Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mediastinum is the visceral compartment between the two lungs and includes the mediastinal part of the parietal pleura. It is commonly defined as the region between the two pleural sacs, bounded laterally by the mediastinal parietal pleura, anteriorly by the sternum and posteriorly by the thoracic vertebral column, and extending vertically from the superior thoracic aperture (thoracic inlet) to the respiratory diaphragm (see Fig. 52.1 ). It communicates freely with the neck spaces and with the extraperitoneal, extrapleural and epidural spaces. Beyond each lung hilum, the mediastinum merges into the lung interstitium, incorporating the bronchial tree with its accompanying neurovascular bundles and the pulmonary vasculature.
The posterior mediastinal boundary is longer than the anterior as a result of the oblique orientation of the superior thoracic aperture and the posteroinferior curvature of the respiratory diaphragm. With respect to the median plane (midline), the mediastinum appears asymmetric.
The mediastinum is divided into superior and inferior mediastina by the sternal plane, which passes horizontally from the manubriosternal joint to intersect the vertebral column from the fourth to the superior half of the fifth thoracic vertebral body ( ). This boundary is sometimes defined as a fixed plane passing from the manubriosternal joint to the inferior limit of the fourth thoracic vertebral body ( Fig. 56.1 ). The inferior mediastinum is subdivided into anterior, middle and posterior parts.
Detailed accounts of mediastinal contents are included with descriptions of the respiratory viscera ( Ch. 54 ), heart ( Ch. 57 ) and great vessels ( Ch. 58 ).
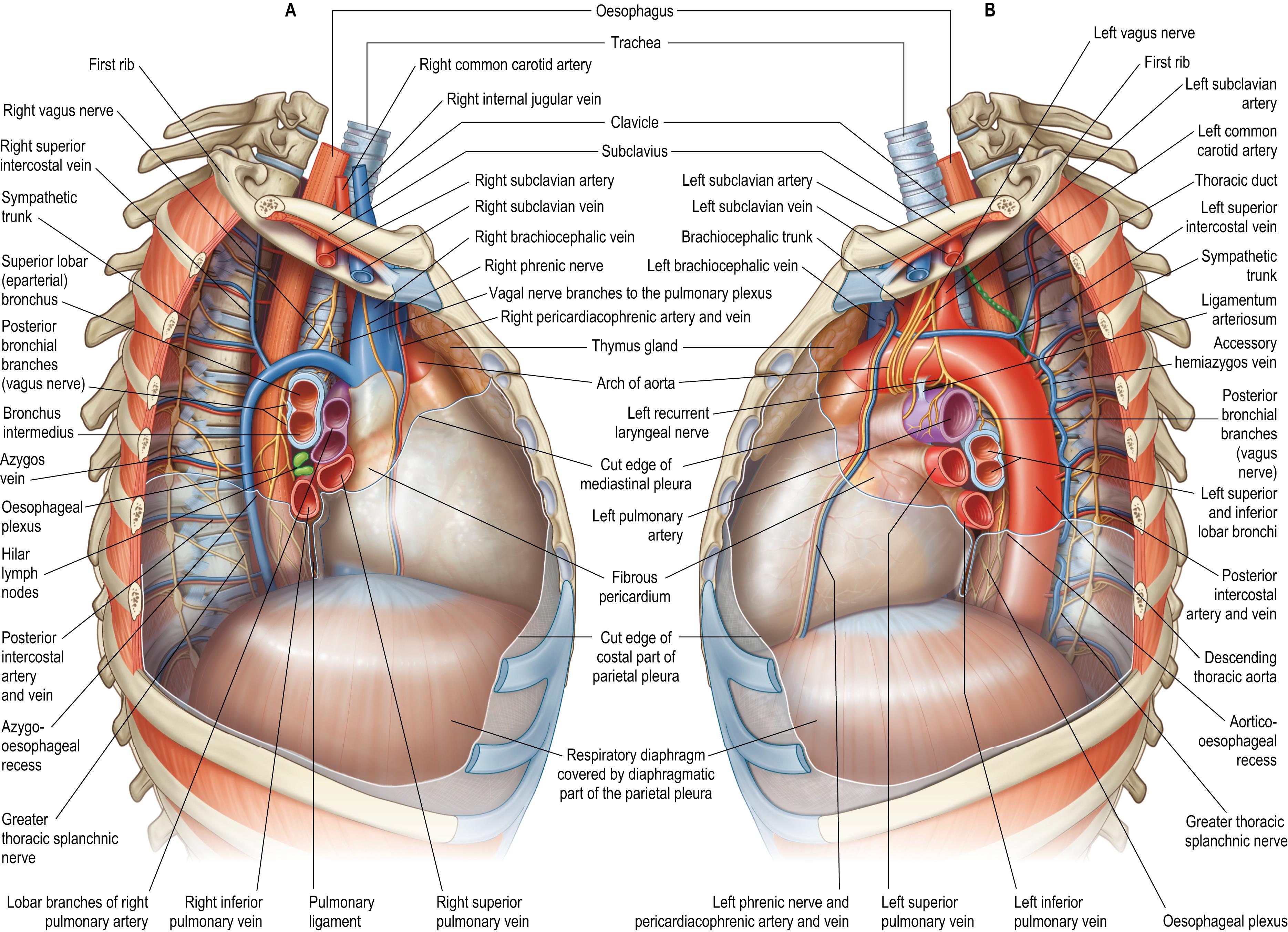
The superior mediastinum lies between the manubrium of the sternum anteriorly and the bodies of the upper four or five thoracic vertebrae and the intervening intervertebral discs posteriorly. It is bounded laterally by the mediastinal parietal pleurae (see Fig. 56.1 ). The reflections of the left and right costal parietal pleura onto the superior mediastinum each follow a line that passes from the sternoclavicular joint in an inferomedial direction, to meet each other in the midline posterior to the sternal angle (see Fig. 53.4B ). The resultant triangular space on the posterior surface of the manubrium of the sternum and between the lines of pleural reflection is occupied by the thymus or its remnants. The superior mediastinum contains the trachea, oesophagus, aortic arch, brachiocephalic trunk, left common carotid and subclavian arteries (the vertebral artery sometimes arises from the aortic arch between them), the internal thoracic arteries, the supreme intercostal, left superior intercostal, accessory hemiazygos, internal thoracic and inferior thyroid veins, and numerous lymph nodes, including those of the para-aortic, upper paratracheal, retrotracheal and prevascular groups (stations). Laterally, the superior mediastinal structures have the appearance of being ‘shrink-wrapped’ by the tightly applied mediastinal parietal pleura. The superior mediastinum transmits the sympathetic trunks, the sympathetic and parasympathetic cardiac nerves, the phrenic nerves laterally, the vagus nerves medially and the left recurrent laryngeal nerve. The terminal part of the thoracic duct emerges from the posterior aspect of the aortic arch and oesophagus, and passes superiorly between the left subclavian and left common carotid arteries to enter the neck (see Fig. 56.7B ). The left and right brachiocephalic veins join to form the superior vena cava. The origins of sternothyroid and sternohyoid are attached to the posterior surface of the manubrium of the sternum. An enlarged thyroid gland may extend inferiorly between the manubrium and the brachiocephalic veins; its surgical excision requires care due to possible venous adherence. The origins of longus colli (inferior oblique and vertical intermediate portions) are the deepest contents of the superior mediastinum.
