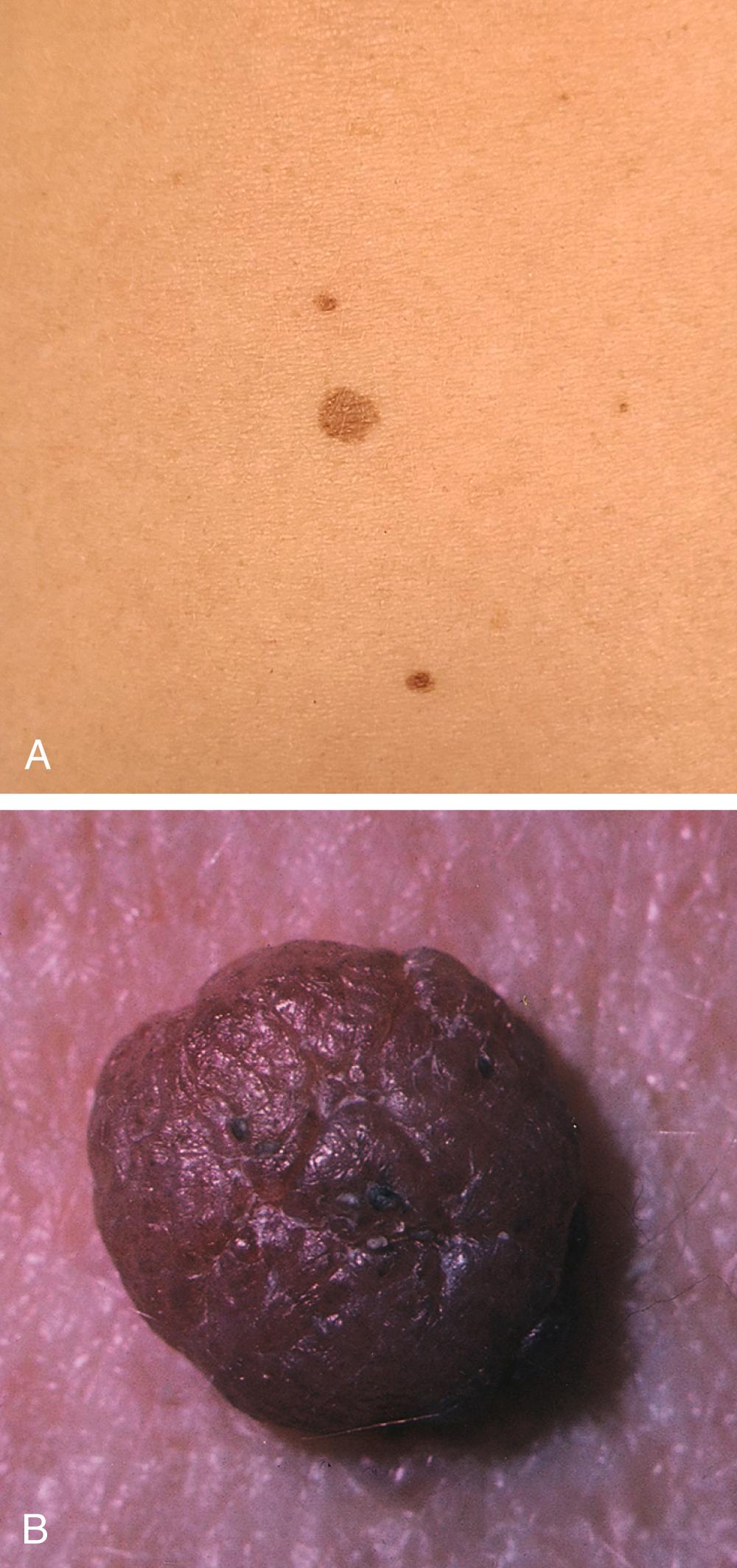
Individuals with an increased number of typical or benign moles, atypical moles, or dysplastic nevi ( Figs. 188-1 and 188-2 ) also have an increased risk for melanoma. Atypical moles or dysplastic nevi are important precursor lesions of melanoma and serve as markers for increasing risk. For example, individuals with dysplastic nevi have a 6% lifetime chance of developing melanoma, and this risk increases to as high as 80% in individuals who have dysplastic nevi and a strong family history of melanoma.
