Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Amazingly, ventilation via tracheal cannulation was performed as early as 1543, when Vesalius demonstrated the ability to maintain the beating heart in animals with open chests. This technique was first applied to humans in 1780, but there was little progress in positive-pressure ventilation until the development of the Fell–O’Dwyer apparatus. This device provided translaryngeal ventilation using bellows and was first used in 1887 ( Fig. 7.1 ). The Drinker–Shaw iron lung, which allowed piston-pump cyclic ventilation of a metal cylinder and concomitant negative-pressure ventilation, became available in 1928 and was followed by a simplified version built by Emerson in 1931. Such machines were the mainstays in the ventilation of victims of poliomyelitis in the 1930s through the 1950s.
In the 1920s, the technique of tracheal intubation was refined by Magill and Rowbotham. In World War II, the Bennett valve, which allowed cyclic application of high pressure, was devised to allow pilots to tolerate high-altitude bombing missions. Concomitantly, the use of translaryngeal intubation and mechanical ventilation became common in the operating room as well as in the treatment of respiratory insufficiency. However, application of mechanical ventilation to newborns, in both the operating room and the intensive care unit (ICU), lagged behind that for children and adults.
The use of positive-pressure mechanical ventilation in the management of respiratory distress syndrome (RDS) was described in 1962. It was the unfortunate death of Patrick Bouvier Kennedy at 32 weeks gestation in 1963 that resulted in additional National Institutes of Health (NIH) funding for research in the management of newborns with respiratory failure. The discovery of surfactant deficiency as the etiology of RDS in 1959, the ability to provide positive-pressure ventilation in newborns with respiratory insufficiency in 1965, and demonstration of the effectiveness of continuous positive airway pressure (CPAP) in enhancing lung volume and ventilation in patients with RDS in 1971 set the stage for the development of continuous-flow ventilators specifically designed for neonates. The development of neonatal intensive care units (NICUs), hyperalimentation, and neonatal invasive and noninvasive monitoring enhanced the care of newborns with respiratory failure and increased survival in preterm newborns from 50% in the early 1970s to more than 90% today. In fact, as the evolution of mechanical ventilation continues, the future is focused on the use of noninvasive approaches, automated modes and weaning strategies, and early mobilization.
The approach to mechanical ventilation is best understood if the two variables of oxygenation and carbon dioxide (CO 2 ) elimination are considered separately.
The primary purpose of ventilation is to eliminate CO 2 , which is accomplished by delivering tidal volume ( V t ) breaths at a designated rate. The product ( V t × rate) determines the minute volume ventilation (
). Although CO 2 elimination is proportional to
E , it is, in fact, directly related to the volume of gas ventilating the alveoli because part of the
E resides in the conducting airways or in nonperfused alveoli. Therefore, the portion of the ventilation that does not participate in CO 2 exchange is termed the dead space ( V d ). In a patient with healthy lungs, this dead space is fixed based on anatomy and consists of about one-third of the tidal volume (i.e., V d / V t = 0.33). In a setting of respiratory insufficiency, the proportion of dead space ( V d / V t ) may be augmented by the presence of nonperfused alveoli and a reduction in V t . Furthermore, dead space can unwittingly be increased through the presence of extensions of the trachea such as an endotracheal tube, a pneumotachometer to measure tidal volume, an end-tidal CO 2 monitor, or an extension of the ventilator tubing beyond the Y .
V t is a function of the applied ventilator pressure and the volume/pressure relationship (compliance), which describes the ability of the lung and chest wall to distend. At the functional residual capacity (FRC), the static point of end expiration, the tendency for the lung to collapse (elastic recoil) is in balance with the forces that promote chest wall expansion. As each breath develops, the elastic recoil of both the lung and chest wall work in concert to oppose lung inflation. Therefore, pulmonary compliance is a function of both the lung elastic recoil (lung compliance) and that of the rib cage and diaphragm (chest wall compliance).
