Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
BLEEDING IS AN INEVITABLE CONSEQUENCE of surgery and trauma. Provided the coagulation processes are normal, a meticulous hemostatic surgical technique is usually adequate to achieve hemostasis for most surgical procedures. However, if the degree of injury is more extensive, major blood loss can occur, particularly if there is a coexistent deficiency of the normal coagulation process. Since the early part of the 20th century, it has been appreciated that transfusion of fluids and human (allogeneic) blood can prevent many of the adverse effects of blood loss. Despite this, it is important to appreciate the risks and limitations of transfusion: packed red blood cells (PRBCs) will fail to correct any existing coagulopathy and immunologic and pathophysiologic adverse effects of exogenous stored blood products may occur.
This chapter examines the specific interventions to correct coagulopathy and reduce the requirement for transfusion of blood products by encouraging hemostasis. Although similar, these objectives are not synonymous. During many types of surgery, in vitro tests may demonstrate only mild coagulation defects, and yet the use of hemostatic medications may reduce blood loss and obviate the need to transfuse PRBCs. In the absence of these medications, bleeding itself is often unlikely to be life-threatening. In comparison, severe coagulopathic bleeding is, by its nature, life-threatening. Transfusion of blood products cannot be avoided and the hemostatic medications used are often the blood products themselves. The balance of risk and benefit is very different in these two situations. In the former case, benefit will occur if the risk reduction associated with the avoidance of blood products is greater than the risk from the medication itself. In the latter case, the use of medications that carry a high risk of causing adverse effects is more acceptable if the aim is to prevent death from uncontrolled bleeding.
Avoiding the transfusion of human blood products seems to be a fundamentally good notion. However, many common ideas about the potential harm of transfusion may be mistaken. An audit of the Serious Hazards of Transfusion (SHOT) organization in the United Kingdom reported 3239 adverse events over a 10-year period; this is only 0.013% of all blood transfusions. These adverse events were more common in infants but were reported in only 0.037% of infants who had transfusions. The true incidence of events is likely to be more common because of underreporting, though many of these “events” were procedural errors not associated with actual harm. It is clear that the risk of immediate harm directly attributable to transfusion is exceptionally low. The risk of direct transmission of infection (the most feared risk in the public perception) is particularly small; the risk of transmission of HIV from transfusion of blood in the United Kingdom is 1 in 5 million. The greatest hazard of immediate harm is transfusion of incompatible blood. However, subtle negative effects of transfusion on children's outcomes may be considerably more important. Transfusion of blood may lead to deterioration in pulmonary function and immunologic effects that predispose children to infection. In addition, if the objective of transfused red cells is to boost tissue oxygenation by improving oxygen-carrying capacity, then transfused blood is less effective than the child's own blood for this purpose. Additional considerations include the increasing costs of transfused blood products, the logistical difficulties of maintaining a secure blood supply (in both well-resourced and less well-developed health systems), substantially greater risks of transfusion in poorly developed health systems, and the possibility of new, unrecognized, infective agents entering the blood supply.
There are 2.3 million transfusions each year (37 per 1000 population) in the United Kingdom; 4.2% are given to children younger than 18 years of age (7.1 per 1000) and 1.7% to infants less than one year of age (52 per 1000). An audit of pediatric blood transfusion from Australia revealed 41% of blood transfused was given perioperatively, and blood was transfused during 6.3% instances of anesthesia. The majority of units were used during heart surgery (58% of perioperative use), while a very small minority, 4%, were used during major trauma surgery. Heart surgery (on and off bypass), craniosynostosis surgery, and liver transplantation were all commonly associated with blood transfusion. The epidemiology of major bleeding is less certain. Reports of blood use may be misleading (e.g., neonates undergoing cardiac surgery to correct congenital defects may receive few blood units despite significant bleeding), and blood is also used for purposes such as priming the bypass circuit. Observational studies have reported very heavy blood loss associated with heart surgery in children, with increased (per kilogram) blood loss in smaller children and more complex surgery. Cardiac surgery can probably be considered the main cause of major blood loss in children and account for most severe bleeding events in children younger than 1 year.