The anterior mediastinum lies between the sternal body and fibrous pericardium (see Fig. 56.1 ). It narrows above the level of the fourth costal cartilages where the lines of pleural reflection converge (see Fig. 53.4B ), and contains a variable amount of adipose tissue, loose connective tissue, sternopericardial ligaments, a few lymph nodes, the mediastinal branches of the internal thoracic artery, and sometimes part of the thymus gland or its remnants. The paired sternocostal triangles (of Morgagni) are openings between the sternal and costal attachments of the respiratory diaphragm: they are filled with areolar tissue and crossed by the superior epigastric vessels and by some lymph vessels from the abdominal wall and liver. A retrosternal hernia may pass through the triangles. The pericardium and the heart are routinely approached by either a complete or partial median sternotomy. The sternopericardial ligaments and the pleural reflections are easily separated by blunt dissection. In case of pericardial tamponade, the pericardial cavity can be drained through a left parasternal or subxiphoid approach, normally without entering the pleural cavity.
The middle mediastinum is the broadest part of the inferior mediastinum (see Fig. 56.1 ) and contains the pericardia, heart and ascending aorta, the intrapericardial (inferior) half of the superior vena cava (receiving the azygos vein posteriorly), the tracheal bifurcation and main bronchi, the pulmonary trunk, right and left pulmonary arteries and veins, phrenic nerves, the deep part of the cardiac plexus and the subcarinal and lower paratracheal (tracheobronchial) lymph nodes. The short thoracic part of the inferior vena cava, both the extra- and intrapericardial segments, extends between the caval foramen of the respiratory diaphragm, through which it passes, and its termination in the right atrium. The structures forming the root of the lung converge at the hilum of the lung. The mediastinal part of the parietal pleura forms the lateral boundary of the middle mediastinum and is continuous with the visceral pleura at the level of the root of the lung and pulmonary ligament; the parietal pleura folds back (reflects) on itself to continue as the visceral pleura. The fibrous pericardium lies on, and is fused with, the middle folium of the central tendon of the respiratory diaphragm (see Fig. 55.3A ).
The posterior mediastinum is the longest and narrowest part of the inferior mediastinum (see Fig. 56.1 ). It is bounded anteriorly by the tracheal bifurcation, fibrous pericardium and pulmonary vessels, and posteriorly by the bodies of the fourth or fifth to the twelfth thoracic vertebrae, and the intervening intervertebral discs. Numerous structures pass freely between the superior and posterior mediastina. The curved posterior third of the median (midline) region of the respiratory diaphragm constitutes the anteroinferior limit of the posterior mediastinum. Two pleural recesses, the aortico-oesophageal and azygo-oesophageal, project medially between the aorta and oesophagus on the left side and the azygos vein and oesophagus on the right side (see Fig. 56.7 ).
The posterior mediastinum contains the descending thoracic aorta (on the left side of the vertebral column), the oesophagus (median, but positioned anterior to the aorta inferiorly) and, more posteriorly, the azygos and hemiazygos venous systems, the thoracic duct, prevertebral and para-oesophageal lymph nodes, right and left sympathetic trunks and associated ganglia, and thoracic splanchnic nerves. The vagal trunks are located adjacent to the oesophagus; the anterior trunk is constituted mainly from the left vagus nerve and the posterior mainly from the right vagus nerve. The descending thoracic aorta gives off the intercostal arteries (see Fig. 52.3 ) and the largest anterior segmental medullary artery, the ‘great radicular artery of Adamkiewicz’ ( ) (see Fig. 47.11 ).
There are communications between the posterior mediastinum and the abdomen either through, or posterior to, the respiratory diaphragm ( Ch. 55 ). The oesophageal hiatus at the level of the body of the eleventh thoracic vertebra (conventionally reported as at the level of the body of the tenth thoracic vertebra), transmits the oesophagus, anterior and posterior vagal trunks, gastric nerves and oesophageal branches of the left gastric artery. The aortic hiatus at the level of the body of the twelfth thoracic vertebra transmits the aorta, thoracic duct and occasionally the azygos and hemiazygos vein. Apertures within the diaphragmatic crura transmit the thoracic splanchnic nerves; apertures deep to the medial arcuate ligament transmit the sympathetic trunks; minute openings in the central tendon of the respiratory diaphragm transmit small veins. All openings represent potential communication sites for suppurative or neoplastic processes.
Pathways exist between the oral cavity and the mediastinum via the parapharyngeal space and along other fascial planes/spaces of the neck. The parapharyngeal space is more likely to be infected than any of the other potential tissue spaces in the head and neck. It communicates with the retropharyngeal and pretracheal spaces, eventually reaching the superior mediastinum and then the inferior mediastinum ( Chapter 35, Chapter 37, Chapter 40 ). The planes between the buccopharyngeal, alar and prevertebral fasciae, the retropharyngeal and danger spaces, respectively, are a highway for the spread of air and gastric contents between the neck and mediastinum after oesophageal injury ( ). The carotid sheath, containing the carotid arteries, internal jugular veins and vagus nerves, represents another potential route of communication.
The mediastinum contains the great vessels (ascending aorta, aortic arch and its branches, descending thoracic aorta, superior vena cava and the thoracic part of the inferior vena cava), the pulmonary arteries and veins, the internal thoracic and posterior intercostal arteries and veins, the azygos and hemiazygos venous systems, and the thoracic duct.
The ascending aorta, aortic arch and its branches, descending thoracic aorta, pulmonary trunk and superior vena cava are described in Chapter 58 .
