Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There are many excellent textbooks and articles on the mediastinum. This chapter references these articles and a review of the recent literature as the basis for a discussion of the anatomy, pathology, and radiologic manifestations of mediastinal diseases.
Comprehensive knowledge of cross-sectional anatomy is required to accurately evaluate mediastinal abnormalities. Interpretation of mediastinal anatomy can be assisted by analyzing computed tomography (CT) images at specific levels within the chest that are easily identifiable because of their characteristic anatomic landmarks and appearance.
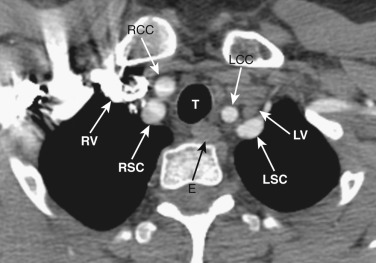
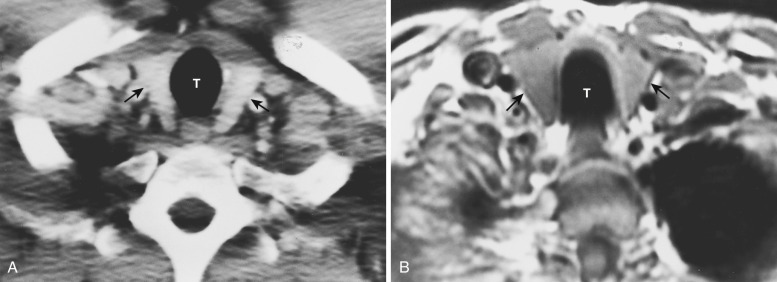
At the junction of the neck and the thorax, most of the mediastinal structures are vascular. The two brachiocephalic veins are formed as the internal jugular veins join the subclavian veins and are located posterior to the clavicular heads ( Fig. 38-1 ). These veins are the most anterior and lateral of the six major vessels at this level. More medial are the two common carotid arteries, and just posterior to these are the subclavian arteries. The subclavian arteries and veins exit the mediastinum to enter the axilla after crossing over the first rib. The esophagus is posterior or posterolateral to the trachea.
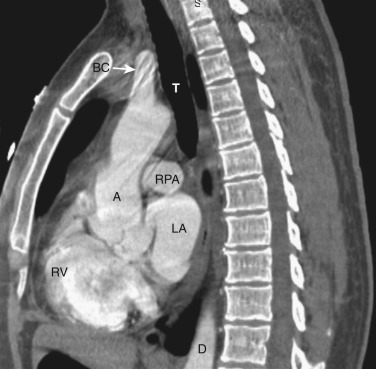
The left brachiocephalic vein crosses the midline anterior to the arterial branches of the aorta and joins the more vertically orientated right brachiocephalic vein to form the superior vena cava (SVC) ( Fig. 38-2 ). The internal mammary veins can often be identified coursing posteriorly from a parasternal location to join the brachiocephalic veins.
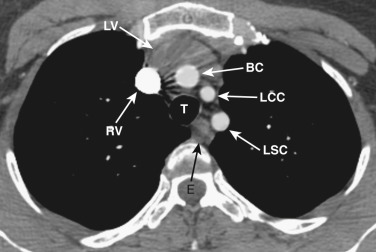
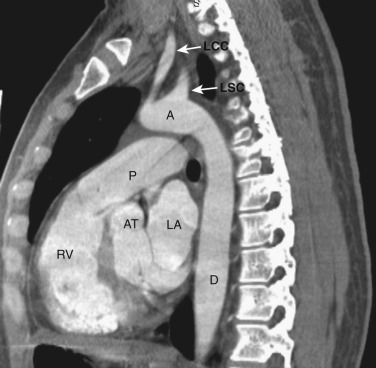
The innominate, left common carotid, and left subclavian arteries are located posterior to the left brachiocephalic vein and anterior to the trachea. The innominate artery is the more centrally located artery; the left common carotid artery (smallest of the three arteries) and the left subclavian artery are located to the left of the midline in a more lateral and posterior position.
The esophagus maintains its position posterior or posterolateral to the trachea and anterior to the spine.
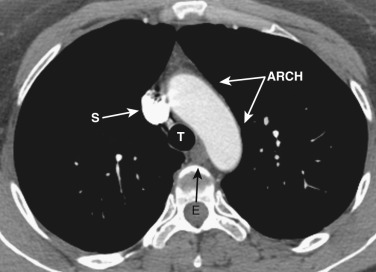
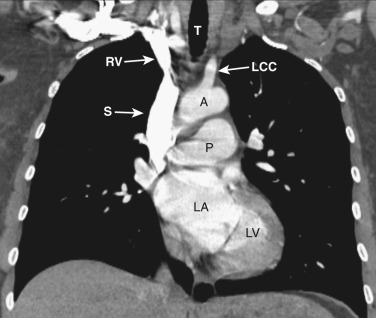
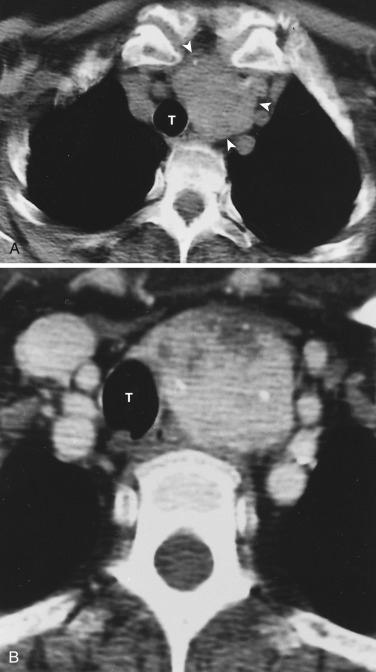
The transverse arch crosses the mediastinum anterior to the trachea, coursing obliquely from right to left and from anterior to posterior ( Fig. 38-3 ). The SVC is located adjacent to the anterior aspect of the transverse aorta to the right of the trachea. The fat-filled region posterior to the SVC, anterior to the trachea, and lateral to the aorta is the pretracheal or anterior paratracheal space. Anterior to the transverse aorta is the fat-filled prevascular space, a compartment of the anterior mediastinum that extends cephalad anterior to the great vessels of the aorta and the brachiocephalic veins. If present, the thymus gland is located in this space. Both of these spaces often contain a few small lymph nodes.
The esophagus maintains its position posterior or posterolateral to the trachea and anterior to the spine.