Aprotinin, recombinant factor VIIa (rFVIIa), and fibrinogen concentrate have all been proposed as “magic bullets” able to restore hemostasis at some stage. However, all of these agents have proved to have limitations; each is of value when they address a specific deficit in coagulation. In many respects, the management of severe bleeding has not greatly changed in recent years. Newer approaches are now emerging, although at the time of writing, robust evidence of their risks and benefits for children is not available. Optimal management of severe bleeding requires the following:
Good surgical hemostasis
Adequate preparation, including availability of blood products and the means to administer them
Targeting of coagulation defects that are either likely (given the clinical context) or have been identified by appropriate coagulation tests
Avoidance (and recognition) of hypothermia, acidosis, and electrolyte disturbance
The understanding of coagulation has shifted greatly in the past few years. The “traditional” model, which emphasizes the importance of a cascade of proteolytic enzymes, has given way to the “cell-based” model of coagulation that emphasizes the importance of cellular elements in coagulation and presents coagulation as a complex web of interactions rather than a linear process. This complexity can be confusing and abstract compared with the decisions made in clinical care. However, there are lessons that can be drawn from this, and a basic knowledge of current models of coagulation is of value.
To achieve effective hemostasis, a platelet plug must form at the site of vessel injury. To prevent widespread thrombosis, this process needs to remain localized to the injured site. This is achieved through changes at the cell surface and localization of the procoagulant reactions on the surfaces of specific cells. Different cells possess different procoagulant and anticoagulant properties; these are incompletely understood, but platelets and cells bearing tissue factor (TF) are central to the process. Endothelium is vital to inhibition of coagulation, both by forming a physical barrier between components of the coagulation system (principally preventing activated factor VII and platelets from contacting collagen and TF bearing cells) and by playing a more active role in the expression of inhibitory proteins such as thrombomodulin (TM). The phases of coagulation are often described as initiation, amplification, and propagation, though in reality there is considerable overlap.
Coagulation is initiated by an interaction between circulating factor VIIa and TF (a membrane-bound lipoprotein expressed on subendothelial cells such as fibroblasts). A complex is formed between TF and factor VIIa (TF/VIIa), which activates factors IX and X. Factor Xa, in association with cofactor Va, also forms “prothrombinase” complexes on the surface of the TF-bearing cell, which activates a small amount of thrombin (factor IIa) ( Fig. 20.1 ), leading to activation of platelets and factors V and VIII.
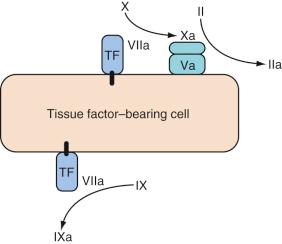
This low level of thrombin production occurs constantly and is not sufficient to initiate widespread clot formation. Inhibitors, such as TF pathway inhibitor (TFPI) and antithrombin (AT), provide a localizing function on factor Xa by inhibiting any factor Xa that becomes dissociated from the TF-bearing cell.
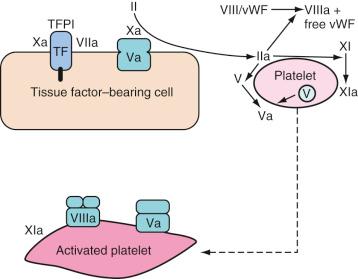
More extensive damage to the vasculature allows greater interaction between TF and factor VIIa and contact between platelets and extravascular components (including collagen and von Willebrand factor [vWF]). There is a strong positive interaction between these two processes that leads to recruitment of further platelets and production of thrombin in large quantities.
Small quantities of thrombin are generated on the TF-bearing cells. This sets up the subsequent propagation phase, during which thrombin is generated in larger quantities ( Fig. 20.2 ). This thrombin has several functions:
Activation of platelets, exposing receptors and binding sites for clotting factors
Activation of cofactors V and VIII on the activated platelet surface, thereby releasing vWF to mediate additional adhesion and aggregation at the injury site
Activation of factor XI to XIa
Activation of factor XIII (fibrin-stabilizing factor) and promotion of fibrin cross-linking
Cleaving fibrinopeptides A and B from fibrinogen (forming fibrin)
The concept of platelet activation is important. Circulating platelets are discoid in shape. Upon activation, they change shape dramatically to increase their surface area, increase expression of a variety of receptors and binding proteins, and release a series of chemicals (including clotting factors and platelet activators). These complex intracellular changes are central to normal coagulation and alterations in this process are central to the coagulopathy seen during surgery and major bleeding. Once formed into a clot, the platelets will undergo a further change spreading out to form a physical plug.