Exposure to sunlight, especially ultraviolet (UV) radiation ( Chapter 18 ), is the major causative factor for the development of melanoma. Melanomas originate from melanocytes, which are located predominantly in the basal cell layer of the epidermis and use the enzyme tyrosinase to synthesize melanin pigment, which serves to protect against UV damage ( Chapter 403 ). In persons of color, however, exposure to UV light may not be an important risk factor.
Somatic mutations in primary and metastatic melanoma primarily involve the mitogen activated protein kinase pathway (MAPK). The point-mutation burden increases from benign through intermediate precursor lesions to melanoma, with strong genetic evidence of the effects of UV radiation detectable at all evolutionary stages. Activating mutations in the BRAF gene can be found in approximately 50% of melanomas, and 20% of melanomas are associated with a mutation in the NRAS gene. Distinct patterns of genetic alterations are found in primary melanomas based on anatomic location and extent of sun exposure. For example, melanomas that originate on mucous membranes, acral skin (soles, palms), and skin with chronic sun damage (i.e., lentigo maligna melanoma) have frequent mutations in KIT . Uveal melanoma is associated with mutations in GNAQ/GNA11 . Monosomy of chromosome 3 and somatic mutations in the gene encoding BRCA1-associated protein (BAP1) on chromosome 3 are associated with worse outcome and the development of metastatic melanoma. The discovery of somatic mutations in melanoma and associated aberrant signal transduction pathways has provided the basis for the development of molecularly targeted therapy for patients with advanced melanoma.