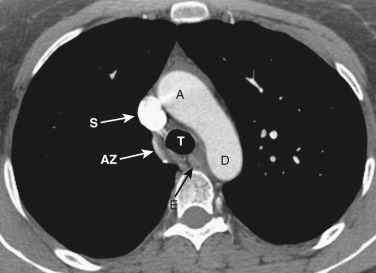
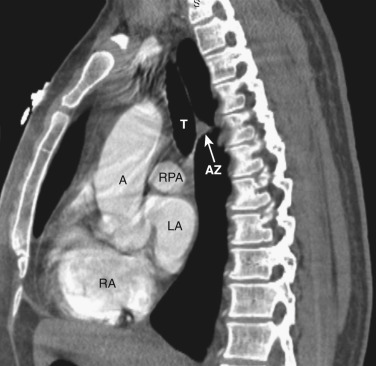
The azygos vein, located anterior and slightly to the right of the spine, arches anteriorly and joins the SVC at this level ( Figs. 38-4 and 38-5 ). The azygos arch may not be seen in its entirety on one slice and may be confused with lymphadenopathy. The ascending and descending aortas are visible as separate structures at this level. The ascending aorta is anterior to the trachea, and the descending aorta is posterolateral to the trachea on the left. Typically the ascending aorta (mean diameter 3.5 cm) is larger than the descending aorta (mean diameter 2.5 cm).
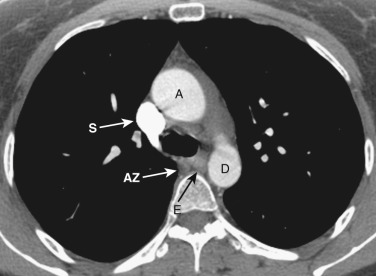
The aortopulmonary window is a space located between the inferior aspect of the aortic arch and the superior aspect of the left main pulmonary artery. In some patients the close anatomic contiguity of these two structures prevents visualization of this space. The space is otherwise fat-filled and contains a few small lymph nodes, the left recurrent laryngeal nerve, and the ligamentum arteriosum.
The esophagus maintains its position posterior to the trachea, anterior to the spine, and medial to the descending aorta.
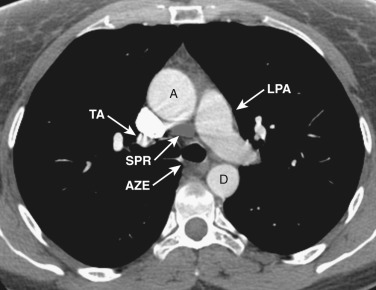
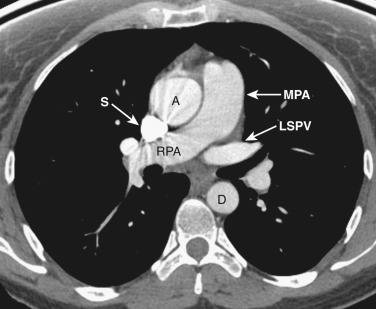
The main pulmonary artery is anterior and to the left of the ascending aorta ( Figs. 38-6 and 38-7 ). The left pulmonary artery curves posteriorly from its origin from the main pulmonary artery and is located anterolateral to the left main bronchus at the level of the carina. The left superior pulmonary veins are located lateral to the posterior portion of the left pulmonary artery. On the right at the level of the carina is the origin of the right upper lobe bronchus. Anterior to the right upper lobe bronchus lies the right upper lobe pulmonary artery, or the truncus anterior. The right superior pulmonary veins are located anterior and lateral to the truncus anterior. The azygos vein is located posterior and to the right of the esophagus, while the hemiazygos vein parallels the course of the azygos but is to the left of the spine.
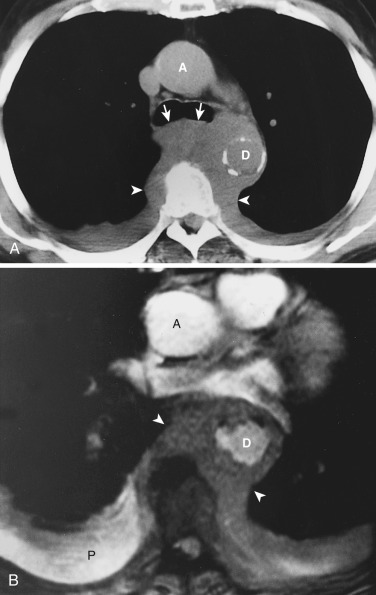
The superior pericardial recess, a crescent-shaped extension of the pericardial space, is contiguous to the posterior aspect of the ascending aorta. The space often contains a small amount of fluid and may occasionally be confused with a lymph node; however, its characteristic location, shape, and low attenuation allow confident identification. Occasionally the superior pericardial recess extends more cephalad to the level of the brachiocephalic vessels and may be mistaken for a mass or mediastinal cyst. The use of thin sections can be helpful to distinguish this so-called high-riding pericardial recess from pathologic conditions by showing that the “mass” is of water attenuation and by demonstrating continuity with the superior pericardial recess at the level of the ascending aorta.
The concave extension of the right lung into the mediastinum anterior to the spine is the azygoesophageal recess, which extends inferiorly from the subcarinal region to the level of the diaphragm.
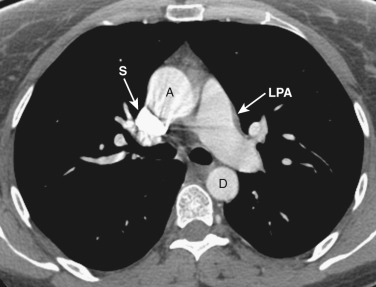
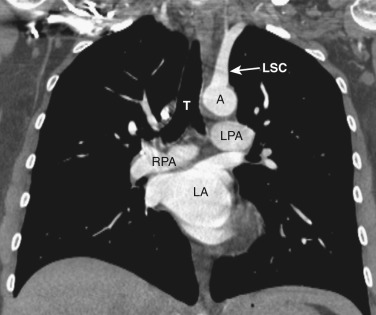
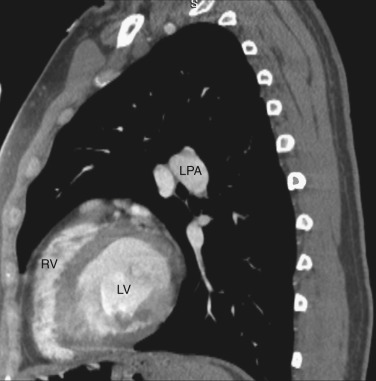
The main pulmonary artery is anterior and to the left of the ascending aorta ( Figs. 38-8 and 38-9 ). The right pulmonary artery extends posteriorly and to the right from the main pulmonary artery, passing anterior to the bronchus intermedius and posterior to the SVC. The right superior pulmonary vein is to the right of and lateral to the intrapulmonary portion of the right pulmonary artery. Anterior to the left main and upper lobe bronchi is the left superior pulmonary vein, and posterior to the left upper lobe bronchus is the left lower lobe pulmonary artery.
