Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Normal cerebral autoregulation maintains stable cerebral blood flow over a range of systemic perfusion pressures and metabolic demands.
Atherosclerosis is an inflammatory disease characterized by the progressive deposition of atheromatous plaques that narrow affected arteries.
The prevalence of moderate to severe carotid stenosis is common, and duplex ultrasonography is the most common initial screening method.
Antihypertensive, statin, and antiplatelet medications are recommended for the medical treatment of extracranial carotid and vertebral atherosclerosis.
Carotid endarterectomy (CEA) is indicated for symptomatic patients with average to low surgical risk with internal carotid artery stenosis > 70%. Carotid artery stenting is an alternative to CEA for symptomatic patients with operative comorbidities, contralateral carotid occlusion, radiation-induced stenosis, or recurrent stenosis after previous CEA.
CEA is indicated in asymptomatic patients with > 60% stenosis and a low risk of perioperative complication.
Symptomatic steno-occlusive disease of the vertebral artery is less common than carotid artery stenosis and portends a high risk of ischemic events.
Medical management with antiplatelet therapy is the mainstay of treatment for intracranial atherosclerotic disease.
Surgical revascularization is the preferred method of treatment of symptomatic moyamoya disease.
Screening protocols for traumatic blunt cerebrovascular injury are cost effective for preventing stroke.
Approximately 780,000 people each year experience a new or recurrent stroke in the United States; approximately 87% are ischemic. Stroke is the third leading cause of death behind heart disease and cancer and a leading cause of disability. Neurologic symptoms from a transient ischemic attack (TIA) or ischemic stroke occur due to arterial occlusion, and the symptomatology depends on the location, duration, and severity of occlusion as well as presence of collateral supply and compensatory flow. Chronic steno-occlusive disease can lead to compensatory flow from nearby arterial territories and adequate perfusion of the affected territory under normal conditions due to the development of arterial collaterals. In acute steno-occlusive disease, neurologic symptoms may develop unless immediate collateral flow is available.
Cerebrovascular occlusive disease is a broad category of pathology involving the extracranial and intracranial vasculature of the carotid and vertebral arteries. These vascular territories are afflicted by a variety of pathologic mechanisms including atherosclerotic disease, moyamoya vasculopathy, and dissection, all of which can cause a disabling or fatal stroke. Treatment options include medical, surgical, and endovascular therapy alone or in combination and can be directed at the prevention of initial stroke in an asymptomatic patient or prevention of future events in a symptomatic patient. The goal of this chapter is to cover this spectrum of steno-occlusive disease commonly treated by neurologic surgeons and to outline areas of controversy.
The prevalence of moderate to severe carotid stenosis (ie, luminal stenosis > 50%) occurs in 6% of men > 70 years old regardless of a history of coronary artery or cerebrovascular disease. In the Framingham Study, the prevalence of > 50% carotid stenosis was 7% in females and 9% in males based on duplex ultrasonography. Extracranial atherosclerotic disease accounted for 17% of ischemic strokes in the Northern Manhattan Stroke Study.
The progression of carotid atherosclerosis from plaque growth, increasing stenosis, and resultant ischemic symptoms (TIA or stroke) is complex. In the North American Symptomatic Carotid Endarterectomy Trial (NASCET), the degree of stenosis (as measured by ultrasonography) correlated with the risk of stroke in symptomatic patients, from 19% with 70% to 79% stenosis to 33% with 90% to 99% stenosis after 18 months of medical therapy. In asymptomatic patients the relationship between stroke risk and degree of stenosis was less clear, as both the Asymptomatic Carotid Atherosclerosis Study (ACAS) and the Asymptomatic Carotid Surgery Trial (ACST) showed higher stroke rates with 60% to 80% stenosis than stenosis > 80%. With advances in medical therapy, ipsilateral stroke rates in asymptomatic patients with > 50% carotid artery stenosis are 0.3% to 1% per year.