Approximately 10% of patients with melanoma have a family history of melanoma. Several chromosomal loci determine susceptibility to melanoma. The most important locus is p16/CDKN2A , which is on chromosome 9p21. This gene is a member of a class of molecules that play a central role in cell cycle regulation. Of the members of melanoma-prone families, 25 to 40% have mutations in this gene. The risk for developing cutaneous melanoma in an individual who is a CDKN2A carrier is between 30 and 90% by age 80 years and varies by geographic location.
Genetic variability in the melanocortin-1 receptor (MC1R) plays a key role in pigmentation of skin and hair, and this variability has been implicated in a predisposition to melanoma. Carriers of a pathogenic mutation in the BRCA2 familial breast cancer gene have an increased risk of developing cutaneous melanoma. Germline mutations in BAP1 gene are linked to the BAP1 tumor predisposition syndrome. Both uveal and cutaneous melanoma have been described in this syndrome, which is also associated with mesothelioma and renal cell cancer.
Early detection and recognition of melanoma are key to improving survival. The signs of early melanoma are based on the clinical appearance of the pigmented lesion and a change in the shape, color, or surface of an existing mole. Most patients report a preexisting mole at the site of the melanoma. Itching, burning, or pain in a pigmented lesion should increase suspicion, although melanomas often are not associated with local discomfort. Bleeding and ulceration are signs of a more advanced melanoma. Most melanomas are varying shades of brown, but they may be black, blue, or pink. The ABCDEs for the recognition of melanoma are asymmetry, border irregularity, color variation, diameter greater than 6 mm, and evolution or a change in a skin lesion. The “ugly duckling” sign is recognizing a skin lesion that looks different from other skin lesions and is therefore suspicious.