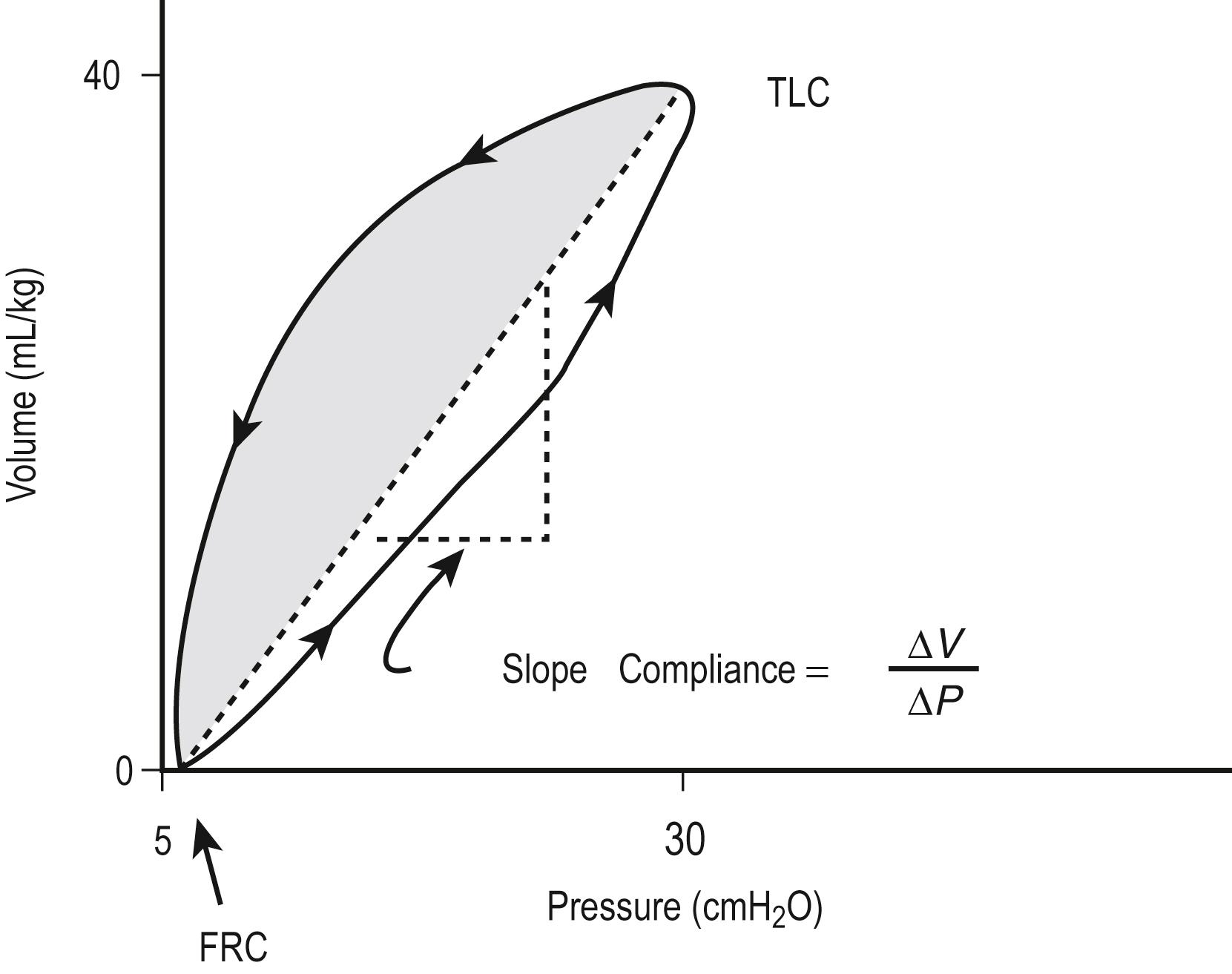
The compliance can be determined in a dynamic or static mode. Figure 7.2 demonstrates the dynamic volume/pressure relationship for a normal patient. Note that application of 25 cmH 2 O of inflating pressure (Δ P ) above static FRC at positive end-expiratory pressure (PEEP) of 5 cmH 2 O generates a V t of 40 mL/kg. The lung, at an inflating pressure of 30 cmH 2 O when compared with ambient (transpulmonary) pressure, is considered to be at total lung capacity (TLC) ( Table 7.1 ). Note that the loop observed during both inspiration and expiration is curvilinear. This is due to the resistance in the airways and describes the work required to overcome resistance to airflow. As a result, at any given point of active flow, the measured pressure in the airways is higher during inspiration and lower during expiration than at the same volume under zero-flow conditions. Pulmonary compliance measurements, as well as alveolar pressure measurements, can be effectively performed only when no flow is present in the airways, which occurs at FRC and TLC. The change observed is a volume of 40 mL/kg and pressure of 25 cmH 2 O or 1.6 mL/kg/cmH 2 O. This is termed effective compliance because it is calculated only between the two arbitrary points of end inspiration and end expiration.