TIA is defined as a syndrome of acute neurologic dysfunction due to the distribution of a single artery with symptom duration < 24 hours although the typical symptom duration is < 15 minutes. Patients with acute ischemic stroke have symptoms lasting > 24 hours. TIA predicts stroke with a 5% incidence in the first week, 13% in 90 days, and 30% at 5 years. The benefit of surgical revascularization diminishes after 2 weeks due to the high rate of early recurrent ischemia. After 4 weeks in females and 12 weeks in males, the benefit of revascularization for symptomatic patients is similar to asymptomatic patients, and it may be harmful.
Atherosclerosis is an inflammatory disease characterized by the progression deposition of atheromatous plaques. Risk factors include cigarette smoking, diabetes, hypertension, and hypercholesterolemia. Plaque growth leads to steno-occlusive disease and typically occurs at an arterial bifurcation. The bifurcation creates shear stress from turbulent flow, which causes endothelial damage and focal inflammation. Initially, lipoprotein particles accumulate on the intimal lining and undergo oxidative modification, which facilitate uptake by monocytes and migration into the arterial wall. Monocytes become lipid-laden macrophages as modified lipoproteins accumulate, which release additional inflammatory mediators. As a result, smooth muscle cells migrate from the media to the intima where they proliferate and produce extracellular matrix. Meanwhile, extracellular lipid accumulates in a central core surrounded by a fibrous cap of connective tissue. This fibrous cap can become calcified over time. The lesion initially grows outward from the lumen, but as the plaque grows, the lumen is narrowed and causes stenosis. Disruption of the plaque can result from rupture or erosion of the fibrous cap, which initiates the coagulation cascade and thrombus formation. Thrombus formation and intraplaque hemorrhage further contribute to progressive plaque expansion and luminal narrowing.
Stroke and TIA result from multiple mechanisms. Embolism of thrombus formed on a plaque can occlude distant arteries. Atheromatous debris such as cholesterol crystals can embolize to retinal arteries (ie, Hollenhorst plaques). Plaque rupture can lead to acute occlusion of the artery. Dissection or subintimal hematoma can result from structural weakening of the arterial wall and exposure to thrombogenic subintimal tissue. Chronic, progressive plaque growth can reduce blood flow and hence cerebral perfusion.
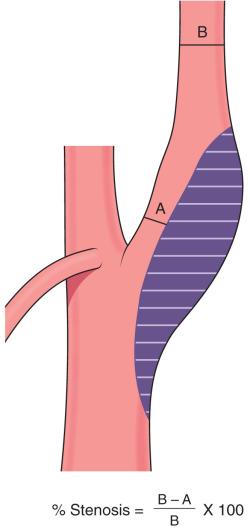
Duplex ultrasonography, computed tomography angiography (CTA), and magnetic resonance angiography (MRA) provide information about the plaque and degree of stenosis and help guide therapy. Duplex ultrasonography is typically the initial screening method and characterizes both macroscopic plaque appearance and flow characteristics. The NASCET and the European Carotid Surgery Trial (ECST) determined carotid stenosis severity on catheter-based angiography. By NASCET criteria (most commonly used in clinical or research settings), the percentage of carotid stenosis is defined by the following formula: % stenosis = (normal distal cervical internal carotid artery [ICA] diameter − narrowest ICA diameter/normal distal cervical carotid diameter) × 100 ( Fig. 15.1 ). Plaques in carotid vessels are divided into four types: type 1 is predominantly hemorrhage, lipid, cholesterol, and proteinaceous material; type 2 is dense fibrous connective tissue with > 50% volume of hemorrhage, lipid, cholesterol, and proteinaceous material; type 3 is dense fibrous connective tissue with < 50% volume of hemorrhage, lipid, cholesterol, and proteinaceous material; and type 4 is dense fibrous connective tissue.