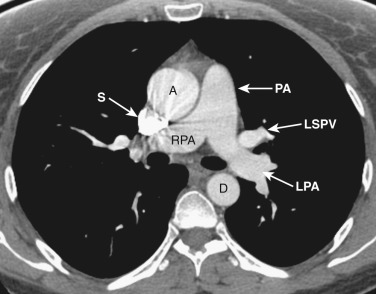
The anatomy of the mediastinum is complex at this level ( Figs. 38-10 and 38-11 ). Anterior to the SVC is the right atrial appendage, curving laterally around the ascending aorta. The aorta is located centrally within the mediastinum, and anterior and to the left of the aorta is the pulmonary outflow tract. Posterolateral and to the left of the pulmonary outflow tract is the left atrial appendage. Within the fat between the left atrial appendage and the aortic root is the left main coronary artery. The left superior pulmonary vein enters the left atrium immediately posterior to the left atrial appendage. The right superior pulmonary veins enter the left atrium just posterior to the SVC.
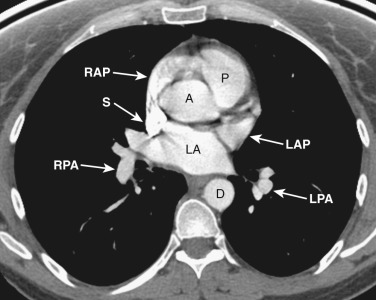
The esophagus position at this level is variable and may be midline or to the right or left of midline along the posterior wall of the left atrium.
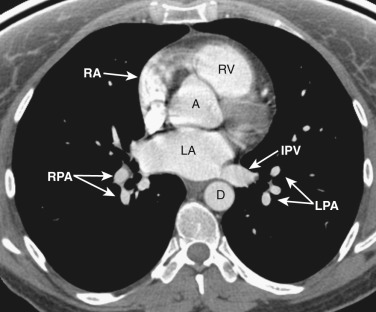
All four chambers of the heart are identified at this axial level ( Figs. 38-12 and 38-13 ) and should not be confused with a four-chamber cardiac reconstructed image. The posteriorly located left atrium is the most cranial of all the chambers. The right and left inferior pulmonary veins enter the posterolateral aspect of the left atrium. Anterior and to the right is the right atrium. To the left and anterior to the right atrium is the right ventricle, located behind the sternum. The left ventricle is to the left and posterior to the right ventricle.
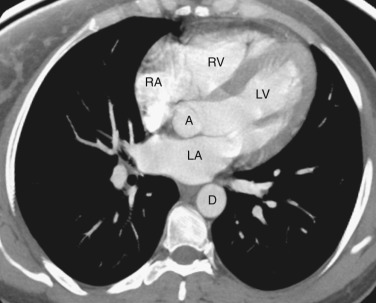
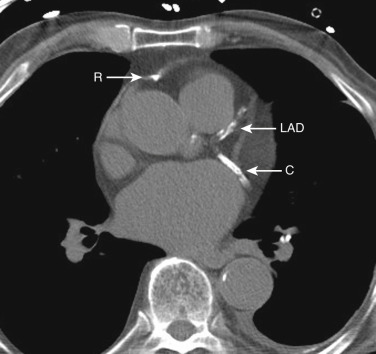
The coronary arteries can be detected if they are calcified or if there is fat surrounding the heart. The right coronary artery lies in the right atrioventricular groove. The circumflex coronary artery lies in the left atrioventricular groove, and the left anterior descending coronary artery lies in the interventricular groove between the left and right ventricles.
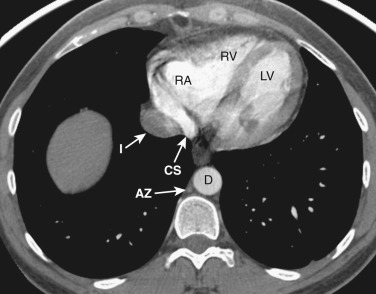
The ventricles and right atrium are identified at this level ( Fig. 38-14 ); the left atrium, because of its more cranial position, is not visible. The right atrium is located to the right. To the left and anteriorly, the thin-walled right ventricle is located just beneath the sternum. The thick-walled left ventricle makes up the posterolateral left portion of the mediastinum. Between the left ventricle and the inferior vena cava's (IVC's) opening into the right atrium is the coronary sinus.
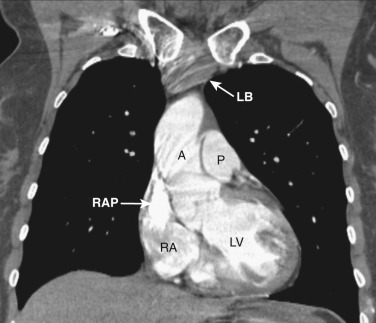
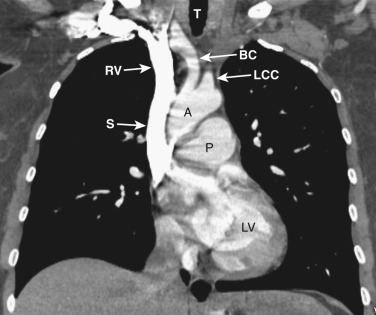
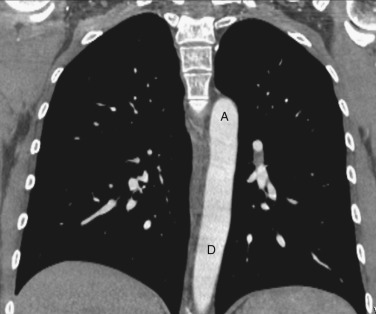
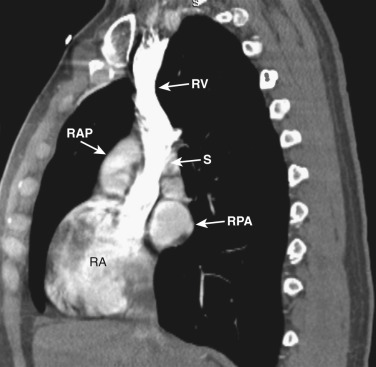
As CT imaging and workstation technologies have advanced, the use of multiplanar imaging reconstructions for interpretation has become routine. It is important for radiologists to recognize the normal mediastinal anatomy in the coronal and sagittal planes. Given the long axis of several structures in the mediastinum, axial and sagittal planes are particularly useful when assessing the aorta, great vessels, vena cava, trachea, esophagus, and heart. Coronal ( Figs. 38-15 to 38-19 ) and sagittal ( Figs. 38-20 to 38-24 ) reformations at different levels are provided for correlation. The same nomenclature as in the axial plane is used, along with the same abbreviations to identify the different structures.
The mediastinum extends craniocaudad from the thoracic inlet to the diaphragm, and historically it has been divided into compartments (on the chest radiograph) to facilitate lesion localization and aid in the differential diagnosis. Although this division of the mediastinum into anatomic regions or compartments is still important, it is not essential, because CT and magnetic resonance imaging (MRI) can accurately localize and in many instances characterize masses. Despite these advances, however, mediastinal masses are still discussed, classified, and grouped by their position on chest radiographs.