| Variable | Definition | Normal Value |
|---|---|---|
| TLC | Total lung capacity | 80 mL/kg |
| FRC | Functional residual capacity | 40 mL/kg |
| IC | Inspiratory capacity | 40 mL/kg |
| ERV | Expiratory reserve volume | 30 mL/kg |
| RV | Residual volume | 10 mL/kg |
| V t | Tidal volume | 5 mL/kg |
| E |
Minute volume ventilation | 100 mL/kg/min |
| VA | Alveolar ventilation | 60 mL/kg/h |
| V d | Dead space | mL = wt in lb |
| V d / V t | % Dead space | 0.33 |
| C St | Static compliance | 2 mL/cmH 2 O/kg |
| C eff | Effective compliance | 1 mL/cmH 2 O/kg |
As can be seen from Figure 7.3 , the volume/pressure relationship is not linear over the range of most inflating pressures when a static compliance curve is developed. Such static compliance assessments are most commonly performed via a large syringe in which aliquots of 1–2 mL/kg of oxygen, up to a total of 15–20 mL/kg, are instilled sequentially with 3- to 5-second pauses. At the end of each pause, zero-flow pressures are measured. By plotting the data, a static compliance curve can be generated. This curve demonstrates how the calculated compliance can change depending on the arbitrary points used for assessment of the effective compliance ( C eff ).
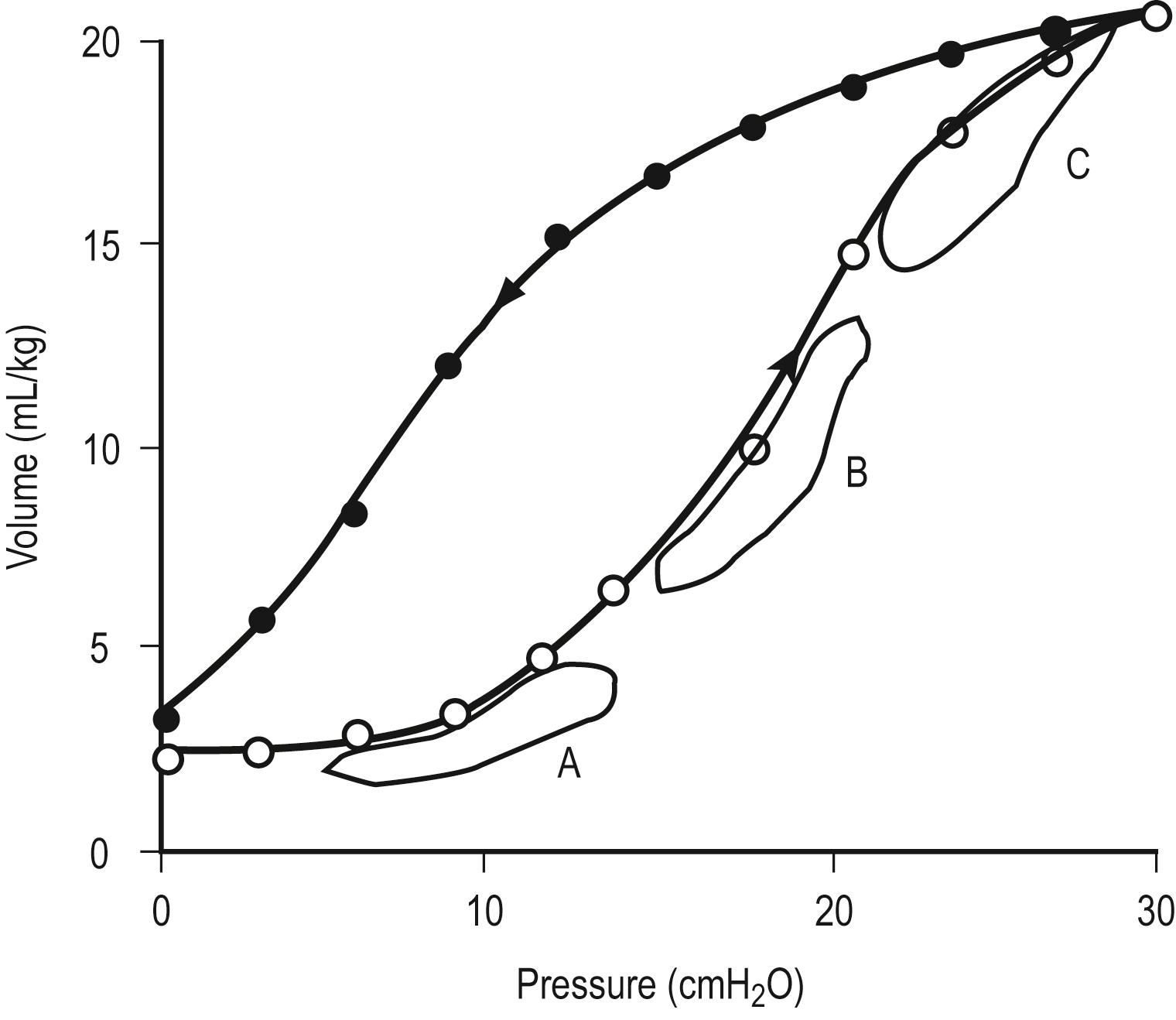
Alternatively, the pulmonary pressure/volume relationship can be assessed by administration of a slow constant flow of gas into the lungs with simultaneous determination of airway pressure. A curve may be fitted to the data points to determine the optimal compliance and FRC. The compliance will change as the FRC or end-expiratory lung volume (EELV) increases or decreases. For instance, as can be seen in Figure 7.3 , at low FRC (point A), atelectasis is present. A given Δ P will not optimally inflate alveoli. Likewise, at a high FRC (point C), because of air trapping or application of high PEEP, the lung is already distended. Application of the same Δ P will result only in overdistention and potential lung injury, with little benefit in terms of added V t . Thus, optimal compliance is provided when the pressure/volume range is on the linear portion of the static compliance curve (point B). Clinically, the compliance at a variety of FRC or PEEP values can be monitored to establish optimal FRC.