The Society of Radiologists in Ultrasound consensus established recommendations for the diagnosis and stratification of carotid stenosis based on ultrasonography. Table 15.1 includes the criteria for peak systolic velocity (PSV) and end diastolic velocity (EDV) for the ICA and common carotid artery (CCA). CTA and MRA are generally reserved for hemodynamically significant stenosis and treatment planning. In cases of discordant findings or extensive calcification, catheter-based angiography may be necessary. A meta-analysis of MRA characterization of carotid plaque found that intraplaque hemorrhage, lipid-rich necrotic core, and thinning/rupture of the fibrous cap are associated with an increased risk of future TIA or stroke. Asymptomatic patients with > 60% carotid stenosis with > three plaque ulcerations may be nine times more likely to develop stroke or death in 3 years compared to those without ulcerations (18% vs 2%, p = .03) as evaluated by three-dimensional ultrasound. In the same study, patients with microembolic signals on transcranial Doppler (TCD) had a significantly higher 3-year risk of stroke or death compared to those without microemboli (20% vs 2%, p = .003). The Asymptomatic Carotid Emboli Study (ACES) also demonstrated that asymptomatic patients with > 70% carotid stenosis and microemboli are at higher risk of ipsilateral stroke and TIA at 2 years (7.13% vs 3.04%, hazard ratio [HR] 2.54) and for ipsilateral stroke alone (3.62% vs 0.7%, HR 5.57). As such, asymptomatic patients with microemboli or evidence of infarction on MRI are at higher risk of stroke or TIA and may be considered symptomatic for the purposes of risk stratification and treatment strategy.
| Diagnosis | Criteria |
|---|---|
| Normal | ICA PSV < 125 cm/sec and no plaque or intimal thickening, ICA/CCA PSV ratio < 2 and ICA EDV < 40 cm/sec |
| <50% ICA stenosis | ICA PSV < 125 cm/sec with visible plaque or intimal thickening, ICA/CCA PSV ratio < 2 and ICA EDV < 40 cm/sec |
| 50%–69% ICA stenosis | ICA PSV 125-230 cm/sec with visible plaque, ICA/CCA PSV ratio 2–4 and ICA EDV 40–100 cm/sec |
| ≥70% ICA stenosis but less than near occlusion | ICA PSV is > 230 cm/sec and visible plaque and luminal narrowing, ICA/CCA PSV ratio > 4 and ICA EDV > 100 cm/sec |
| Near occlusion of the ICA | Velocity may be high, low, or undetectable and parameters may not apply, diagnosis established primarily by markedly narrowed lumen |
| Total occlusion of the ICA | No detectable patent lumen and no flow |
Recommendations for medical therapy were published in the 2011 multisociety guidelines for extracranial carotid and vertebral atherosclerosis and are class I recommendations unless indicated. Antihypertensive treatment is recommended to maintain a blood pressure below 140/90 mm Hg in asymptomatic patients. Except in hyperacute period, antihypertensive treatment is probably indicated for symptomatic atherosclerosis, but a target has not been established due to risk of exacerbating cerebral ischemia (class IIa). Treatment with statin medications is recommended for asymptomatic and symptomatic patients to reduce low-density lipoprotein (LDL) below 100 mg/dL and is reasonable to consider below 70 mg/dL (class IIa). Diet, exercise, and glucose-lowering drugs are useful for patients with diabetes mellitus; however, the stroke prevention benefit for aggressive therapy to maintain a glycosylated hemoglobin A1c level has not been established (class IIa). In patients not undergoing revascularization, antiplatelet therapy with aspirin (75 to 325 mg daily) is recommended to prevent ischemic cardiovascular disease but has not been established for prevention of stroke in asymptomatic patients; however, in symptomatic patients, aspirin alone, clopidogrel alone (75 mg), or aspirin in combination with extended-release dipyridamole is recommended and preferred over the combination of aspirin and clopidogrel. Antiplatelet agents are recommended rather than oral anticoagulants (class I), unless a contraindication to antiplatelet medications exists or there is an indication for anticoagulation (class IIa). Hyperhomocysteinemia, physical inactivity, and obesity are risk factors for stroke, but specific recommendations were not made. Notably, early trials comparing medical therapy alone to revascularization with medical therapy included the use of antiplatelet agents alone and did not include statin medications or other medical interventions currently considered standard of care. A meta-analysis for asymptomatic carotid stenosis showed that annual ipsilateral stroke rates were 2.38% before 2000 and decreased to 1.13%.