In this chapter we use the widely accepted division of the mediastinum into superior, anterior, middle, and posterior compartments. The superior mediastinum is the space between the thoracic inlet and the superior aspect of the aortic arch (i.e., all mediastinal structures above an imaginary line drawn between the sternal angle and the fourth intervertebral disk on the lateral chest radiograph). The anterior mediastinum is the space anterior to the heart and great vessels on the lateral chest radiograph. It is bordered anteriorly by the sternum and posteriorly by the pericardium. Using this designation, normal anterior mediastinal structures include the thymus, branches of the internal mammary artery and vein, lymph nodes, and fat. There is controversy concerning the division between the middle and posterior mediastinum. Some authors believe the dividing line should be the posterior aspect of the pericardium, whereas others divide the two by an imaginary line drawn 1 cm posterior to the anterior border of the vertebral bodies. The latter method places most aortic and esophageal lesions in the middle mediastinum and reserves the posterior mediastinum for neurogenic or other paraspinal lesions. Other methods divide the middle and posterior mediastinum based on the azygos arch and aorta. For purposes of this discussion, we define the middle mediastinum as the space between the anterior border of the pericardium and an imaginary line drawn 1 cm posterior to the anterior border of the vertebral bodies on the lateral radiograph. As such, it contains the heart, aorta, SVC, IVC, brachiocephalic arteries and veins, pulmonary arteries and veins, thoracic duct, azygos and hemiazygos veins, phrenic and vagus nerves, trachea and proximal bronchi, esophagus, mediastinal fat, and lymph nodes. The posterior mediastinum is defined as the space between the imaginary line drawn 1 cm posterior to the anterior border of the vertebral bodies and the posterior paravertebral gutters. Structures in the posterior mediastinum thus include nerves, fat, vertebral column, and lymph nodes.
Although the chest radiograph is usually the initial study that reveals a mediastinal abnormality, CT is used to assess the location and extent of mediastinal disease; because of its superior contrast resolution, it is also used to characterize the tissue components of masses ( Fig. 38-25 ). CT is useful for distinguishing vascular variants or benign processes of the mediastinum (e.g., lipomatosis) from true pathologic conditions.
Although CT is usually used to further evaluate an abnormality detected on the chest radiograph, in certain clinical situations CT may be performed when the chest radiograph is normal, such as in patients with myasthenia gravis, owing to the strong association between that disease and thymoma ( Fig. 38-26 ). Also, certain malignancies such as lung cancer have a marked predilection to metastasize to mediastinal lymph nodes. Because these lymph node metastases may not be visible on chest radiographs, CT is used by most thoracic surgeons and oncologists to assess the mediastinal nodes in patients with lung cancer.
Recent advances in CT imaging, such as multidetector and dual-source technologies, have improved its ability to image the mediastinum. Significant shortening of scanning time limits respiratory and cardiac motion artifacts, and in some instances the total dose of iodinated contrast material can be reduced. Dual-energy CT applications for imaging the mediastinum are not yet well established but may be helpful in quantifying perfusion, a potentially helpful marker in differentiating benign from malignant processes.
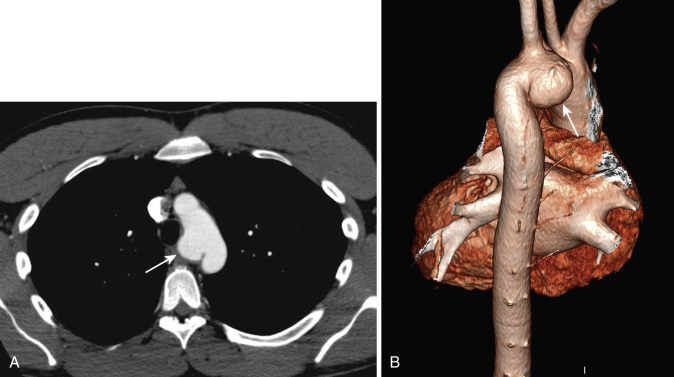
Volumetric CT data sets can be effectively reconstructed in a variety of nonaxial planes, often facilitating interpretation of mediastinal abnormalities. Application of nonaxial two- and three-dimensional (2D and 3D) reconstruction techniques has proved most useful for imaging abnormalities of the central airways and great vessels ( Figs. 38-27 and 38-28 ). For example, in the evaluation of stenoses in obliquely oriented bronchi, these reconstruction techniques can improve diagnostic accuracy and confidence in the interpretation. It should be emphasized, however, that axial CT images are usually sufficient for diagnosis. Nevertheless, because 2D and 3D images present anatomic information in a context more familiar to clinicians, these methods may demonstrate the location and extent of an abnormality in a way that radiologic reports and axial CT images often do not and can be helpful in treatment planning.
Most routine CT scans of the mediastinum can be performed without intravenous (IV) contrast enhancement unless the primary indication is a suspected vascular abnormality ( Fig. 38-29 ). Interpretation of non–contrast-enhanced (NCE) CT scans can be more difficult and time-consuming than interpretation of contrast-enhanced scans and requires a thorough knowledge of mediastinal anatomy and normal variants. There are, however, several advantages to NCE CT: (1) scan time is decreased because there is no need to establish IV access, set up the contrast injector, or delay the scan as the bolus is administered; (2) there is no risk of contrast reaction; and (3) the cost is lower.
When needed, IV contrast is given through a large forearm or antecubital vein using a power injector. The contrast injection rate and total injection volume depend on the indication. For routine purposes a rate of 3 mL/sec for a total volume of 100 mL is sufficient. For vascular imaging (aorta and pulmonary artery), higher injection rates (4-6 mL/sec) are often necessary. In such cases, injection through a relatively large-bore IV catheter (18 or 20 gauge) into a large antecubital vein is mandatory. Timing of the bolus in relationship to the scan also varies and depends on both the indication (routine vs. arterial imaging) and the time required to complete the scan.
CT is usually performed without esophageal contrast; however, oral contrast can be helpful in identifying the relationship between the esophagus and other mediastinal structures and occasionally in depicting intrinsic esophageal abnormalities ( Fig. 38-30 ). Commercial preparations of esophageal paste made of a dilute barium mixture are available. The patient must swallow several teaspoons of the paste and refrain from further swallowing during the examination.
CT scans of the mediastinum are usually reconstructed and interpreted in an axial format. Until recently, sagittal, coronal, and off-axis reconstructions of the thorax were not routinely performed because of slice misregistration and respiratory motion artifacts. However, with the advent of multiplanar scanning, continuous volume data sets can be acquired during a single breath hold, and excellent nonaxial 2D and 3D reconstructed images of mediastinal vascular structures and airways can be generated (see Figs. 38-3 and 38-4 ). The different reconstruction methods, such as multiplanar reformat imaging, multiplanar volume reformat imaging, and external and internal 3D renderings, vary significantly in computational complexity and time required to generate the images.