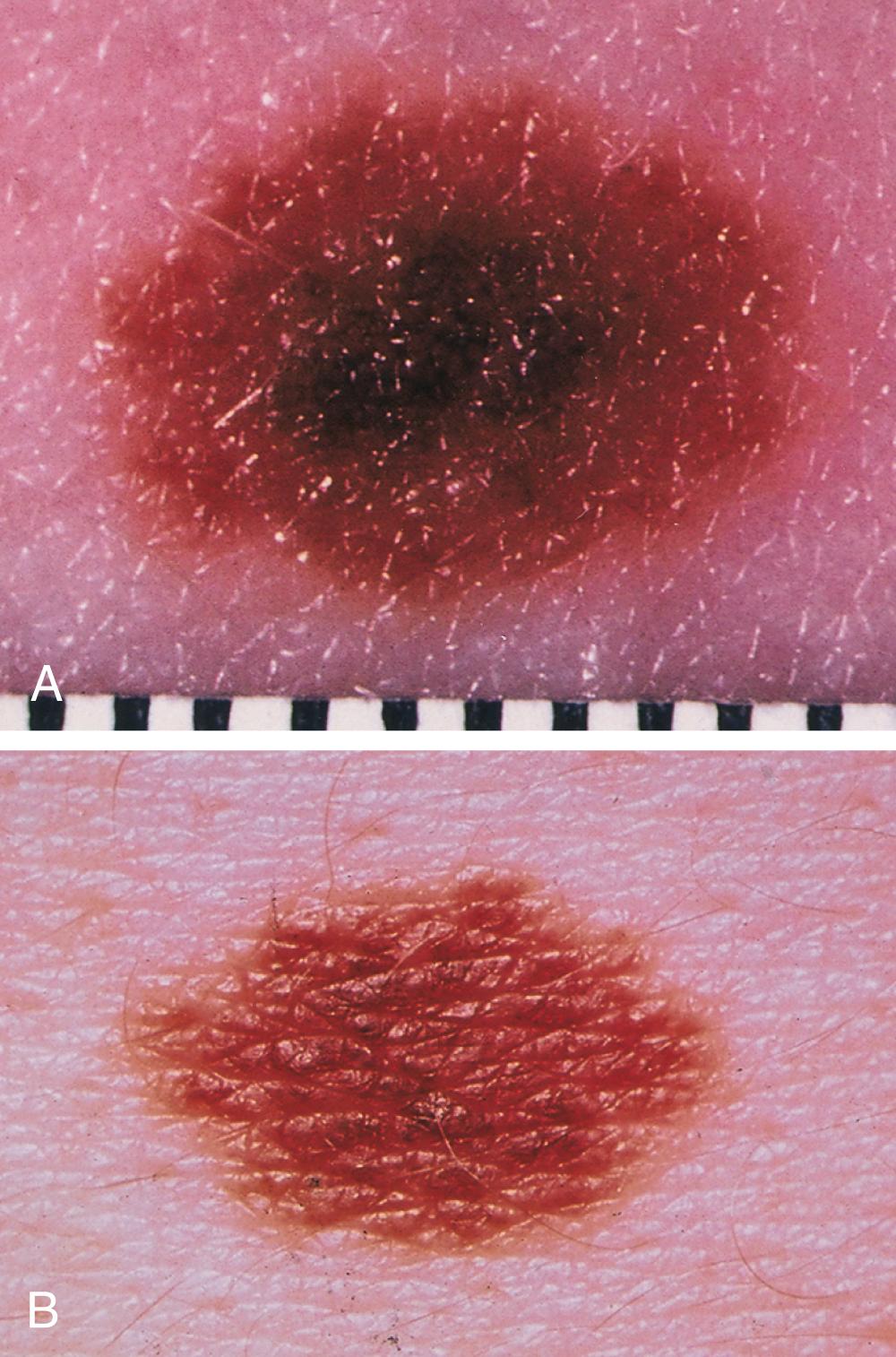
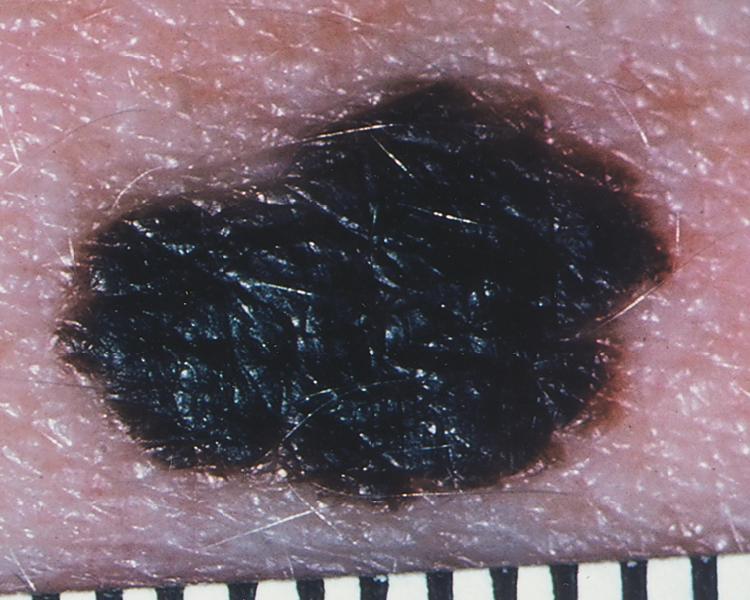
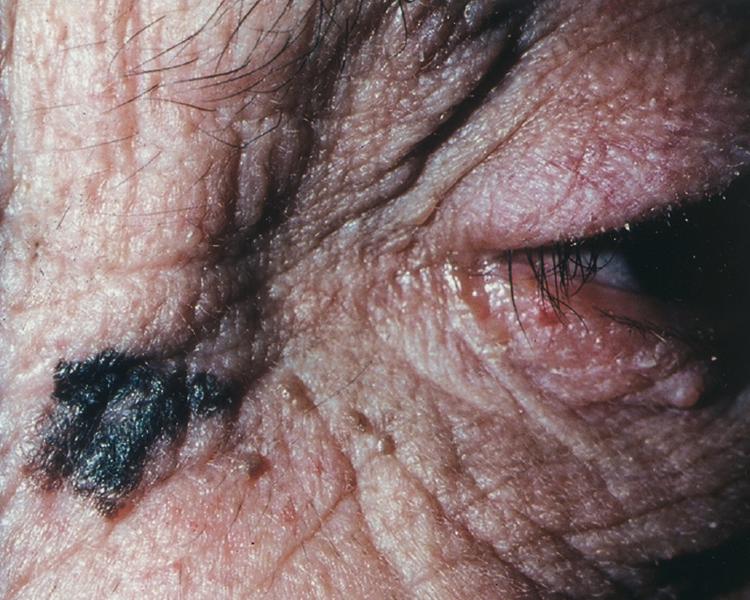
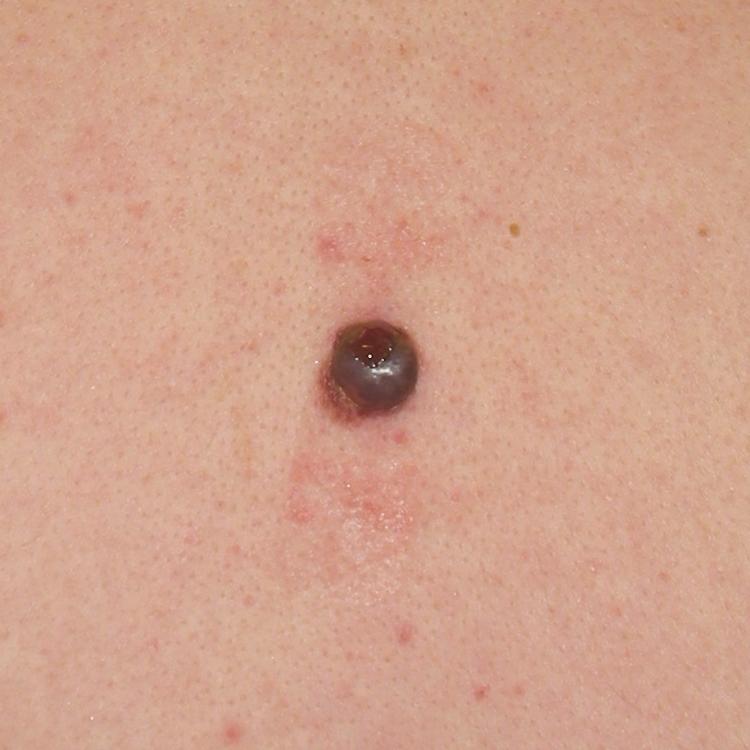
Cutaneous melanoma has been divided into four subtypes. Superficial spreading melanoma, which accounts for 70% of all melanomas, can be located on any anatomic site ( Fig. 188-3 ). Lentigo maligna melanoma, which represents 4 to 10% of all melanomas, tends to occur more commonly in chronically sun-exposed skin in older patients, frequently on the head and neck ( Fig. 188-4 ); clinically, it appears as a macular (flat) lesion, with varying degrees of color, usually brown. Nodular melanoma ( Fig. 188-5 ) accounts for 15 to 30% of melanomas and manifests as a rapidly enlarging, elevated, or polypoid lesion, often blue or black. These lesions can also be amelanotic and lack pigment and have more of a pink color. The ABCDE rule does not always apply as well to nodular melanomas. Acral lentiginous melanoma manifests as a darkly pigmented, flat to nodular lesion on the palm, on the sole, or subungually; sunlight is not thought to play a causative role in this form of melanoma. Histologic subtype does not directly correlate with clinical behavior. However, recent data suggest that histologic subtype may correlate with specific genetic abnormalities. For example, superficial spreading melanoma may be associated with BRAF mutation, and lentigo maligna melanoma may be associated with KIT mutation.