In 2011, the multisociety guidelines for revascularization were published ( Table 15.2 ).
| Recommendation | Description |
|---|---|
| Class I | Carotid endarterectomy (CEA) is indicated for symptomatic patients (ie, stroke or TIA within 6 months) with average to low surgical risk with ipsilateral ICA stenosis > 70% by noninvasive imaging or > 50% by catheter-based angiography and < 6% perioperative risk of stroke or death. Carotid artery stenting (CAS) is an alternative to CEA for symptomatic patients meeting aforementioned criteria at average to low risk of complication with endovascular intervention. Selection of asymptomatic patients is guided by assessment of comorbidities, life expectancy, and other individual factors. |
| Class IIa | CEA is reasonable in asymptomatic patients with > 70% stenosis and low risk of perioperative complication, CEA is reasonable over CAS when indicated in older patients and those with unfavorable anatomy for endovascular treatment, CAS is reasonable in patients with unfavorable neck anatomy, and revascularization in patients with TIA or stroke within 2 weeks is reasonable unless contraindicated. |
| Class IIb | Prophylactic CAS may be considered in patients with > 60% angiographic stenosis or > 70% by ultrasound, but effectiveness has not been established compared to medical therapy alone and in symptomatic and asymptomatic patients at high risk of complication by CEA or CAS, the effectiveness of revascularization over medical therapy alone is not well established. |
| Class III | CEA or CAS is not recommended in patients with < 50% stenosis, chronic total occlusion of the ICA, or in patients with severe disability. |
In symptomatic patients, NASCET and ECST demonstrated the efficacy of carotid endarterectomy (CEA) over medical therapy alone to reduce ipsilateral stroke in patients with > 70% carotid stenosis. NASCET randomized 328 patients to CEA and 331 to medical therapy before the study was stopped after 18 months of follow-up. In patients with 70% to 99% stenosis undergoing CEA, the rate of 30-day perioperative stroke and mortality was 2.1%, and the cumulative risk of ipsilateral stroke at 2 years including perioperative events was 9%. In patients assigned to medical therapy alone, the cumulative risk of ipsilateral stroke at 2 years was 26%, leading to an absolute risk reduction (ARR) of 17% in favor of CEA. NASCET investigators also demonstrated a benefit for patients with 50% to 69% stenosis; however, the perioperative rate of stroke or death was 6.7%.
ECST randomized 2518 patients over a 10-year period and found a benefit for CEA in patients with 70% to 99% stenosis (remeasured by NASCET criteria) including an ARR of ipsilateral stroke or death of 18.7% at 5 years and no benefit for 50% to 69% stenosis. The 30-day stroke and mortality rate after CEA was 7.1% from a pooled analysis of the NASCET, ECST, and Veteran Affairs Cooperative Study (VACS) involving > 3000 symptomatic patients.
ACAS and ACST demonstrated the benefit of CEA over medical therapy in asymptomatic patients with carotid stenosis > 60%. In ACAS, after a mean follow-up of 2.7 years, the projected 5-year rates of ipsilateral stroke, perioperative stroke, and death were 5.1% for surgical patients and 11% for medical therapy alone. In ACST, the 5-year rates including perioperative events were 6.4% for CEA and 11.7% for medical therapy. The complication rate was 3% with CEA. As with NASCET, medical therapy in these studies was limited by current standards, and the benefit of CEA may be reduced when compared to modern medical management.