Interpretation of mediastinal CT images is most commonly performed at a PACS (picture archiving and communication system) or workstation that permits dynamic adjustment of window and level settings for thorough assessment of the mediastinum; a wide range of attenuation values (−800 Hounsfield units [HU] for lung, 600 HU for bone) are encountered. Appropriate image settings are essential for accurate depiction of air, fat, fluid, calcification, IV contrast, and bone. If hard-copy (film) format is used, care should be taken to ensure adequate window and level settings are used. When filming CT studies of the chest, lung settings should have a wide width to encompass air-filled lung and soft tissue and are usually viewed at a level of −600 and a width of 1200. Because of the smaller density range among fat, soft tissue, bone, and contrast-enhanced vessels, mediastinal settings are usually viewed at a level of 50 and a width of 350. These levels and widths can be varied to aid in evaluation of any lesion that cannot be adequately differentiated on standard settings. Bone windows, with a wide width and a level of near 400 HU, are often needed to evaluate for possible bone metastases.
Although CT imaging usually provides the requisite information in most patients with mediastinal abnormalities, MRI, because of its multiplanar capability and high contrast resolution, is occasionally used to evaluate the location and extent of disease. MRI is the modality of choice for imaging neurogenic tumors, because it not only demonstrates the number and nature of the lesions but also depicts intraspinal extension. Additionally MRI is useful in confirming the cystic nature of mediastinal lesions that appear solid on CT ( Fig. 38-31 ) and demonstrating vascular structures in patients in whom use of iodinated IV contrast is contraindicated ( Fig. 38-32 ). The role of MRI in characterizing mediastinal pathology is evolving, with recent literature suggesting potential applications in differentiating benign from malignant nodal and thymic disease.
Two potential disadvantages of MRI of mediastinal abnormalities, compared with CT, are its poor demonstration of calcification and comparatively poor spatial resolution.
Whereas CT and MRI provide morphologic information, positron emission tomography (PET) and integrated PET/CT provide important functional information in the evaluation and management of mediastinal disease. Several studies have shown the value of fluorine-18 fluorodeoxyglucose ( F FDG) PET in differentiating benign from malignant mediastinal masses. FDG uptake is associated with thymoma, thymic carcinoma, carcinoid, lymphoma, esophageal carcinoma, and metastatic disease among other entities. Of particular interest in oncologic imaging is the identification of mediastinal nodal disease for staging purposes, which affects treatment decisions and potential resectability of the primary tumor. On CT, lymph node size is the primary determinant of disease, with a short-axis dimension greater than 1 cm indicative of pathology. Unfortunately, metastatic involvement can be associated with normal-sized lymph nodes and go undetected at CT imaging. FDG PET, however, is highly sensitive and specific for detection of nodal disease relative to CT alone. Accuracy, sensitivity and specificity improves even more when PET is performed in conjunction with CT (PET/CT). Furthermore, PET is superior to CT in detecting distant metastases, which has been shown to improve staging accuracy and reduce futile treatments.
FDG PET also plays an important role in monitoring treatment response following initiation of therapy. PET has also been found to provide prognostic information in certain mediastinal pathology; for example, a negative PET obtained following treatment for lymphoma is associated with a lower risk of recurrent disease and longer progression-free survival than in patients with a positive posttreatment PET examination.
Although PET is primarily used in the evaluation of suspected malignancy, FDG uptake can also be associated with infectious and inflammatory conditions of the mediastinum, such as acute mediastinitis or sarcoidosis. Examples of the utility of FDG PET in the evaluation of mediastinal processes are provided in reference to specific disease entities throughout this chapter.
A useful approach for discussing mediastinal abnormalities is to classify them into processes that predominantly affect specific compartments (superior, anterior, middle, or posterior) and those that can affect multiple compartments ( Table 38-1 ).
| Location | Lesion |
|---|---|
| Superior mediastinum | Thyroid goiter, tortuous great vessels |
| Anterior mediastinum | Thymic lesions, germ cell tumors, parathyroid adenoma, lymphatic malformations, hemangioma |
| Middle mediastinum | Esophageal lesions, airway lesions, foregut cysts, pericardial cysts |
| Posterior mediastinum | Neurogenic tumors, paraspinal abscess, extramedullary hematopoiesis |
| Multiple compartments | Mediastinitis, lipomatosis, lymphadenopathy (lymphoma, metastases, Castleman's disease, infection), mesenchymal tumors, vascular abnormalities and anomalies, diaphragmatic hernias |
Common superior mediastinal abnormalities include intrathoracic extension of thyroid goiters or masses and tortuous great vessels.
On CT the normal thyroid gland is typically visible at or just below the level of the cricoid cartilage ( Fig. 38-33A ). It appears on NCE CT as two wedge-shaped structures of homogeneous attenuation on either side of the trachea, separated by a narrow anterior isthmus. It typically enhances homogeneously following administration of IV contrast. On MRI T1-weighted images the normal thyroid gland usually has intermediate signal intensity, slightly greater than that of adjacent muscle, and on T2-weighted images it has higher signal intensity (see Fig. 38-33B ).
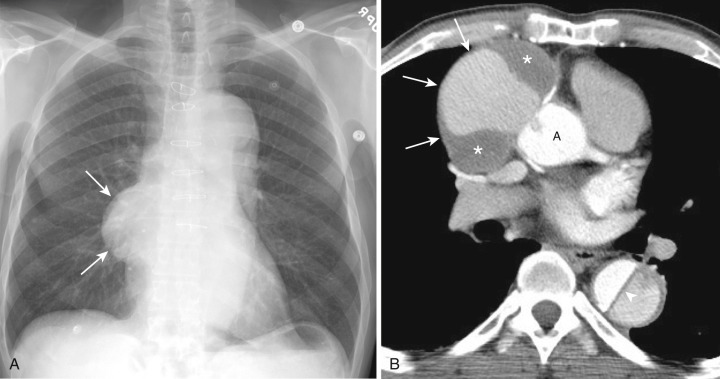
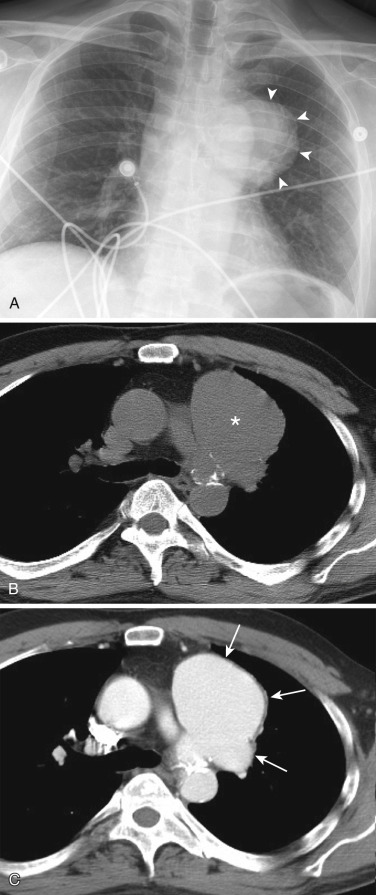
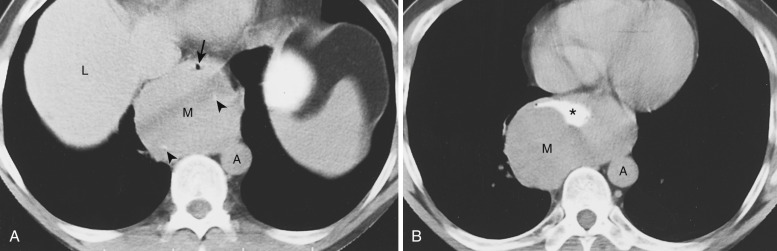
Most thyroid masses in the mediastinum are caused by intrathoracic extension of thyroid goiters; these account for up to 10% of mediastinal masses resected at thoracotomy. True ectopic thyroid masses in the mediastinum are rare. Typically a thyroid goiter extends into the thyropericardiac space anterior to the recurrent laryngeal nerve and brachiocephalic vessels, although posterior extension adjacent to the trachea occurs in 20% of cases. Rarely a thyroid mass extends behind the esophagus and presents as a posterior mediastinal mass.