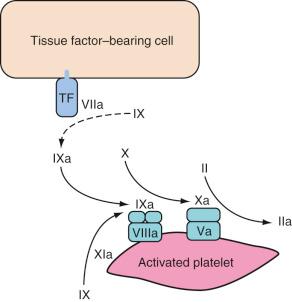
Propagation occurs on the surface of activated platelets that are recruited to the site in large numbers. Activated factor IX (from both initiation phase and provided by factor XI on the platelet) binds to factor VIIIa. The resultant IXa/VIIIa complex activates factor X on the platelet surface. This factor Xa associates with factor Va and forms the prothrombinase complex. The prothrombinase complex causes a “burst” of thrombin generation to cause clotting via fibrinogen ( Fig. 20.3 ). An inability to form the IXa/VIIIa complex and therefore produce this sustained burst in thrombin production explains the bleeding tendency of children with hemophilia A.
Traditional models of coagulation have also described an alternative pathway initiated by contact factors (XII, XI, prekallikrein, and high–molecular-weight kininogen [HMWK]). This pathway is of no physiologic importance in terms of coagulation activation; however, it provides important acceleration loops through feedback activation of factors VIII, IX, and XI and is important in fibrinolytic and inflammatory pathways.
The clot is confined to the site of injury by direct and indirect thrombin inhibitory systems. The direct system consists of AT, α 2 -macroglobulin, and heparin cofactor II (HCII). AT and HCII activities are accelerated in the presence of heparin.
Several indirect systems inhibit thrombin, including the protein C-protein S-TM system and TFPI. Thrombin binds to TM on the surface of intact endothelial cells and can no longer cleave fibrinogen to form fibrin. The TM/thrombin complex is neither able to activate platelets nor activate factors V and VIII. Instead, this complex activates protein C, which binds to the cofactor protein S and inactivates factors Va (on the surface of endothelial cells and platelets) and VIIIa.
Fibrinolysis, the breakdown of fibrin into soluble degradation products, is mediated by the proteolytic enzyme, plasmin. Plasmin is formed from an inactive zymogen, plasminogen, which is produced in the liver. This process is controlled by activators and inhibitors. The principal intravascular plasminogen activator is tissue plasminogen activator (tPA). The main inhibitory proteins are plasminogen activator inhibitor-1 (PAI-1), antiplasmins (α 2 -PI and α 2 -macroglobulin), and thrombin-activated fibrinolysis inhibitor (TAFI) ( Fig. 20.4 ).
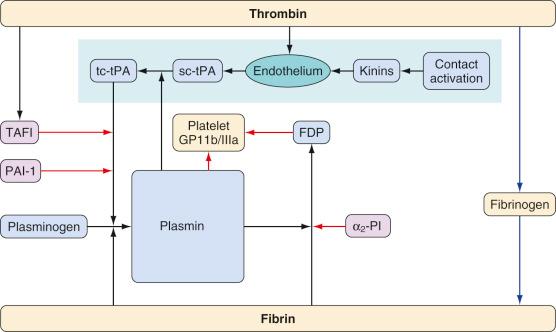
In addition to cleaving fibrin, plasmin metabolizes a number of other proteins, including the platelet receptor for fibrinogen (glycoprotein IIb/IIIa) and fibrinogen. Furthermore, plasmin accelerates its own production by metabolizing the conversion of single-chain plasminogen activators to more active two-chain versions. The action of plasmin on fibrin produces a series of degradation products, some of which convey anticoagulant properties; this effect is achieved by preventing polymerization of fibrinogen and by inhibition of platelet function.
tPA is released by vascular endothelium of small blood vessels. Release is increased in the presence of stimuli such as trauma, endotoxins, ischemia, or normal exercise. This effect is mediated via contact activation (through the kallikrein system) and by a series of other substances, including thrombin. Once released, tPA is rapidly metabolized by the liver with a half-life of approximately 5 minutes. Fibrin binds both plasminogen and tPA and greatly accelerates the conversion of plasminogen to plasmin (facilitating its own degradation, but also localizing the process to areas of clot). An alternative mechanism for plasminogen activation by tPA exists through binding to receptors expressed by certain cells (endothelium, white cells, and some tumor cells); the importance of this is unclear.