Risks associated with CEA include hemorrhage, acute arterial occlusion, stroke, myocardial infarction, venous thromboembolism, cranial nerve palsy, wound infection, arterial restenosis, and death. Patients with symptoms undergoing urgent operations and reoperations are at higher risk of complication.
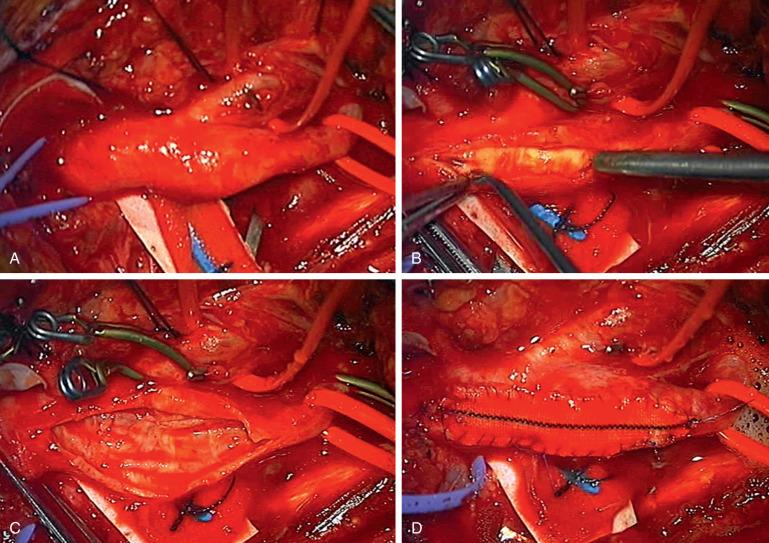
A variety of factors may affect the outcome and risks of CEA including technique, operator experience, and clinical factors ( Fig. 15.2 , see Operative Techniques section). Controversies regarding technique include the type of anesthesia, the role of shunting, and the method of arteriotomy closure. Cerebral dysfunction can be directly observed in patients undergoing CEA under local anesthesia, whereas neurophysiologic monitoring is used for patients under general anesthesia. Cerebral function is monitored to determine which patient may benefit from shunting during arterial clamping. Routine shunting versus selective shunting is controversial, and no study has shown a difference in perioperative complication. Closure with patch angioplasty may be preferred over primary closure. Hospitals performing < 100 CEA operations annually typically have worse results; however, ACAS did not demonstrate a difference with case volume and had a 30-day complication rate of 1.5%.
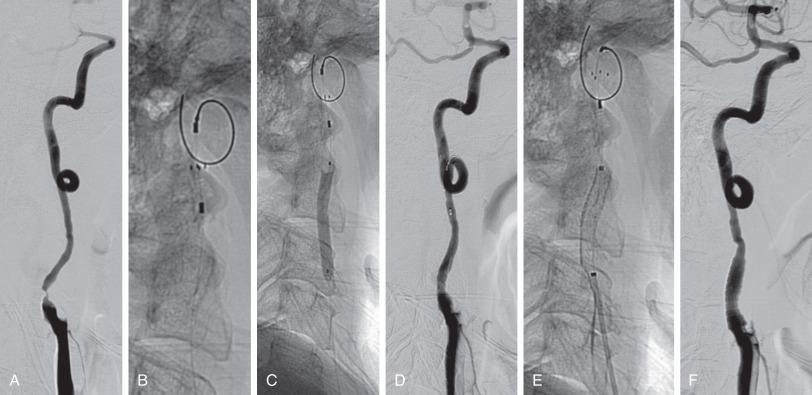
Randomized trials have not demonstrated consistent composite outcome differences between carotid artery stenting (CAS) and CEA (discussed later). Risks of CAS include neurologic deficit (1%–5%), arterial injury (<1%), arterial restenosis (3%–5%), device malfunction (< 1%), medical complications, access-site complications, and mortality. Cerebral protection devices are used to decrease risk of perioperative stroke and include distal filters, which trap plaque debris, and proximal protection devices, which stop or reverse blood flow by balloon inflation. The use of embolic protection devices (EPDs) has been correlated with decreased rates of stroke and death and improved overall outcomes in trials and registries, but no randomized trials have confirmed these results ( Fig. 15.3 , see Operative Techniques section).