Finally, it is important to recognize that a portion of the V t generated by the ventilator is actually compression of gas within both the ventilator tubing and the airways. The ratio of gas compressed in the ventilator tubing to that entering the lungs is a function of the compliance of the ventilator tubing and the lung. The compliance of the ventilator tubing is 0.3–4.5 mL/cmH 2 O. A change in pressure of 15 cmH 2 O in a 3-kg newborn with respiratory insufficiency and a pulmonary compliance of 0.4 mL/cmH 2 O/kg would result in a lung V t of 18 mL and an impressive ventilator tubing/gas compression volume of 15 mL if the tubing compliance were 1.0 mL/cmH 2 O. The relative ventilator tubing/gas compression volume would not be as striking in an adult. The ventilator tubing compliance is characterized for all current ventilators and should be factored when considering V t data. The software in many ventilators corrects for ventilator tubing compliance when displaying V t values.
Typical ventilator rate requirements in patients with healthy lungs range from 10 breaths/min in an adult to 30 breaths/min in a newborn. The V t is maintained at 5–10 mL/kg, resulting in a
E of about 100 mL/kg/min in adults and 150 mL/kg/min in newborns. In healthy lungs, these settings should provide sufficient ventilation to maintain normal Pa CO 2 levels of approximately 40 mmHg, and should generate peak inspiratory pressures between 15–20 cmH 2 O above an applied PEEP of 5 cmH 2 O. Clinical assessment by observing chest wall movement, auscultation, and evaluation of gas exchange determines the appropriate V t in a given patient. In the near future, use of diaphragmatic monitoring will play a role in synchronizing the tidal volumes required for a given patient.