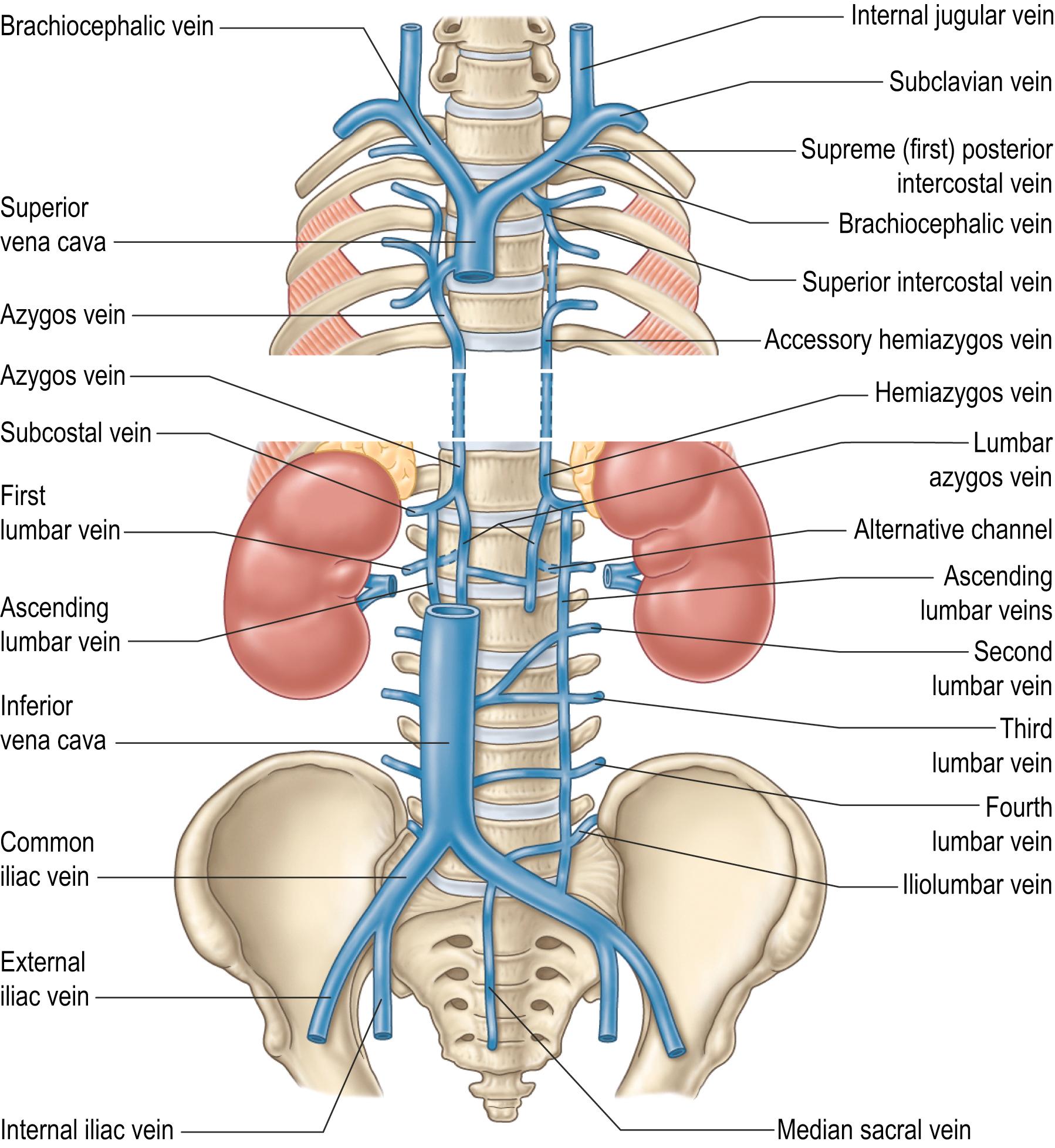
The azygos vein has a variable origin at or below the level of the renal veins ( Figs 56.2 – 56.3 ; see Fig. 56.14B ). When present, the azygos lumbar vein ascends anterior to the bodies of the upper lumbar vertebrae and passes either posterior to, or through, the right diaphragmatic crus or traverses the aortic hiatus to the right of the cisterna chyli. Anterior to the body of the twelfth thoracic vertebra, it is joined by a venous trunk, formed by the union of the right ascending lumbar and subcostal veins, to form the azygos vein. In the absence of a lumbar azygos vein, this common venous trunk continues as the azygos vein. The azygos vein passes to the right, posterior to the right crus of the respiratory diaphragm, and ascends in the posterior mediastinum to the level of the body of the fourth thoracic vertebra, where it arches anteriorly, superior to the right lung hilum and root, and joins the posterior aspect of the superior vena cava, just superior to the point where it passes into the pericardium.
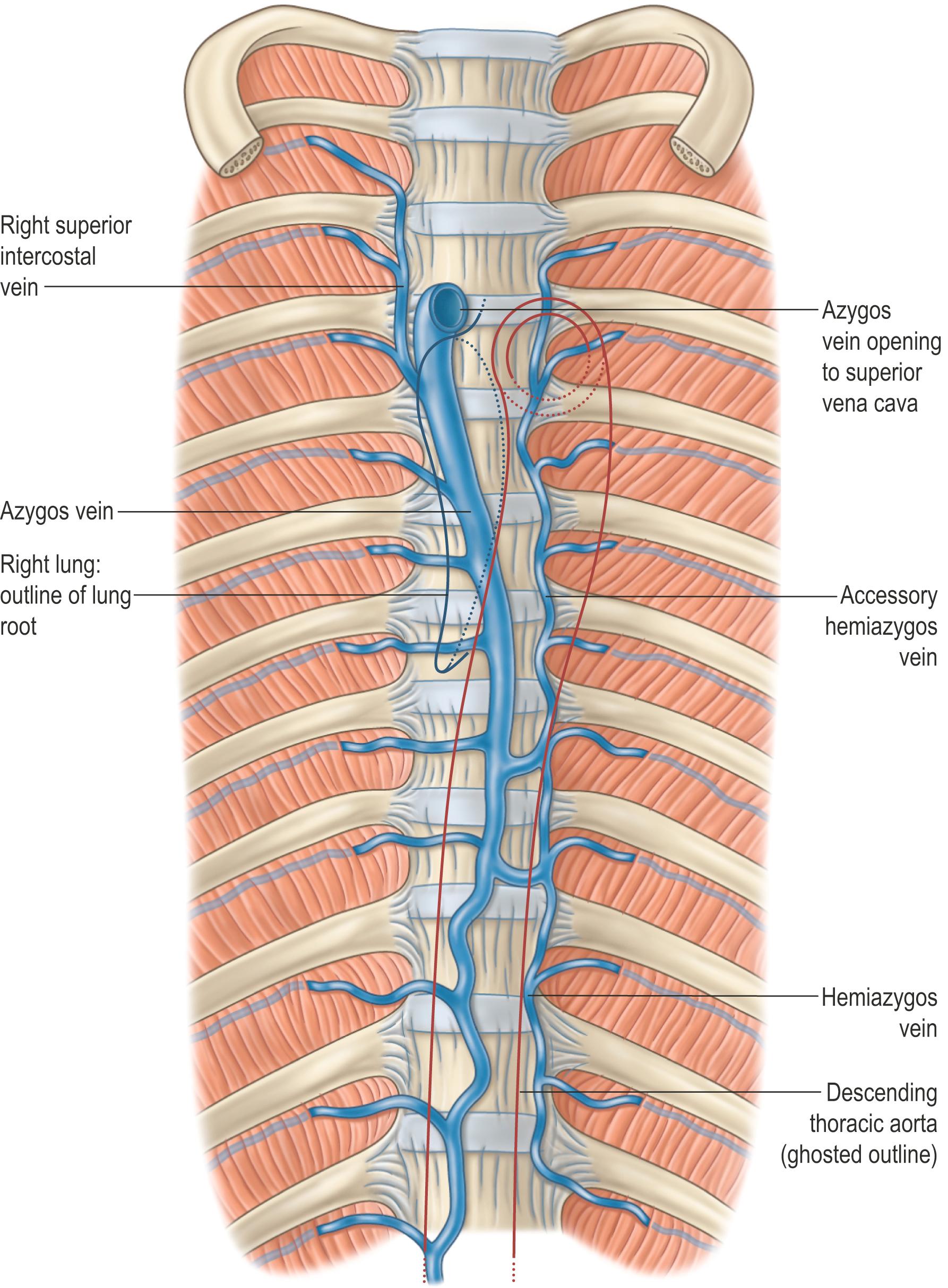
In its course, the azygos vein lies anterior to the anterior longitudinal ligament covering the bodies of the lower eight thoracic vertebrae and the intervening intervertebral discs, and the right posterior intercostal arteries. Right lateral relations include the right sympathetic trunk and associated ganglia, the right greater thoracic splanchnic nerve, the right lung and parietal pleura. Left lateral relations include the thoracic duct, descending thoracic aorta and, where the vein arches anteriorly, the oesophagus, trachea and right vagus nerve. Aortic pulsations may assist venous return in the azygos and hemiazygos veins, although only a small and variable number of imperfect valves are present. Inferiorly, the azygos vein lies posterior to the oesophagus, from which it is separated by a right-sided pleural recess, the azygo-oesophageal recess. There are age-related differences in the appearance of the contour of this recess on computed tomography (CT); the recess is dextroconvex below the age of 6 years, non-concave (equally divided between straight and convex) between 6 and 12 years of age and concave, as seen in adults, after the age of 12 years of age ( ).