High-risk candidates for CEA may be candidates for CAS. High-risk anatomic features include contralateral carotid occlusion, restenosis after prior CEA, history of neck radiation, lesions above the C2 vertebral body or below the clavicle, tandem intracranial stenosis, and difficult surgical necks due to obesity or other conditions. Medical comorbidities can also pose a high risk for CEA, including congestive heart failure class III or IV, an ejection fraction < 30%, and recent cardiac ischemia. Patients considered at high risk for CAS may have difficult anatomic arterial access, allergy or adverse reaction to antiplatelet agents, concentric plaque calcifications, common carotid artery stenosis, and intraluminal thrombus.
Multiple studies have compared outcomes after CEA and CAS in symptomatic and asymptomatic patients and have been published since the 2011 multisociety guidelines. The International Carotid Stenting Study (ICSS) trial randomized 1713 symptomatic patients to CAS (n = 855) or CEA (n = 858) with a median follow-up of 4.2 years. The majority (90%) of patients had 70% to 99% stenosis, with similar risk factors and comorbidities. The cumulative 5-year risk of disabling and fatal strokes was similar between CAS and CEA (6.4% vs 6.5%, respectively); however, the cumulative 5-year risk of any stroke (including nondisabling stroke) was higher with CAS (15.2% vs 9.4%, HR 1.71, 95% confidence interval [CI] 1.28–2.30, p < .001). Functional outcomes per modified Rankin scale (mRS) at 1-year, 5-year, or final follow-up were similar.
The Asymptomatic Carotid Trial (ACT) I was a prospective randomized trial of 1453 patients and compared CAS with EPD to CEA in patients < 79 years with 70% to 99% carotid stenosis with < 60% contralateral stenosis with follow-up up to 5 years. The primary composite end points were death, stroke, or myocardial infarction (MI) within 30 days after the procedure or ipsilateral stroke within 1 year. CAS was noninferior to CEA (event rate 3.8% and 3.4%, respectively, p = .01). The rate of stroke or death at 30 days was 2.9% for CAS and 1.7% for CEA ( p = .33). Ipsilateral stroke-free survival from 30 days to 5 years was 97.8% with CAS and 97.3% with CEA ( p = .51) and cumulative 5-year rate of stroke-free survival was 93.1% with CAS and 94.7% with CEA. The overall survival rates were 87.1% for CAS and 89.4% for CEA.
The original Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), published in 2010, randomized 2502 symptomatic and asymptomatic patients to CEA or CAS, with a median follow-up of 2.5 years. The primary composite end point was stroke, MI, or death during the periprocedural period or ipsilateral stroke within 4 years of randomization. The estimated 4-year ipsilateral stroke rates were similar between CAS and CEA (7.2% and 6.8%, p = .51). Overall, rates of 4-year stroke and death were higher with CAS (6.4% vs 4.7%, HR 1.50, p = .03). In symptomatic patients, the 4-year rate of stroke of death was 6.4% with CAS and 4.7% with CEA (HR 1.37, p = .14) and in asymptomatic patients 4.5% and 2.7%, respectively (HR 1.86, p = .07). Perioperative stroke was higher with CAS (4.1% vs 2.3%, p = .01), and perioperative myocardial infarction was higher with CEA (1.1% vs 2.3%, p = .03). At 10-year follow-up, there was no significant difference in the rate of primary composite end point between CAS and CEA (11.8% vs 9.9%, HR 1.10, 95% CI 0.83–1.44). Postprocedural ipsilateral stroke occurred in 6.9% of CAS and 5.6% of CEA patients (HR 0.99, 95% CI 0.64–1.52). No significant differences were found when symptomatic and asymptomatic patients were analyzed separately.