In contrast to CO 2 determination, oxygenation is determined by the fraction of inspired oxygen ( Fi O 2 ) and the degree of lung distention or alveolar recruitment, determined by the level of PEEP and the mean airway pressure ( P aw ) during each ventilator cycle. If CO 2 was not a competing gas at the alveolar level, oxygen within the pulmonary capillary blood would simply be replaced by that provided at the airway, as long as alveolar distention was maintained. Such apneic oxygenation has been used in conjunction with extracorporeal CO 2 removal (ECCO 2 R) or arteriovenous CO 2 removal (AVCO 2 R), in which oxygen is delivered at the carina, whereas lung distention is maintained through application of PEEP. Under normal circumstances, however, alveolar ventilation serves to remove CO 2 from the alveolus and to replenish the P O 2 , thereby maintaining the alveolar/pulmonary capillary blood oxygen gradient.
Rather than depending on the degree of alveolar ventilation, oxygenation predominantly is a function of the appropriate matching of pulmonary blood flow to inflated alveoli (ventilation/perfusion [
] matching). In normal lungs, the PEEP should be maintained at 5 cmH 2 O, a pressure that allows maintenance of alveolar inflation at end expiration, balancing the lung/chest wall recoil. An Fi O 2 of 0.50 should be administered initially. However, one should be able to wean the Fi O 2 rapidly in a patient with healthy lungs and normal
matching. Areas of ventilation but no perfusion (high
), such as in the setting of pulmonary embolus, do not contribute to oxygenation. Therefore, hypoxemia supervenes in this situation once the average residence time of blood in the remaining perfused pulmonary capillaries exceeds that necessary for complete oxygenation. Normal residence time is threefold that required for full oxygenation of pulmonary capillary blood.
However, the common pathophysiology observed in the setting of respiratory insufficiency is that of minimal or no ventilation, with persistent perfusion (low
), resulting in right-to-left shunting and hypoxemia. Patients with the acute respiratory distress syndrome (ARDS) have collapse of the posterior, or dependent, regions of the lungs when supine. As the majority of blood flow is distributed to these dependent regions, one can easily imagine the limited oxygen transfer and large shunt secondary to
mismatch and the resulting hypoxemia that occurs in patients with ARDS. Attempts to inflate the alveoli in these regions, such as with the application of increased PEEP, can reduce
mismatch and enhance oxygenation. Infant and child positioning also may be effective at improving oxygenation, but oxygenation is not sustained and requires constant changes to the position.
Just as partial pressure of CO 2 in the pulmonary artery ( Pa CO 2 ) is used to evaluate ventilation, partial pressure of oxygen in pulmonary arterial blood ( Pa O 2 ) and arterial oxygen saturation ( Sa O 2 ) levels are the measures most frequently used to evaluate oxygenation. Lung oxygenation capabilities are also frequently assessed as a function of the difference between the ideal alveolar and the measured systemic arterial oxygen levels (A–a gradient), the ratio of the Pa O 2 to the Fi O 2 (P/F ratio), the physiologic shunt (
ps /
t ), and the oxygen index (OI).
where Fi O 2 is the fraction of inspired oxygen, P B is the barometric pressure, P H 2 O is the partial pressure of water, and RQ is the respiratory quotient or the ratio of CO 2 production ( V CO 2 ) to oxygen consumption ( V O 3 ).
where C v O 2 , C a O 2 , and C i O 2 are the oxygen contents of venous, arterial, and expected pulmonary capillary blood, respectively.
where P aw represents the mean airway pressure.
The overall therapeutic goal of optimizing oxygenation parameters is to maintain oxygen delivery ( D O 2 ) to the tissues. Three variables determine D O 2 : cardiac output ( Q ), hemoglobin concentration (Hgb), and arterial blood oxygen saturation ( Sa O 2 ). The product of these three variables determines D O 2 by the relation:
Note that the contribution of the Pa O 2 to D O 2 is minimal and may be disregarded in most circumstances. If the hemoglobin concentration of the blood is normal (15 g/dL) and the hemoglobin is fully saturated with oxygen, the amount of oxygen bound to hemoglobin is 20.4 mL/dL ( Fig. 7.4 ). In addition, approximately 0.3 mL of oxygen is physically dissolved in each deciliter of plasma, which makes the oxygen content of normal arterial blood equal to approximately 20.7 mL O 2 /dL. Similar calculations reveal that the normal venous blood oxygen content is approximately 15 mL O 2 /dL.