A mediastinal goiter is usually detected on chest radiographs as a thoracic inlet or superior mediastinal mass that deviates and occasionally narrows the trachea. Although scintigraphy can detect mediastinal goiters, uptake of technetium or iodine is variable and may not be visible. The CT appearance of a mediastinal goiter is variable, but it can be confidently diagnosed when lesion continuity with the thyroid gland is visible. Additional useful features to establish the diagnosis are heterogeneous attenuation with areas of both high and low attenuation on NCE scans, marked enhancement following administration of IV contrast, and focal punctate or curvilinear calcifications ( Figs. 38-34 and 38-35 ). The cystic and adenomatoid composition of goiters results in a heterogeneous appearance on both T1- and T2-weighted MRIs ( Fig. 38-36 ). On T1-weighted images goiters may have a lower signal intensity than normal thyroid, but high signal intensity may be seen in areas of subacute hemorrhage, colloid cysts, and adenomas. Goiters have high signal intensity on T2-weighted images.
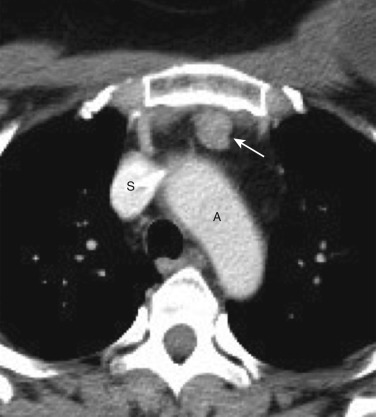
Tortuosity of the great vessels is a common cause of superior mediastinal abnormality on chest radiographs. The most common location of the radiographic abnormality is in the right paratracheal region; this is true because in older patients the right brachiocephalic artery often has a tortuous course before giving rise to the right subclavian and common carotid arteries. NCE CT can often determine that tortuous vessels are the cause of the apparent mass. Calcification within vessel walls allows confident identification of tortuous arteries. If any doubt exists as to the vascular nature of the mass, IV contrast enhancement readily identifies the vessels ( Fig. 38-37 ).
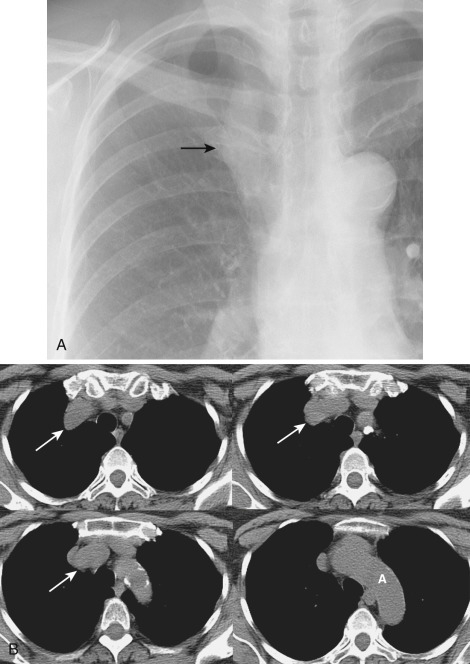
Approximately half of all mediastinal masses occur in the anterior mediastinum. Lesions that occur preferentially in the anterior mediastinum include thymic lesions (cysts, thymolipoma, thymoma, thymic carcinoma), germ cell tumors, parathyroid adenoma, lymphatic malformations, and hemangioma. Lymphoma is another common cause of an anterior mediastinal mass, but it is discussed in the section on diffuse mediastinal disease.
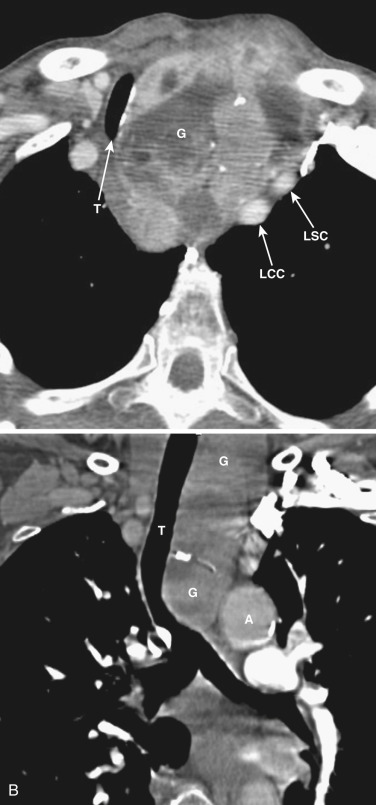
The thymus is a bilobed triangular gland that occupies the thyropericardiac space of the anterior mediastinum and extends inferiorly to the heart. The normal morphology and size of the thymus change markedly with age. There is wide variation in the normal size of the thymus, particularly in children and young adults. In newborns the thymus gland is often larger than the heart. Thymic size decreases with age as the gland undergoes fatty infiltration. An atrophied thymus is often visualized on CT in patients in the fourth decade of life but is seen in less than 50% of patients older than 40 years ( Fig. 38-38 ). The most useful measurement is the thickness of the lobes, measured perpendicular to the long axis of the gland. The normal maximal thickness before age 20 is 18 mm; it is 13 mm in older individuals. Although these measurements are useful indicators of thymic abnormality, thymic shape is also important; focal contour abnormality of the normal thymus gland suggests an underlying abnormality.