ICSS, updated CREST, and ACT I have confirmed that CAS is comparable to CEA in composite outcomes; however, higher rates of perioperative stroke appear to occur with CAS. Each method is presumed superior to historical medical therapy, but decreased rates of ipsilateral stroke in asymptomatic carotid stenosis have been seen with improved medical therapy. CREST-2 (NCT02089217) is in progress and will reevaluate revascularization in asymptomatic patients. The trial is organized as two independent randomized controlled trials comparing revascularization (both CAS and CEA) to best medical therapy in asymptomatic patients and is expected to end in 2020. The SPACE-2 trial had similar objectives but was terminated in 2015 due to inadequate recruitment. Before recruitment stopped, 513 patients were enrolled and the 30-day event rate was 1.97% for CEA, 2.54% for CAS, and 0% with medical management.
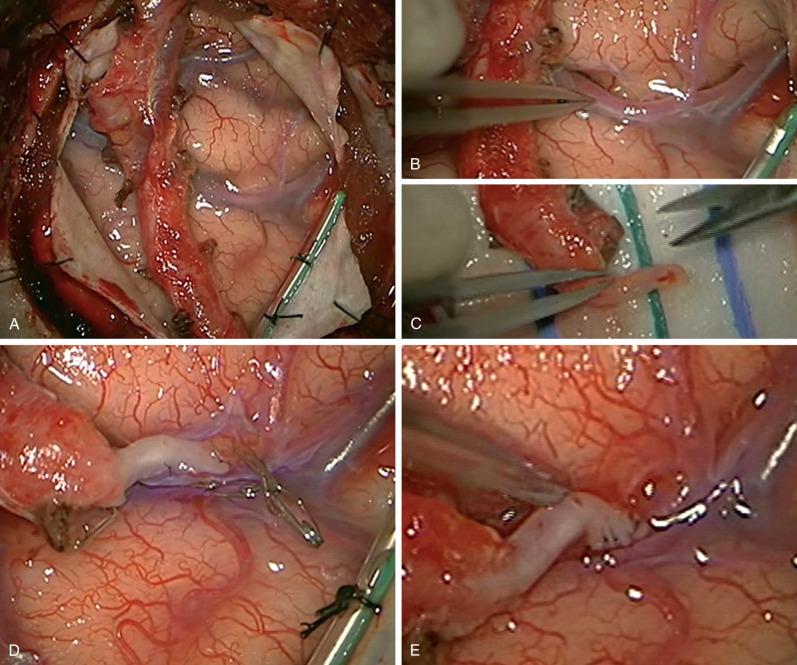
The Extracranial-Intracranial (EC-IC) Bypass Study and the Carotid Occlusion Surgery Study (COSS) have evaluated surgical revascularization to treat ischemia in patients with anterior circulation atherosclerotic disease. Neither study demonstrated that EC-IC bypass surgery reduces the risk of recurrent ipsilateral ischemic events over time. In COSS, the 30-day ipsilateral ischemic strokes rates were 14.4% in the surgical group and 2% in the medical group, and the 2-year rates were 21% and 22.7%, respectively. The postoperative morbidity and mortality in COSS was similar to those in the EC-IC Bypass Study (15% vs 12%). Graft patency was 98% at 30 days and oxygen extraction fraction (OEF) improved in surgical patients. In surgical patients without perioperative complications, the improved OEF and hemodynamics was associated with a lower risk of recurrent stroke. As such, surgical revascularization can be considered in patients with hemodynamic insufficiency, refractory symptoms despite aggressive medical therapy, and at institutions where the revascularization can be performed with low perioperative morbidity ( Fig. 15.4 , see Operative Techniques Section).