The major tributaries of the azygos vein are the right intercostal veins (excluding the right supreme (first) intercostal vein that drains into the right brachiocephalic vein), the right superior intercostal vein (usually formed by the union of the second to fourth intercostal veins), the right ascending lumbar and subcostal veins, mediastinal, oesophageal, superior phrenic, pericardial and right bronchial veins, and the hemiazygos and accessory hemiazygos veins.
Numerous anastomoses exist between the tributaries and the main trunks of the azygos, hemiazygos and accessory hemiazygos veins, the inferior vena cava and the vertebral venous plexuses. These allow for alternate drainage pathways in case of segmental agenesis (‘interruption’) or thrombosis of the inferior vena cava.
The hemiazygos vein represents the left-sided equivalent of the more inferior part of the azygos vein. It originates in a similar fashion, from the union of the left lumbar ascending and subcostal veins, but exhibits greater variability, and may also connect with the left renal vein via the equivalent of the lumbar azygos vein. The hemiazygos vein crosses the midline anterior to the body of the eighth or ninth thoracic vertebra, passing posterior to the descending thoracic aorta and oesophagus, and either anterior or posterior to the thoracic duct, to join the azygos vein (see Figs 56.2, 56.5B ). It receives mediastinal, oesophageal and the ninth to eleventh left posterior intercostal veins.
The accessory hemiazygos vein functions as the left-sided mirror image of the superior portion of the azygos vein. It drains the left fifth to eighth posterior intercostal veins and occasionally the left bronchial veins, and usually crosses the midline anterior to the body of the seventh thoracic vertebra to join the azygos vein. The supreme (first) left posterior intercostal vein drains directly into the left brachiocephalic vein, while the second to the fourth may connect indirectly to the accessory hemiazygos vein via the left superior intercostal vein (see Fig. 56.7 ). The accessory hemiazygos vein typically joins the azygos vein directly, but it can join the hemiazygos vein before draining into the azygos vein. One or more prevertebral transversal anastomoses can be present.
The origin, course, tributaries, anastomoses and termination of the azygos system of veins are all highly variable ( ). The arrangement shown in Fig. 56.2 represents the most common pattern with a right-sided azygos vein. The left-sided hemiazygos vein can be absent or underdeveloped, allowing direct drainage of the left intercostal veins into the azygos vein and/or the termination of the accessory hemiazygos vein into the left brachiocephalic vein either directly or by joining the left superior intercostal vein. In such cases, the azygos vein is located in a median position. In cases of common cardinal or anterior cardinal vein malformation, the azygos vein may enter the right atrium, right brachiocephalic vein or right subclavian vein rather than joining the superior vena cava.
In congenital interruption of the inferior vena cava or acquired thrombosis, the azygos system provides an alternative pathway for venous drainage. A higher than usual azygos arch may travel over the apical region of the right superior lobe within an accessory azygos fissure, covered by four pleural layers (two parietal and two visceral), and isolate a portion of the superior lobe of the right lung medially: the ‘azygos lobe’ ( ). If present, the azygos fissure can be identified on chest radiograph as a fine radiopaque line. The azygos vein located within the azygos fissure often presents as a lung ‘pseudo-nodule’ on a chest radiograph; it is typically comma-shaped and should not be confused with a more rounded appearance of early lung carcinoma or metastasis.
The internal thoracic vessels are described in Chapter 53 .
The pulmonary vessels are described in Chapter 54 .
The posterior intercostal veins accompany their arteries in 11 pairs; they are described in Chapter 53 .
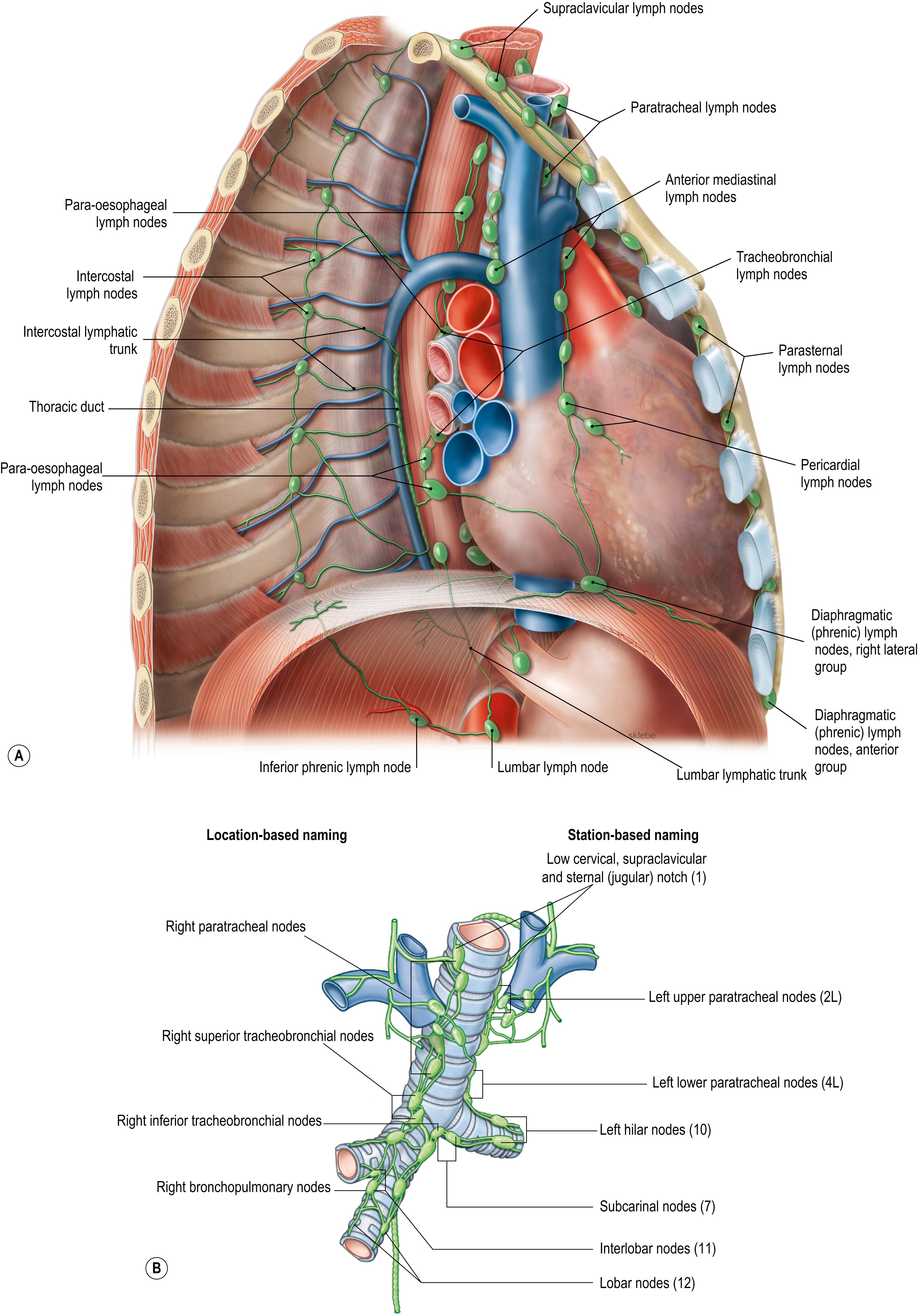
There are multiple groups of lymph nodes throughout the mediastinum. Conventionally they have been named according to the nearest mediastinal viscera or structure (which they often drain): parietal node groups drain the thoracic wall and visceral groups drain the thoracic viscera ( Fig. 56.4A ). However, this naming system implies that the mediastinal node groups are separate and distinct, whereas in reality they are part of a continuum. To better reflect their regional disposition, and for the purposes of cancer staging and management, mediastinal lymph node groups are best categorized into regional lymph node stations and zones ( ) (see Fig. 54.12 B, Fig. 56.4B , Table 56.1 ).