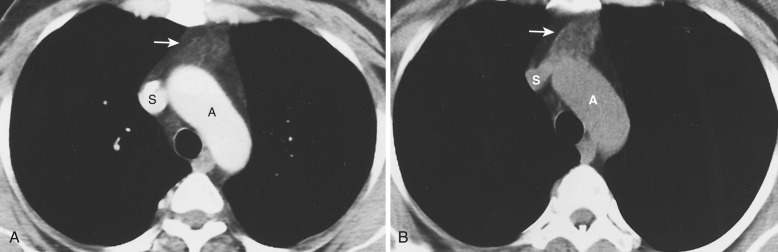
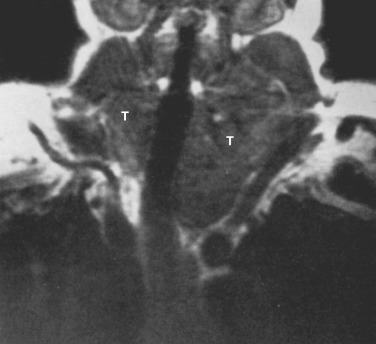
On CT the normal thymus appears as a homogeneous bilobed structure of soft tissue attenuation in the anterior mediastinum ( Fig. 38-39 ). It is usually seen at the level of the aortic arch and the origin of the great vessels. The left lobe is usually slightly larger than the right. Rarely a lobe is congenitally absent. The normal thymus is easily demonstrated on MRI ( Fig. 38-40 ). Characteristically the thymus is homogeneous with intermediate signal intensity (less than that of fat) on T1-weighted images. Because the thymus begins to involute at puberty and is replaced by fat in older patients, the T1-weighted signal intensity of the thymus increases with age. On T2-weighted images the thymus has high signal intensity similar to fat in all age groups; this can make identification of the thymus difficult in patients with abundant mediastinal fat. The normal thymus in patients younger than 20 years typically has diffuse increased FDG uptake on PET. After that age, significant FDG accumulation is less common. Nevertheless, significant FDG uptake in the thymus, sometimes simulating that seen in malignancy, is occasionally seen in adults (see Fig. 38-39 ). A standardized uptake value (SUV) of less than 3.1 is typical for normal thymic tissue, whereas SUV greater than 3.4 is suspicious for malignancy.
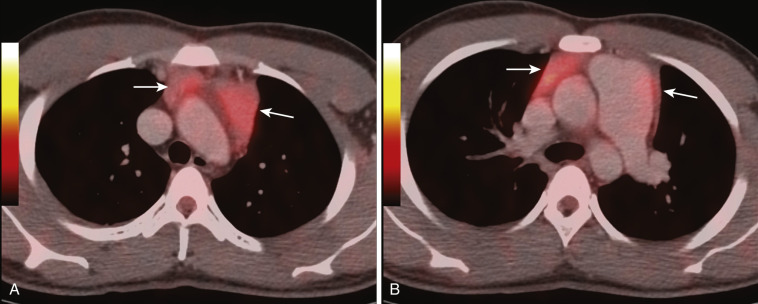
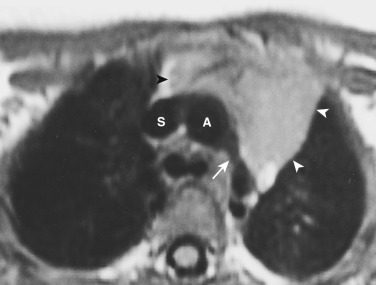
Thymic masses may sometimes be difficult to distinguish from normal thymus. As a rule, however, thymic masses usually manifest on CT or MRI as round masses and do not conform to the shape of the normal thymus. In addition, thymic masses are usually of heterogeneous attenuation on CT, with areas of decreased attenuation and possibly calcification.
Thymic hyperplasia can occur in association with hyperthyroidism, acromegaly, Addison's disease, or stress, as well as in patients receiving chemotherapy or radiation therapy and in those with myasthenia gravis. Differentiating between hyperplasia and other causes of thymic enlargement on CT can be difficult because there are no specific attenuation characteristics associated with hyperplasia. Because thymic rebound or hyperplasia occurs in patients after chemotherapy or radiation therapy, it can be difficult to distinguish this enlargement from recurrent tumor. Generally, however, the thymus in rebound hyperplasia has a normal shape, with homogeneous attenuation on CT ( Figs. 38-41 and 38-42 ). Asymmetry of the lobes, focal contour abnormalities, and heterogeneity suggest tumor.
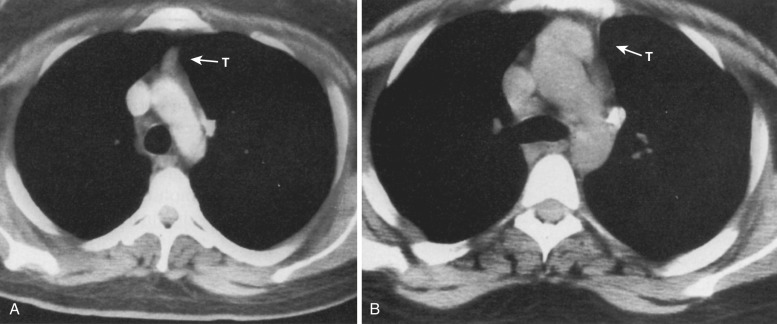
MRI may be beneficial in distinguishing normal thymus and thymic hyperplasia from thymic neoplasms. Unlike thymic tumors, normal and hyperplastic thymic tissue suppresses on out-of-phase gradient echo imaging because of the presence of interspersed microscopic fat. In addition, thymic rebound or hyperplasia is typically of homogeneous normal intensity on MRI, whereas tumors demonstrate more heterogeneous signal properties and enhancement.
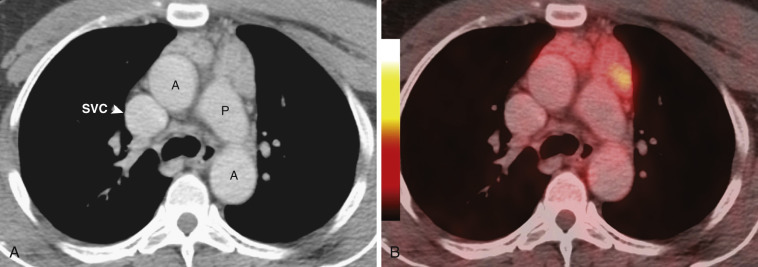
Preliminary data suggest that FDG PET imaging may be helpful for differentiating thymic hyperplasia from thymoma in patients with myasthenia gravis. Patients with thymic hyperplasia show homogeneous FDG accumulation in the thymus (see Fig. 38-42 ), whereas patients with thymoma tend to have more focal FDG uptake. The degree of uptake tends to be greater in thymoma than in thymic hyperplasia.
Thymic cysts account for approximately 3% of all anterior mediastinal masses and are either congenital or secondary to inflammation or malignancy.