Symptomatic steno-occlusive disease of the vertebral artery (VA) is less common than carotid artery stenosis, and as a result it is less studied and understood but portends a high risk of ischemic events. Annual stroke rates for patients with symptomatic intracranial vertebrobasilar stenosis are around 10%. Many studies combine extracranial and intracranial VA stenosis as well as basilar artery stenosis. In a study of patients with vertebrobasilar TIA or stroke, the incidence of > 50% atherosclerotic stenosis of the VA or BA is about 25%. In the same study, > 50% vertebrobasilar stenosis was unrelated to age, sex, or vascular risk factors and was associated with higher rates of ischemic events and recurrent stroke. Extracranial VA stenosis typically affects the origin of VA but can occur within the transverse foramina of the vertebral bodies due to osteophyte formation. Dynamic compression of the extracranial VA in this location or at the craniocervical junction results in episodic vertebrobasilar insufficiency (bow hunter syndrome). Duplex ultrasonography of the vertebrobasilar system is unreliable due to technical limitations on insonation, so CTA or MRA is often recommended for initial diagnostic evaluation.
Medical therapy recommendations for VA disease are similar to those for extracranial carotid stenosis. Antiplatelet therapy with aspirin is recommended for stroke and MI prevention in patients with VA stenosis, and aspirin alone, aspirin plus dipyridamole, or clopidogrel alone is recommended for symptomatic extracranial VA stenosis. Surgical revascularization including bypass for VA disease is rarely performed but favorable outcomes for proximal and distal VA reconstruction for extracranial VA and intracranial vertebrobasilar disease have been reported in case series.
Retrospective studies of endovascular treatment of extracranial VA stenosis demonstrate the safety and efficacy of angioplasty and stenting. A systematic review of 27 studies (n = 993) found a 30-day stroke and TIA rate of 1.1% and 0.8%, respectively. The rate of vertebrobasilar territory stroke was 1.3% during a mean follow-up of 21 months. Restenosis occurred in 30% of bare-metal stents and 11% of drug-eluting stents at mean follow-up of 24 months. The rate of restenosis after stenting correlates with length of stenosis, from 21% in lesions < 5 mm to 50% in lesions > 10 mm in length. These results suggest that stenting of extracranial VA stenosis has good outcomes especially with focal lesions.
Endovascular treatment of VA stenosis has been prospectively evaluated by three randomized controlled trials: CAVATAS, SAMMPRIS, and VAST. In the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), 16 patients with intracranial and extracranial VA disease stenosis were included, and none of the patients had vertebrobasilar ischemic stroke during follow-up (mean 4.7 years). In Stenting versus Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS), 60 of 451 patients had VA stenosis. In a 2-year follow-up, the overall probability of stroke or death after 30 days from the initial stroke or after revascularization was 10% with medical therapy and 21% with endovascular therapy, but in the short-term and long-term SAMMPRIS results, subgroup analysis was not performed for VA stenosis. The Vertebral Arterial Stenting Trial (VAST) was the first randomized controlled control to specifically evaluate the outcomes of endovascular therapy with medical therapy for vertebrobasilar ischemia. VAST enrolled 115 patients with > 50% extracranial and intracranial stenosis before the trial stopped due to regulatory requirements. The primary outcome was vascular death, MI, or any stroke within 30 days after treatment, and the secondary outcomes were symptomatic VA stroke during follow-up, composite outcome, and degree of stenosis after 12 months. The primary outcome occurred in 3 (5%) of the stenting patients and 1 (2%) of the medical therapy patients. During a median follow-up of 3 years, 7 (12%) patients in the stenting group and 4 (7%) patients in the medical therapy group had a VA stroke and 11 (19%) of the stenting patients and 10 (17%) of medical therapy patients had the composite outcome. The results of these endovascular trials show the severe natural history of symptomatic VA stenosis and do not demonstrate the utility of endovascular therapy in addition to best medical management in most cases, though the results are potentially confounded by the inclusion of patients with intracranial stenosis, which according to SAMMPRIS carries higher periprocedural risk. Endovascular treatment should thus be limited to severe cases that have failed medical therapy and should be treated at high-volume centers to reduce the risk of periprocedural complications.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here