| Nodes | Zone | Station | |
|---|---|---|---|
| Supraclavicular | 1 | Low cervical, supraclavicular and sternal (jugular) notch | |
| Superior mediastinal | Upper | 2R | Upper paratracheal (right) |
| 2L | Upper paratracheal (left) | ||
| 3a | Prevascular | ||
| 3p | Retrotracheal | ||
| 4R | Lower paratracheal (right) | ||
| 4L | Lower paratracheal (left) | ||
| Aortic | Aorticopulmonary | 5 | Subaortic |
| 6 | Para-aortic (ascending aorta or phrenic) | ||
| Inferior mediastinal | Subcarinal | 7 | Subcarinal |
| Lower | 8 | Para-oesophageal (below carina) | |
| 9 | Pulmonary ligament | ||
| N1 | Hilar/interlobar | 10 | Hilar |
| 11 | Interlobar | ||
| Peripheral | 12 | Lobar | |
| 13 | Segmental | ||
| 14 | Subsegmental | ||
Metastatic involvement of mediastinal lymph nodes has important prognostic implications and influences the choice of treatment ( , ). The staging system for lung cancer classifies node involvement using a nodal descriptor (N-’Stage’), where N0 indicates no regional lymph node metastases; N1 indicates involvement of ipsilateral peribronchial or hilar lymph nodes and intrapulmonary nodes; N2 indicates involvement of ipsilateral mediastinal and/or subcarinal nodes; and N3 indicates involvement of contralateral mediastinal or hilar nodes, and ipsilateral or contralateral scalene or supraclavicular nodes/scalene nodes ( , , ).
Pulmonary nodes within the lung parenchyma are continuous with the bronchopulmonary node groups (in order, these include subsegmental, segmental, lobar, interlobar and hilar nodes) (see Figs 56.4B , 54.12 ). Bronchopulmonary node groups are continuous with subcarinal (inferior tracheobronchial) nodes located inferior to the tracheal bifurcation and the lower paratracheal (superior tracheobronchial) nodes. In turn, these groups are continuous with the upper paratracheal groups of nodes that drain the lungs, bronchi, the thoracic part of the trachea, heart, pericardium and also receive efferent vessels from prevertebral nodes that receive lymph from the posterior respiratory diaphragm, thoracic oesophagus and intercostal lymph nodes. Efferent vessels ascend on the trachea to unite with efferents from the parasternal and brachiocephalic nodes to become the right and left bronchomediastinal trunks. The right trunk may occasionally join a right lymphatic duct or another right-sided lymph trunk, and the left trunk may join the thoracic duct, but usually they open independently in or near the ipsilateral junction of the internal jugular and subclavian vein (see Fig. 35.16 ).
The decision to proceed with mediastinoscopic biopsy depends on whether suitable nodal stations (zones) seem involved on CT scanning and whether there is a history of prior neck and mediastinal surgical procedures. Complications of mediastinoscopy include haemorrhage, iatrogenic injury to the trachea or left recurrent laryngeal nerve and pneumothorax. Mediastinoscopy can be performed with video-assisted or direct optic visualization. A transverse incision is made at the level of the jugular (suprasternal) notch; sternohyoid and sternothyroid muscles within the superficial lamina of deep cervical fascia are retracted; the deep lamina of the deep cervical fascia is incised. The pretracheal plane is dissected and a space is created between the anterior aspect of the trachea and the brachiocephalic trunk inferior to the carina and along both sides of the trachea and main bronchi. This space facilitates biopsy of the upper paratracheal 2R and 2L stations, the right lower paratracheal 4R station and higher nodes from the left lower paratracheal 4L station superior to the aortic arch (see Fig. 54.12 B; Table 56.1 ). The anterior part of subcarinal station 7 and bilateral hilar stations 10R and 10L may be accessible but are technically challenging. Lymph nodes can be sampled or removed entirely for histological evaluation.
The anterior part of the superior mediastinum is more difficult to assess by mediastinoscopy and usually requires an anterior mediastinotomy (Chamberlain procedure). This is performed to biopsy nodes from the more inferior aspect of the left lower paratracheal 4L station, subaortic station 5, para-aortic station 6 and subcarinal station 7, and also facilitates anterior mediastinal mass biopsy. Endobronchial ultrasound can be performed to biopsy lymph nodes in stations 1–4, 7 and 10–12, using fine needle aspiration through the tracheal or bronchial walls. In a similar way, transoesophageal ultrasound may help during biopsy of subcarinal station 7, para-oesophageal station 8 and pulmonary ligament station 9 nodes.
A video demonstrating lymph node dissection via mediastinoscopy can be seen in . A video demonstrating mediastinal lymph node dissection via tracheo-oesophageal mediastinoscopy can be seen in .