Excessive fibrinolysis can directly result from excess production of fibrin, as in disseminated intravascular coagulation. This is termed secondary hyperfibrinolysis and in this context, the fibrinolysis is considered beneficial because it prevents widespread vascular occlusion. Therapy is directed at replacement of consumed clotting factors, inhibition of excessive coagulation, and treatment of the underlying cause. Primary hyperfibrinolysis can occur during cardiac bypass, massive blood loss, trauma, and liver transplantation. During the anhepatic stage of liver transplant surgery, there is hyperfibrinolysis because of failure to metabolize tPA. On reperfusion of the liver, a further surge of tPA occurs that can take several hours to return to normal. In coagulopathic patients, reduced thrombin formation may lead to reduced production of TAFI (important in inhibition of membrane-bound plasmin), whereas conversion of single- to two-strand tPA by plasmin may further sustain the process. Individual susceptibility is likely to be important and may have a genetic component.
Two groups of drugs are used clinically to inhibit fibrinolysis:
Synthetic lysine analogs—for example, tranexamic acid (TXA) and ε-aminocaproic acid (EACA)
Protease inhibitors (e.g., aprotinin)
Synthetic lysine analogs are specific inhibitors of plasminogen activation, working by competitively binding to lysine-binding sites on the plasminogen molecule (see Fig. 20.5 ). This blocks the binding of plasminogen to fibrin, a required step for the conversion of plasminogen to plasmin by plasminogen activators. At larger doses, they may have additional effects through direct inhibition of plasmin; this includes inhibition of the plasmin-mediated effects on platelets.
Aprotinin is a less specific inhibitor of proteolytic enzymes; it has actions on the kallikrein-kinin (contact) system, as well as the enzymes involved in coagulation and fibrinolysis. In addition, aprotinin may be associated with greater preservation of platelet function, as well as an antiinflammatory effect. This wider spectrum of effects from aprotinin may have additional benefits over the antifibrinolytic lysine analogs.
The hemostatic system in the neonate rapidly matures toward that of the adult (see also Chapter 10 ). Understanding the rate at which the coagulation system matures with age in childhood is necessary to correctly interpret coagulation tests and to select appropriate interventions to manipulate hemostasis in vivo.
All fetal coagulation factors are produced independently of the mother; fibrinogen starts to be formed as early as 5.5 weeks gestation, and blood can clot at 11 weeks. The development of microassay techniques in the 1980s facilitated the determination of reference ranges for the coagulation factors beginning at 19 weeks gestational age. In general, there are four fundamental differences between the coagulation systems in the infant and the adult :
Concentrations of components of the hemostatic system
The turnover rate of coagulation proteins
The rate of synthesis
Differences in the overall ability to generate and regulate the key enzymes: thrombin and plasmin
The concentration of vitamin K–dependent factors II, VII, IX, and X in the neonate are only 50% those of adult values; this leads to a slightly prolonged prothrombin time (PT) or international normalized ratio (INR). Similarly, the concentrations of contact factors HMWK, prekallikrein, and factors XI and XII are approximately 50% of adult values. The reduced contact factors account for a disproportionally prolonged activated partial thromboplastin time (aPTT). The reduced concentration of factors at birth is probably explained by the reduced synthesis by the liver; however, concentrations increase rapidly after birth, reaching approximately 80% of adult values by 6 months of age.
In contrast, the plasma concentrations of fibrinogen and factors V and VIII at birth are similar to those in adults, although the fetal form of fibrinogen differs in structure from that in the adult; the physiologic significance of this is not clear. Concentrations of vWF in the first 2 months of life are greater than those in adults.