Ocular melanomas arise from the pigmented layer of the eye. Uveal melanoma is the most common intraocular malignancy of adults. Melanomas can also arise from noncutaneous sites, including mucosal epithelium in the gastrointestinal tract, anorectal area, genitourinary tract, and nasal and nasopharyngeal mucosa. Melanomas of the vulva and vagina are relatively rare. In general, mucosal melanomas are diagnosed at a more advanced stage of disease. The mainstay of treatment is surgical.
Any skin lesion suggestive of melanoma should be sampled by biopsy with complete excision, including a 1- to 2-mm margin of normal skin and some subcutaneous fat. Lesions that can be confused with melanoma include blue nevi, pigmented basal cell carcinoma, seborrheic keratosis, and hemangiomas ( Table 188-1 ). Both molecular and cellular criteria can distinguish common nevi from dysplastic nevi.
| DISEASE | CHARACTERISTICS |
|---|---|
| Common acquired nevi (moles) | These tend to be small, flat, and round; the border is regular, smooth, and well defined; the color is homogeneous, usually no more than two shades of brown; any site is affected; lesions are usually <6 mm (see Fig. 188-1 ). |
| Dysplastic nevi (atypical moles) | These occur predominantly on the trunk; they tend to be large, usually >5 mm, with a flat component; the border is characteristically fuzzy and ill defined. The shape can be round, oval, or misshapen. The color is usually brown but can be mottled with dark brown, pink, and tan. Some individuals have only one to five moles; others have more than 100 (see Fig. 188-2 ). |
| Melanoma | The border is more irregular; lesions tend to be larger, often >6 mm; substantial heterogeneity of color is noted, which can be tan-brown, dark brown, black, pink, red, gray, blue, or white. |
An incisional biopsy may be necessary for lesions that are too large for complete excision. Shallow shave biopsies, curettage, cryosurgery, laser, and electrodessication are contraindicated in lesions suggestive of melanoma.
Activating mutations in the BRAF gene are present in approximately 50% of melanomas, and all patients with advanced cutaneous melanoma should have their tumors assessed for the presence or absence of a BRAF mutation. Almost 80% of BRAF mutations are V600E, with most of the remainder consisting of V600K.
Sentinel lymph node mapping is considered a critically important surgical staging tool and should be performed whenever the melanoma is ulcerated or when the original biopsy reveals a melanoma with a depth of more than 1 mm or an increased mitotic rate ( Fig. 188-6 ). For melanomas that are less than 1 mm thick, the likelihood of regional lymph node involvement is approximately 5%, so sentinel lymph node mapping is not routinely recommended for thin melanomas unless patients have adverse prognostic features such as ulceration and/or high mitotic count.
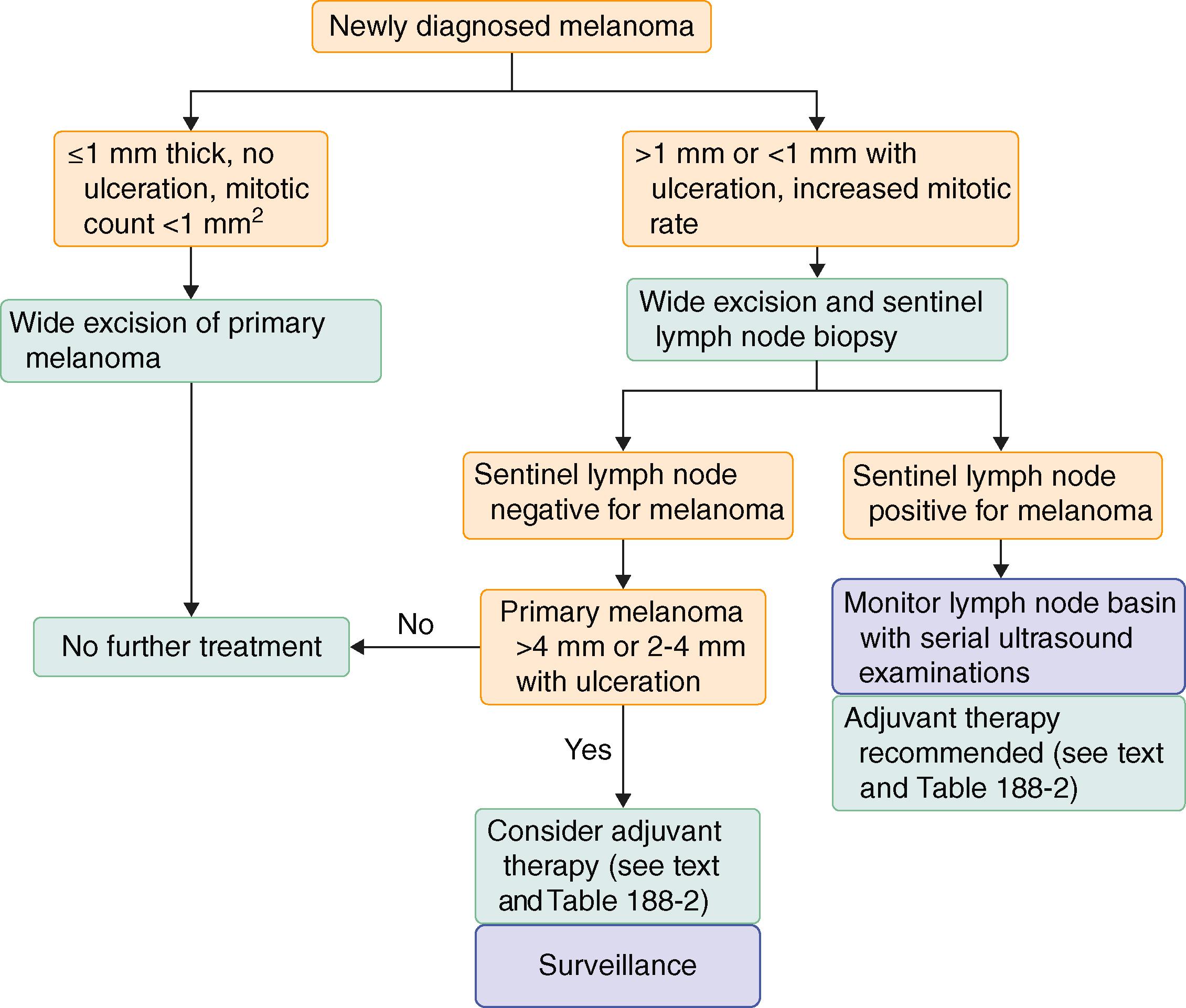