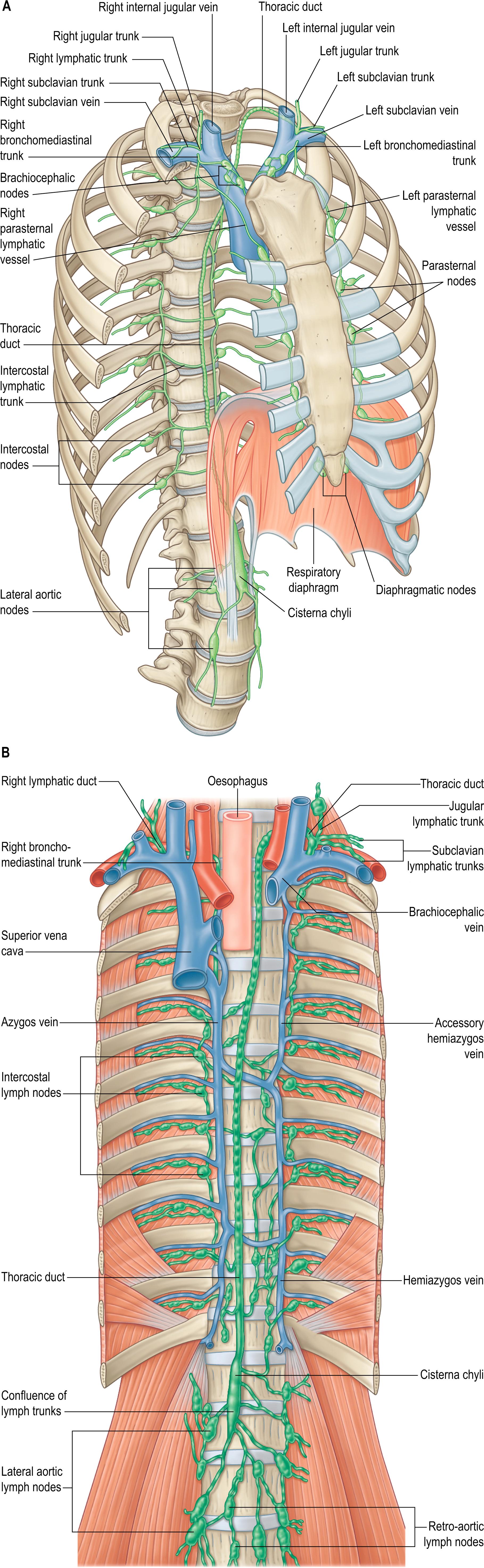
The thoracic duct conveys the lymph from the majority of the entire body back to the venous circulation, except lymph from the right part of the head, neck and heart, right upper limb, parts of the left and right lung and part of the convex surface of the liver. It usually originates anterior to the body of the first or second lumbar vertebra, where the intestinal and two lumbar lymphatic trunks join to form the cisterna chyli. Both the thoracic duct and the cisterna chyli are variable in their origin, course and length; the latter is even more variable in width and shape than the thoracic duct ( ). From the superior aspect of the cisterna chyli, the thoracic duct passes through the aortic hiatus and enters the posterior mediastinum, lying to the right of the midline, between the descending thoracic aorta and the azygos vein, up to the level of the body of the fifth or sixth thoracic vertebra, where it typically passes to the left, over the vertebral column and posterior to the oesophagus (see Fig. 56.7B ). It may branch or even have duplicate trunks that eventually form a single thoracic duct ( ). It continues superiorly, first posterior to the aortic arch adjacent to the left side of the oesophagus, then posterior to the left subclavian artery in the superior mediastinum. It subsequently arches over the left subclavian artery and descends anteriorly to empty into the venous circulation in the region of the left internal jugular and subclavian veins, either at their very confluence or in proximity, or sometimes into the left brachiocephalic vein ( Fig. 56.5 ). In the space between longus colli, scalenus anterior and the suprapleural membrane, the duct usually arches 3–4 cm superior to the clavicle, crossing anterior to the vertebral vessels, the left sympathetic trunk, the vertebral sympathetic ganglion, the left thyrocervical trunk and the left phrenic nerve. The course and position of the thoracic duct determines the location of the effusion seen with duct injury or obstruction. FLOAT NOT FOUND
Two thoracic ducts are sometimes present within the mediastinum and may terminate in veins on their respective side of the body. Occasionally a single thoracic duct may empty into right-sided venous structures, a disposition that is more frequent in individuals with an aberrant right subclavian artery originating from the distal aortic arch and ascending posterior to the oesophagus.
The thoracic duct is commonly 36–45 cm in length, its external diameter varies according to region ( ). When present as a single channel it is 5–8 mm in diameter at its origin, narrows to 2–3 mm at mid-thoracic level and then widens again at its termination: the diameter may fluctuate with lymph flow rate ( ). Alternatively, the thoracic duct may divide into two or three branches that eventually reunite or drain into both the left and right venous systems. The duct appears sinuous and has valves throughout its length; a bicuspid valve at its termination may prevent the backflow of blood. As it passes through the mediastinum, the thoracic duct also receives non-chylous lymph from tributaries that drain regions of the pulmonary parenchyma and parietal pleura. The sum of these sources accounts for a total lymphatic flow through the thoracic duct of 1500–2400 ml per day in proportion to the dietary intake of fat, particularly long-chain triglycerides.
The thoracic duct crosses the mediastinum at, or at about, the level of the body of the fifth thoracic vertebra which means that lymphatic injury or obstruction above or below this level results in a left- or right-sided pleural effusion (chylothorax), respectively ( ). Anomalies and sites of injury to the duct are identified by lymphangiography, with or without post-procedure CT scanning, or lymphoscintigraphy. Lymphangiography is performed after bilateral localization of pedal lymphatics with a subcutaneous injection of methylene blue, followed by a minor surgical procedure to cannulate the lymphatic vessels, and injection of Lipiodol contrast agent. Conventional X-ray and CT imaging (without intravenous contrast) are then undertaken. Lymphangiography can identify areas of chyle leakage and lymphangiectasia in the majority of patients ( ).
Ligation of the thoracic duct via thoracoscopic surgery can be seen in .
Some tributaries of the thoracic duct may terminate directly in the venous system. There are numerous anastomoses between visceral, parietal, limb and mammary lymphatics, as is apparent from observed multiple patterns of dissemination of cancer cells (see Figs 56.4 – 56.5 ). As well as the cisterna chyli and its tributaries, bilateral ascending lumbar lymphatic trunks from the upper lateral aortic (abdominal part) nodes pierce the corresponding diaphragmatic crus and eventually join the thoracic duct in the posterior mediastinum. The inferior six or seven intercostal lymphatic trunks from the corresponding bilateral intercostal nodes descend to terminate in the duct, either directly or by variable common trunks. The left upper intercostal lymphatics may join the cervical portion of the duct or the subclavian trunk. The left subclavian trunk usually joins the thoracic duct but may open independently into the left subclavian vein. These mediastinal trunks provide lymph drainage paths to the thoracic duct from the convex diaphragmatic aspect of the liver, the respiratory diaphragm, the pericardium, heart and oesophagus; many possess terminal bicuspid valves. The left bronchomediastinal trunk occasionally joins the thoracic duct but usually has an independent venous termination. The left jugular trunk usually joins the thoracic duct but may open independently into the left internal jugular vein (see Fig. 35.16 ).
The variable origin, course and length of the thoracic duct, coupled with a failure to identify it during surgery, may lead to inadvertent incision or transection, particularly during trans-hiatal and thoracoscopic oesophageal surgery, with an incidence of 0.2–3% ( ). Thoracic duct laceration is a potentially life-threatening complication; mortality rates are higher with conservative management but remain elevated even after surgical ligation. Rupture of the thoracic duct leads to leakage of chyle, which is rich in lipid, protein, T lymphocytes (ranging from 400 to 6800 cells/μl), immunoglobulins and fat-soluble vitamins, and hence a progressive nutritional and immune deficit develops. Postoperative chylous effusions are usually the result of damage to ductal tributaries rather than to the duct itself, and are usually self-limiting. The greater incidence of injury with trans-hiatal resection may be attributable to shear forces exerted around the region of the duct during the mobilization of the distal oesophagus, whereas the limited field of view probably contributes to the greater incidence of thoracic duct injury during thoracoscopic surgery. Injury should be suspected in the postoperative period if there is either an enlarging mediastinal silhouette on serial chest radiographs or a significant drainage of milky looking fluid from chest or abdominal drains. An electrophoretic confirmation of the presence of chylomicrons in the pleural fluid is diagnostic amongst other tests. The thoracic duct may be injured during the left cervical approach for the exposure of the vertebral and subclavian arteries or the cervicothoracic (stellate) sympathetic ganglion. However, deliberate ligation of the duct at this level represents an accepted surgical manoeuvre with no particular consequences.