The inhibitor systems of coagulation also differ from adults. At birth, plasma proteins C and S are 35% of adult values and do not reach adult values until adolescence. Neonatal concentrations of AV and HCII are 50% of adult values; they reach adult concentrations by 6 months of age. The concentration of α 2 -macroglobulin, however, is increased at birth and remains increased throughout childhood. It has been postulated that this may be one of the mechanisms that protects young children from thromboembolic complications. Thrombin generation in vitro is reduced in children to approximately 75% of adult values.
Despite reduced plasma concentrations of many procoagulant and anticoagulant proteins in infants, there still appears to be an effective hemostatic balance; healthy fetuses, neonates, and children do not suffer excessive hemorrhage in the presence of minor challenges. This is consistent with the thromboelastogram (TEG) studies of healthy children younger than 2 years of age; no defects in coagulation were noted using this test compared with adults, indicating an intact hemostatic system. A further study, conducted using TEG, reported that infants younger than 1 year with complex congenital heart disease have an intact and balanced coagulation-fibrinolytic system but at a “lower level” than healthy children.
The hemophilias are a group of genetic diseases that cause excessive bleeding, often in response to minor trauma. Hemophilia A and von Willebrand disease are the most common variants (see also Chapters 10 and 12 ), associated with low levels of factor VIII and vWF, respectively. A wide range of single-gene defects resulting in deficiencies of single clotting proteins or regulatory proteins has now been described. A further group of single-gene disorders may result in thrombophilic disorders, associated with abnormalities of inhibitory proteins.
Unexplained variation has been observed in bleeding between apparently similar patients in the absence of specific factor deficiency. The causes of this variation are likely to be multifold, according to nuances of surgical technique and subtle difference in disease process and therapy. It might appear counterintuitive that genetic factors have a significant role in acquired bleeding resulting from surgery; however, recently there has been increased interest in the interplay of genetic and environmental factors in the progress of acquired diseases. The genetics of most clotting proteins has been described, and common variations within populations have been revealed for some. Of these, a common polymorphism of PAI-1 has been well described. PAI-1 is an important endogenous inhibitor of fibrinolysis, and deficiency is associated with increased bleeding. The G5 / G5 polymorphism is common (about 20% in European populations) and is associated with reduced concentrations of PAI-1. It has been linked (though not consistently) to bleeding after heart surgery and to increased benefit from use of antifibrinolytics. Should these findings be substantiated, then PAI-1 may still be an exceptional example. In general, the influence and consequences of genetic determinants of bleeding are likely to be more subtle. In infancy, an additional factor may be the relationship between developmental and genetic factors. Many clotting proteins are present in infants as isoforms distinct from those in adults; this implies a different gene expression in the young. It is possible that understanding genetically determined variations in bleeding will increase our understanding of bleeding and allow us to guide therapy for the individual child. The practical applications of such observations remain speculative.
Bleeding is an inevitable consequence of invasive surgery. Severe bleeding can be associated with derangement of coagulation, which may increase the severity of bleeding or, alternatively, may put the child at risk for thrombosis. These two adverse events are not mutually exclusive: patients who bleed more and who demonstrate coagulopathic bleeding may also be at increased risk of thrombosis.
Coagulation changes during major surgery and bleeding are complex and depend on the clinical context in which bleeding occurs. Coagulation changes that occur in surgical patients have some similarities to those who present after severe trauma. However, the balance of pathophysiologic factors is likely to be very different. The factors underlying these coagulation changes include the following:
Dilution. Components of the coagulation system are lost in shed blood. The volume of blood lost is then replaced by crystalloid, colloid, or blood products that lack these components, leading to progressively smaller concentrations of these coagulation components. To some degree such changes are balanced as the concentration of coagulation inhibitors also decreases. In addition, coagulation components may be produced or released in response to trauma, which limits the reduction in concentration. To complicate this issue, a reduction in the concentration of one component of the coagulation system may not have the same clinical effect as the same reduction of another component. For example, substantial decreases in the concentration of many clotting proteins will not result in severe bleeding, whereas even modest decreases in the number of platelets or the fibrinogen concentration may cause clinically noteworthy bleeding (see also Chapters 10 and 12 ).