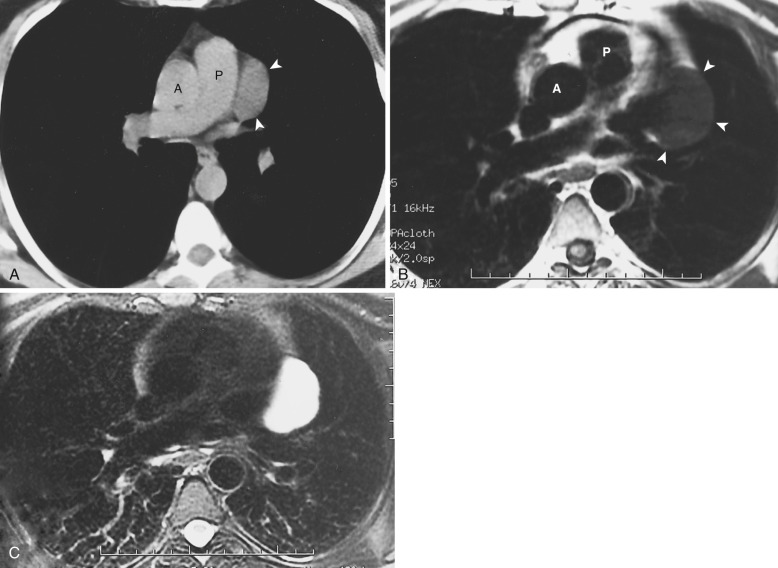
Congenital thymic cysts most likely arise from remnants of the thymopharyngeal duct and are usually thin-walled unilocular lesions less than 6 cm in diameter. On CT they are homogeneous, of water attenuation, with very thin or imperceptible walls. On MRI they manifest as well-circumscribed anterior mediastinal masses of high signal intensity on T2-weighted images. Signal intensity usually increases with increasing repetition time (TR). Occasionally, thymic cysts appear as solid masses on CT because they are filled with proteinaceous fluid. In these cases, MRI can be useful to confirm the cystic nature of the lesion ( Fig. 38-43 ).
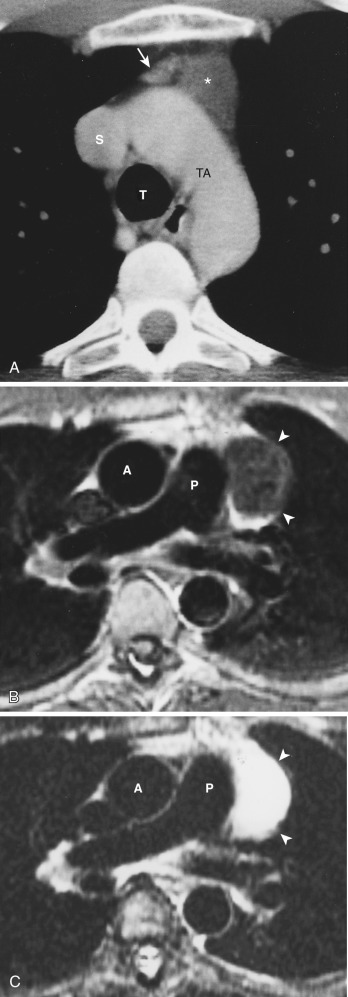
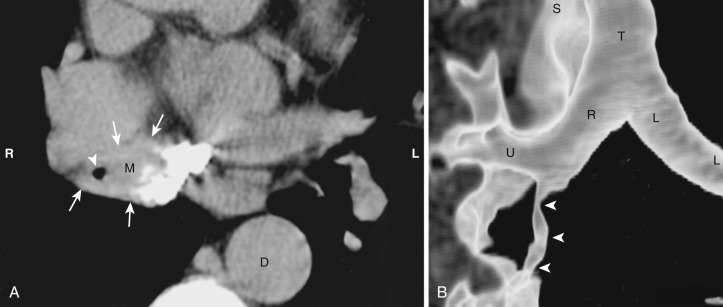
Acquired thymic cysts occur in the setting of inflammation (e.g., Sjögren's syndrome, aplastic anemia, myasthenia gravis) or malignancy. Associated malignancies include Hodgkin's lymphoma, seminoma, thymoma, and thymic carcinoma ( Fig. 38-44 ). These cysts usually have walls of variable thickness, are multilocular, and range in size from 3 to 17 cm in diameter. These cysts can contain hemorrhage or calcification. As such, the CT and MRI features of acquired thymic cysts are more variable than those of congenital thymic cysts. Because of their association with thymic neoplasia, care must be taken when interpreting cystic lesions of the anterior mediastinum. If a thymic cyst is multilocular, heterogeneous, thick walled, or associated with a soft tissue component, it must be evaluated further (biopsy or resection) to exclude malignancy (see Fig. 38-44 ). Furthermore, multiloculated thymic cysts can exhibit FDG uptake on PET/CT, although typically less than that of thymoma or thymic carcinoma ( Fig. 38-45 ).
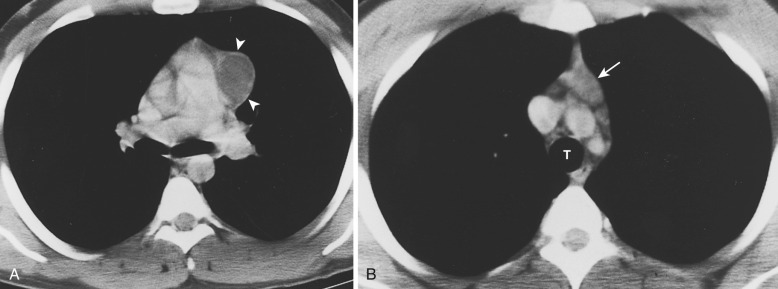
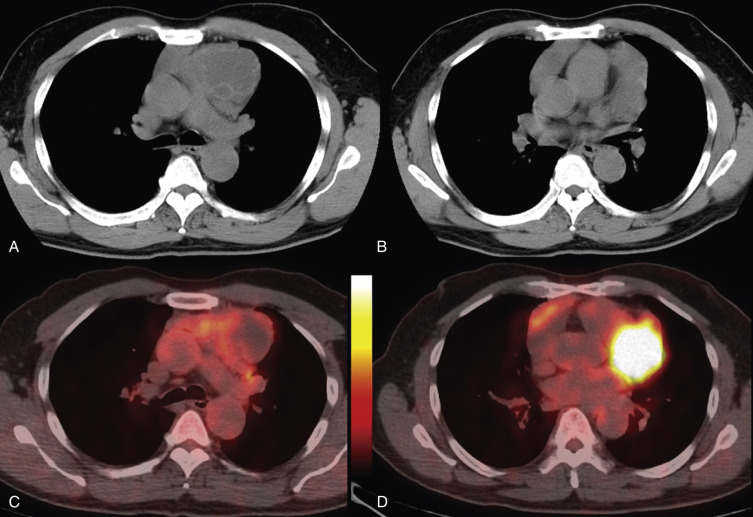
Thymolipomas are rare, benign, slowly growing neoplasms of the anterior mediastinum that can simulate cardiomegaly on the chest radiograph. They occur with equal incidence in men and women, and although there is a wide age range at presentation, most are diagnosed in young adults. On CT these masses are typically large and heterogeneous with a mixture of fat elements and soft tissue attenuation, conforming to adjacent structures. They are not locally invasive, although compression of adjacent structures occurs in approximately 50% of patients. Because they consist of fat and residual thymic tissue, thymolipomas usually have high signal intensity (similar to fat) with interspersed areas of intermediate signal intensity on both T1- and T2-weighted MRIs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here