The sentinel lymph node biopsy is generally performed at the same time as the wide excision of the primary tumor and must be performed prior to the wide excision. The morbidity of sentinel lymph node biopsy is relatively low, and the staging information is critically valuable.
Sentinel lymph node biopsy accurately evaluates whether microscopic melanoma cells involve regional lymph nodes. The technique relies on the concept that specific regions of the skin drain specifically to an initial lymph node within the regional nodal basin through an organized pathway of afferent lymphatic channels. Sentinel lymph node biopsy is performed by injecting the primary melanoma site with blue dye (isosulfan blue), radiolabeled colloid, or both. When both modalities are used in combination, a sentinel node can be identified in 98% of patients; biopsy of the node accurately determines whether melanoma cells have metastasized to that specific lymph node basin.
Staging and prognosis for melanoma are based on the TNM system, in which T refers to tumor, N to nodes, and M to metastasis. Stages I (limited to the epidermis) and II (extending into the dermis) indicate clinically localized primary melanoma, stage III indicates regional involvement (lymph nodes or in-transit metastases), and stage IV is metastatic disease beyond the regional lymph nodes (i.e., lung, liver, brain).
The initial evaluation of a patient with melanoma should include a personal history, a family history, a total skin examination, and palpation of regional (draining) lymph nodes. The focus is to identify risk factors, signs, or symptoms of metastases, dysplastic nevi, and additional melanomas. A chest radiograph and liver enzyme tests may be performed at the discretion of the physician. Most patients who present with melanoma do not have distant metastatic disease at presentation, so extensive evaluations with computed tomography (CT) to search for distant metastases have an extremely low yield and are not indicated in asymptomatic patients. More extensive staging evaluation with CT or positron emission tomography (PET) can be considered in patients who have high-risk disease (primary melanoma >4 mm thick or node-positive disease), in whom the risk for distant metastatic disease is higher, or for mutations in BRAC2 .
In patients who have a family history of melanoma, testing for mutations in the p16/CDKN2A locus can be informative. For patients or family members who are thought to be at risk for a hereditary cancer melanoma syndrome, referral to a genetic counselor should be considered to learn about the risks and benefits of genetic testing, the role of surveillance strategies, and their potential impact on medical management.
Staging guides treatment decisions, including primary treatment, surveillance strategies, adjuvant therapy, and optimal management of patients with advanced melanoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here