Effect of tissue damage. Extensive interactions occur between inflammatory and coagulation pathways. Inflammation following trauma to tissues can be linked to excess activation of fibrinolytic pathways (resulting in excess bleeding) and to activation of procoagulant pathways (resulting in increased risk of thrombosis).
Physiologic derangement associated with blood loss. Acidosis, hypothermia, and hypocalcemia are associated with excess bleeding. Hypothermia slows proteolytic enzyme activity, reduces fibrin synthesis, and reduces platelet function. These effects are largely reversible on rewarming. Acidosis markedly reduces the activity of coagulation proteins. These effects are not fully reversed by correcting acidosis. Dilution and the effect of citrate (contained in many blood products) cause the plasma calcium concentration to decrease. During major bleeding, the concentration of calcium ions should be monitored and replaced as needed. Cryoprecipitate and fresh frozen plasma (FFP) contains high concentrations of citrate and rapid administration may cause a precipitous decrease in the plasma concentration of calcium ( Fig. 12.9 ).
Effects of treatment. The use of some synthetic colloids may worsen bleeding to an extent greater than might be expected by dilution. The use of hydroxyethyl starch increases the risk of coagulation abnormalities and of acute kidney injury when compared with albumin, gelatins, or crystalloids. Whether differences exist in coagulation effects of different starches is controversial. Concerns about safety of starch solutions have limited their use in adults and children.
Use of specific techniques during surgery. The important effects of cardiac bypass and anticoagulation are discussed later. Liver transplant surgery (see also Chapter 31 ) and major trauma (see also Chapter 39 ) are discussed in detail elsewhere in this book.
Without anticoagulation, blood will rapidly form thrombi on the artificial surface of the cardiopulmonary bypass (CPB) circuit. The major mechanism for activation of the coagulation cascade during CPB is thought to be the “extrinsic” TF pathway, which is activated as a result of surgical trauma and inflammation. Inflammatory mediators induce expression of TF on endothelial cells and monocytes. The “intrinsic” coagulation system is also activated when factor XII is adsorbed onto the surface of the CPB circuit, causing activation of complement, neutrophils, and the fibrinolytic system via kallikrein. Although the intrinsic system has little role in initiating coagulation, activations of kinins will lead to increased fibrinolysis and inflammation.
Heparin remains the most effective anticoagulant used to facilitate CPB. The binding of heparin to lysine sites on AT causes a conformational change in AT. This results in an increase in AT potency; the inhibition of thrombin and factors IXa, Xa, XIa, and XIIa is increased by a factor of 1000. In infants, AT concentrations are low until 3 to 6 months of age and other heparin cofactors may have greater importance. However, neonates who require surgery for congenital heart disease have unusually reduced concentrations of all major heparin cofactors, and this may be one reason for the greater concentrations of thrombin produced in these patients.
Heparin therapy is most frequently guided by the activated clotting time (ACT). The ACT is an inexpensive and rapid on-site test in which a small sample of blood is mixed with a coagulation activator such as celite, kaolin, or diatomaceous earth. The ACT is the time to produce a stable clot, with a normal value being between 80 and 140 seconds. A value greater than 400 seconds is required for CPB.
There are limitations to the use of the ACT. First, the ACT is altered by hypothermia, hemodilution, platelet activation, activation of the hemostatic system, and aprotinin therapy. Accordingly, it does not accurately reflect the heparin concentrations. One study reported that the heparin concentration decreased by 50% as soon as the children went on CPB, even though the ACT doubled. The decrease in the heparin concentration was attributed to hemodilution. Second, in the bleeding child, the ACT is unable to differentiate bleeding because of excess heparin from that of other acquired hemostatic defects. The gold standard for measurement of heparin concentration is the antifactor Xa assay; however, this test remains too cumbersome for routine clinical use. An alternative point-of-care test is protamine titration (Medtronic Hepcon HMS Plus, Medtronic, Minneapolis, MN). In adult patients, use of this system reduces thrombin formation. Reduced bleeding and reduced thrombin generation in children, as well as reduced thrombin formation in infants, have also been demonstrated with this system. However, the accuracy of the device has been questioned and a trial, in infants, was terminated early when increased bleeding and increased length of stay were demonstrated in children in whom the Hepcon device was used. The device underestimated heparin concentration in this group, leading to excess dosing of heparin and inadequate reversal with protamine. After modification of their protocol, use of the device demonstrated reduced bleeding, length of stay, and reduced thrombin formation compared to standard treatment. Agreement between protamine titration, measures of heparin concentration, and laboratory measures has been demonstrated.