The right lymphatic trunk has a variable anatomy (see Figs 56.5 , 35.16). Its three main tributaries, the right jugular, subclavian and bronchomediastinal trunks, often empty separately into either the right internal jugular and/or subclavian veins. In up to 20% of individuals, they may aggregate to form the right lymphatic trunk that is usually 5–10 mm in length. The terminal portions of the right lymphatic trunk and of the thoracic duct are concealed by the scalene fat pad, rendering them more vulnerable during surgery.
The thoracic autonomic nervous system consists of the right and left sympathetic trunks and associated ganglia, the vagus nerves, and the cardiac, pulmonary and oesophageal plexuses. The cardiac plexus is described in Chapter 57 , the pulmonary plexus in Chapter 54 , and the oesophageal plexus is described below.
The thoracic sympathetic trunks have ganglia that are almost equal in number to those of the thoracic spinal nerves (usually 11, occasionally 12, rarely 10 or 13), with which they are associated ( Figs 56.6 – 56.7 ). Almost always, the first thoracic ganglion is fused with the inferior cervical ganglion, forming the cervicothoracic (stellate) ganglion; occasionally, the second thoracic ganglion is included in this fusion ( ). The thoracic ganglia sit posterior to the costal part of the parietal pleura and except for the second and lowest two or three, they lie anterior to the heads of the ribs. The second thoracic sympathetic ganglion is commonly located in the second intercostal space, and the lowest two or three thoracic ganglia lie lateral to the bodies of the correspondingly numbered vertebrae. Inferiorly, the thoracic sympathetic trunks pass posterior to the medial arcuate ligament or through the ipsilateral diaphragmatic crus to become the lumbar sympathetic trunks.
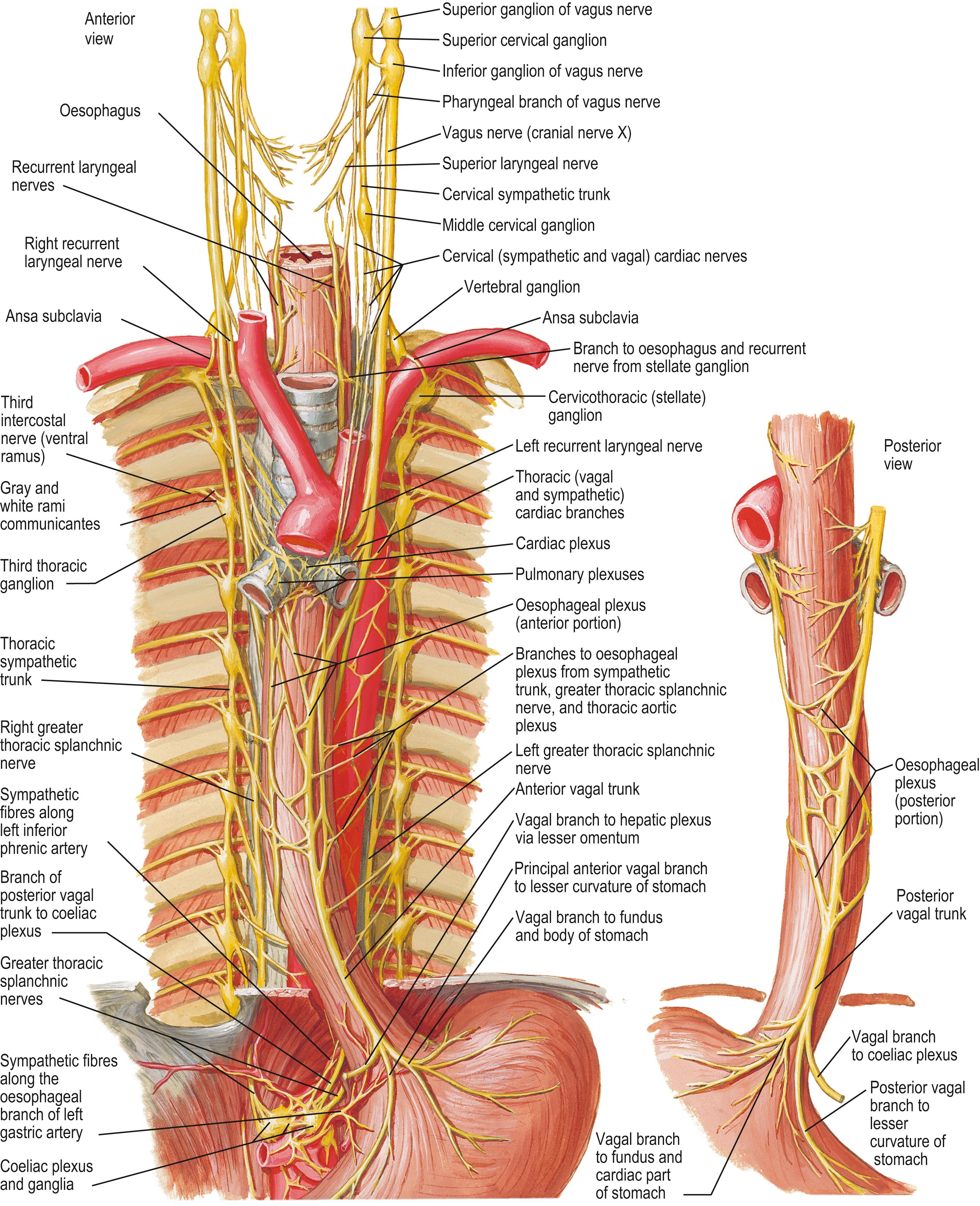
The ganglia are relatively small and interconnected by intervening segments of the trunk. The classic description is that two or more rami communicantes, white (myelinated, preganglionic) and grey (unmyelinated, postganglionic), connect each ganglion with its corresponding spinal nerve, the white rami connecting with the spinal nerve distal to the grey. Variation in this pattern is common, especially at the upper thoracic levels and symmetry is rarely seen. Additional ascending and descending rami communicantes may emerge from the second (54% and 46%, respectively), third (6% and 25%) and fourth ganglia (5% and 8%) ( ). Occasionally, a grey and a white ramus fuse to form a ‘mixed’ ramus. An intrathoracic ramus may join the second intercostal nerve to the ventral ramus of the first thoracic spinal nerve proximal to the point where the latter sends a large branch to the brachial plexus ( ). This intrathoracic nerve of Kuntz is thought to carry sympathetic nerve fibres to the brachial plexus without passing through the sympathetic trunk and has been held responsible for the recurrence of symptoms following surgical sympathectomy. The anatomical variation exhibited by the rami communicantes and the location of the second sympathetic ganglion may also contribute to post-surgical symptom recurrence.
The medial branches from the upper five thoracic ganglia are very small and may supply nerve fibres in a retrograde manner to the thoracic parts of the aorta and its branches as they pass to the cardiac plexuses ( ). Rami of the second to fifth or sixth ganglia enter the posterior pulmonary plexus. Small branches from the pulmonary and cardiac nerves pass to the oesophagus and trachea. The larger medial branches from the lower seven ganglia supply the descending thoracic aorta and then unite to form the greater, lesser and least thoracic splanchnic nerves. The thoracic splanchnic nerves are variable in origin and presence ( ); the greater thoracic splanchnic nerve is always present, the lesser is usually present and the least is often absent. A fourth (accessory) thoracic splanchnic nerve has been described.
The greater thoracic splanchnic nerve consists mainly of myelinated preganglionic efferent and visceral afferent nerve fibres. It is usually described as being formed by branches from the fifth to ninth or tenth thoracic sympathetic ganglia, but most commonly it originates from the seventh to the ninth thoracic sympathetic ganglia. Its roots vary in number from three to ten. It descends obliquely along the vertebral bodies and intervening intervertebral discs, sends branches to the descending thoracic aorta, and perforates the ipsilateral diaphragmatic crus to terminate mainly in the coeliac but also in the aorticorenal ganglion and suprarenal (adrenal) gland. A thoracic splanchnic ganglion is present on the nerve opposite the body of the eleventh or twelfth thoracic vertebra in most individuals. The lesser thoracic splanchnic nerve is formed commonly by rami of the ninth and tenth thoracic sympathetic ganglia (range, ninth to twelfth). It pierces the respiratory diaphragm with the greater thoracic splanchnic nerve, which it may join, and terminates in the aorticorenal ganglion. The least thoracic splanchnic nerve (or renal nerve) from the lowest thoracic sympathetic ganglion enters the abdomen with the sympathetic trunk and terminates in the renal plexus. Some nerve fibres from the greater thoracic splanchnic and vagus nerves form a fine web-like thoracic aortic plexus.
Endoscopic thoracic sympathectomy (ETS) is a surgical procedure used to relieve the symptoms of craniofacial, palmar or axillary hyperhidrosis, facial blushing and Raynaud’s disease with digital ulcers.
ETS may be beneficial to ameliorate cerebral vascularization in patients with moyamoya disease and to treat migraine, hyperactive bronchial states, long QT syndrome, causalgic pain, erythromelalgia, Buerger’s disease, Prinzmetal’s angina, and chronic non-infectious rhinitis. ETS is also effective in frostbite injury, especially if performed within 36–72 hours of cold exposure.
The operation involves making tiny incisions posterior to the anterior axillary (pectoral) fold in the axilla, and insufflating a small amount of carbon dioxide into the thoracic cavity to allow access with a modified thoracoscope. In the treatment of facial blushing, it is sufficient to divide the nerve fibres that run superiorly from the second thoracic ganglion over the neck of the second rib, leaving the second ganglion almost intact. Treatment of palmar hyperhidrosis requires ablation thermocoagulation of the sympathetic trunk over the necks of the third and fourth ribs, taking care to avoid any spreading of thermal energy along the trunk in order to avoid damaging the more superior stellate ganglion (see Horner’s syndrome below). The risk of compensatory sweating is greatly reduced, though not completely excluded, by limiting the number of ganglia treated to an absolute minimum. As an additional procedure, dividing the inconstant sympathetic pathways (nerve of Kuntz, ascending or descending additional rami communicantes) on the second, third and fourth ribs may improve surgical outcomes. The effect is immediately evident: the patient awakes from anaesthesia with dry, warm hands. In many cases, even hyperhidrosis of the feet improves, but the underlying anatomical and physiological mechanisms are not yet properly understood ( ). Surgical complications are rare; Horner’s syndrome is of most concern, and is caused by damage to the stellate ganglion and interruption of the sympathetic nerve fibres from the ventral rami of spinal nerves C8–T1 that ascend around the arteries supplying the head and neck (see Fig. 35.18 ). Side-effects include compensatory sweating (ranging from barely noticeable to profuse) in other locations of the body during exercise or from exposure to high temperatures in up to 70% of patients. Compensatory sweating is severe in 5% of patients, and may be more frequent in those with axillary hyperhidrosis when two more inferior ganglia have to be divided: for this reason, some surgeons do not now consider isolated axillary hyperhidrosis as an indication for this procedure. Gustatory or olfactory sweating may also occur in up to one-third of patients but is rarely considered a problem. Other documented side-effects are the inability to raise the heart rate during increased physical strain/load: in some cases, this has led to decreased ability to perform work and daily activities. Patients can also experience an uncomfortable sensation of not being able to control their body temperature.
The vagus nerve contains branchiomotor, general visceral afferent and preganglionic parasympathetic nerve fibres. The proportion of efferent parasympathetic nerve fibres in the vagus nerve varies at different levels but is small relative to its sensory and sensorimotor content. The branchiomotor fibres arise from neurones in the nucleus ambiguus in the medulla oblongata and are distributed to the constrictor muscles of the pharynx and the intrinsic muscles of the larynx. The general visceral afferent fibres carry sensation from thoracic and abdominal viscera and from aortic arch baroreceptors and chemoreceptors and end in the caudal part of the nucleus solitarius of the medulla oblongata. The preganglionic parasympathetic nerves arise from cell bodies in the vagal dorsal nucleus in the medulla oblongata, travel in the nerve and in its pulmonary, cardiac, oesophageal, gastric and intestinal branches and synapse in minute ganglia in the visceral walls (see Fig. 56.6 ) ( ). Pulmonary branches contain axons that synapse in ganglia of the pulmonary plexuses: they are motor (bronchoconstrictor) to the circular non-striated muscle fibres of the bronchi and bronchioles (and therefore bring about bronchoconstriction), and secretomotor to the mucous glands of the respiratory epithelium. Cardiac branches join the cardiac plexuses and synapse in ganglia that are distributed freely over both atria in the subepicardial tissue. Postganglionic fibres are distributed to the atria and the atrioventricular bundle and are concentrated around the sinuatrial and, to a lesser extent, the atrioventricular nodes ( Ch. 57 ). Cardiac branches slow the heart rate and diminish the force of contraction and may indirectly influence ventricular muscle via their effect on the atrioventricular node. The direct postganglionic parasympathetic innervation of the ventricles is sparse. The smaller branches of the coronary arteries are innervated mainly by parasympathetic vagal neurones, whereas the larger arteries are innervated mainly by sympathetic neurones.
The right vagus nerve descends posterior to the right internal jugular vein in the neck and crosses anterior to the first part of the right subclavian artery to enter the thorax; the ansa subclavia is lateral, and the phrenic nerve is further lateral (see Fig. 55.10 A). After giving off the right recurrent laryngeal nerve, it descends through the superior mediastinum, at first posterior to the right brachiocephalic vein, and then lateral to the trachea and posteromedial to the superior vena cava. Superiorly, the right mediastinal part of the parietal pleura and superior lobe of the right lung are lateral; inferiorly, the azygos vein and its arching terminal portion separate the right vagus nerve from the mediastinal parietal pleura (see Fig. 56.7A ). The nerve next passes posterior to the right main bronchus and lies on the posterior aspect of the right lung root/hilum. Here, it gives off posterior bronchial branches that unite with rami from the second to fifth/sixth thoracic sympathetic ganglia to form the right posterior pulmonary plexus. Two or three branches descend from the inferior part of this plexus on the posterior aspect of the oesophagus to join a left vagal branch and form the posterior oesophageal plexus. The posterior vagal trunk, containing nerve fibres from both vagus nerves, leaves the plexus and passes inferiorly on the posterior surface of the oesophagus, entering the abdomen by passing through the oesophageal hiatus in the respiratory diaphragm (see Fig. 55.6 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here