A common feature of the pediatric studies using the Hepcon device is increased heparin use compared with regimens based on units per kilogram kg dosing or ACT. This is consistent with other studies of traditional dosing regimens. Using common pediatric heparin regimens (300 units/kg before CPB, then 100 units/kg to keep the ACT above 450 seconds), 50% of children on CPB had low levels of heparin (<2 units/mL). It is suggested that reduced heparin concentrations during CPB is a major factor responsible for activation of coagulation and fibrinolysis. It is likely that widely used regimens for dosing of heparin in children lead to inadequate dosing, and that units per kilogram kg dosing fails to allow for important pharmacokinetic (PK) and pharmacodynamic (PD) differences in children.
Even effective dosing of heparin does not completely abolish the production of thrombin. Low-grade ongoing thrombin production leads to ongoing activation of the coagulation cascade, platelets, fibrinolysis, and the endothelium. The continued thrombin generation and activity during CPB reflects the inability of the heparin/AT complex to inactivate fibrin-bound thrombin or to inhibit thrombin-induced platelet activation. Theoretically, direct thrombin inhibition may be free of these limitations. Practically, the use of the current generation of thrombin inhibitors (such as hirudin and bivalirudin) is limited because of a lack of effective monitoring and reversal. Currently few reports exist of the use of thrombin inhibitors in children, although they would be indicated when heparin use is not possible.
Adverse effects of heparin are uncommon; hypotension can result from a reduction in calcium ions or, rarely, anaphylaxis. A benign transient decrease in the platelet count can occur. Heparin-induced thrombocytopenia is a rare but life-threatening prothrombotic condition.
Protamine is a positively charged polypeptide derived from salmon sperm. It neutralizes heparin by forming an ionic bond with heparin. The resultant complex is removed by the reticuloendothelial system. The most appropriate dosing regimen has yet to be determined. Current dosing used in pediatric practice fails to take into account the range of concentrations of heparin that occur in infants and children. The administered heparin dose is often used to guide the dose of protamine; however, it is unclear how this should be modified by various factors, such as additional doses of heparin administered (to prime or during bypass), duration of bypass, ultrafiltration, or developmental coagulation differences in children.
Excessive protamine has been associated with catastrophic pulmonary hypertension and hemorrhagic pulmonary edema. It is also known that protamine can be associated with coagulation abnormalities; an increasing ACT occurs at a protamine/heparin ratio of 2.6 : 1, and platelet aggregation occurs with a minimal excess in protamine. Although some studies of regimens to titrate protamine in adults demonstrated encouraging results in terms of reduced bleeding, others failed to demonstrate differences in transfusion requirements.
The clearance of protamine is greater than that of heparin, and “heparin rebound” is described when tissue-bound heparin redistributes. The diagnosis of residual heparin effect or heparin rebound is challenging. The ACT is not a specific measure of excessive heparin and is also poor at detecting heparin at low concentrations (<0.5 unit/mL). The aPTT and PT are similarly nonspecific and may be increased after CPB in the absence of heparin. An unmodified TEG does not reliably detect heparin rebound if the heparin concentration is small. The sensitivity of these tests may be improved by performing similar tests in parallel and comparing the results, such as a reptilase time (unaffected by heparin), or by eliminating residual heparin in vitro with heparinase or protamine. Protamine titration can be used to guide the protamine dose in children; however, in infants, protocols will require modification (50% greater than the calculated dose). In practice, most anesthesiologists continue to give protamine empirically at a protamine/heparin ratio of between 1 and 1.3 to 1. The author's current practice is to give a standard dose of protamine (4 mg/kg) regardless of heparin dose and to give a further 2 mg/kg in the presence of continued bleeding or unusually high ACT. In the presence of continued bleeding, a TEG is run both with and without heparinase to exclude residual heparin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here