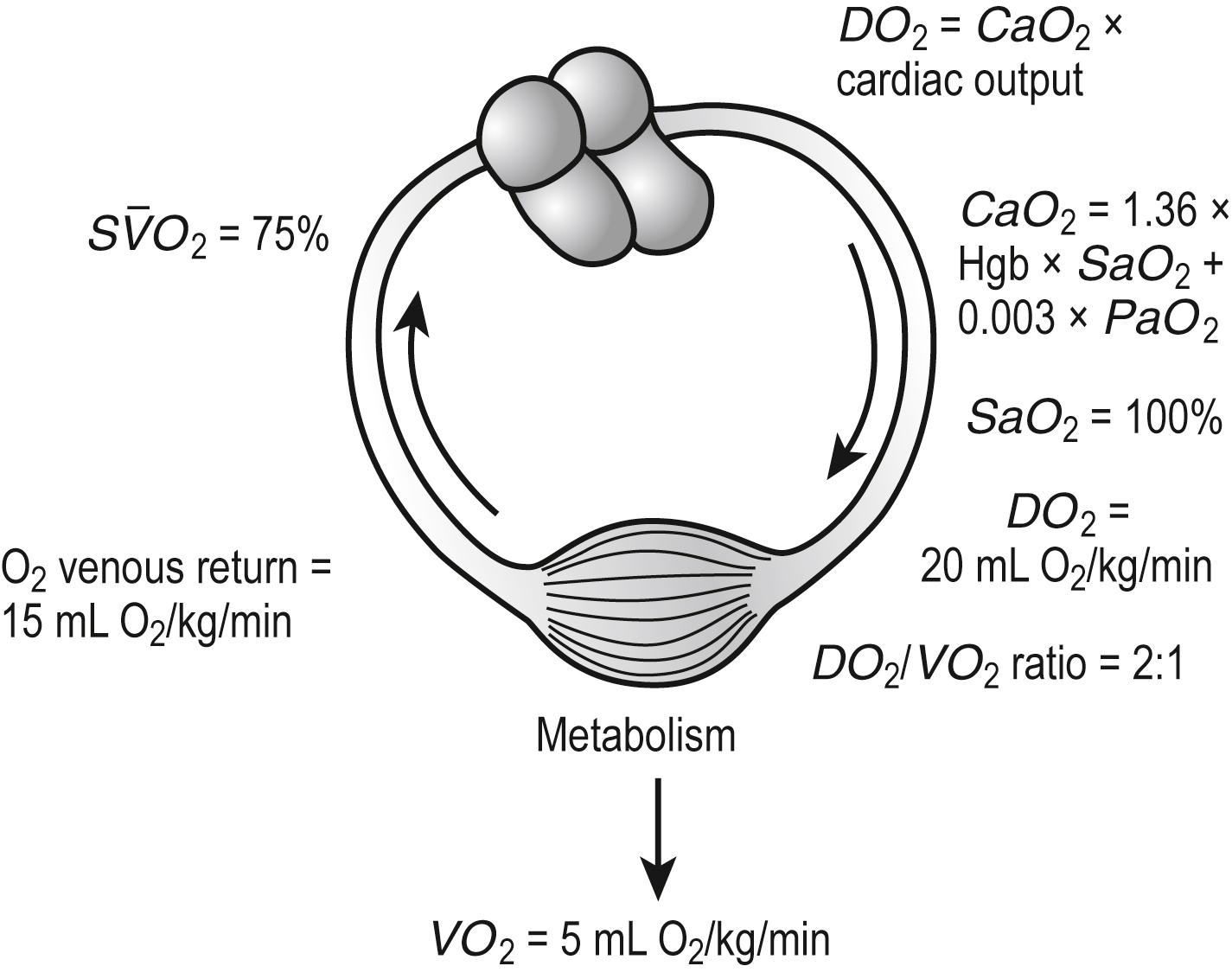
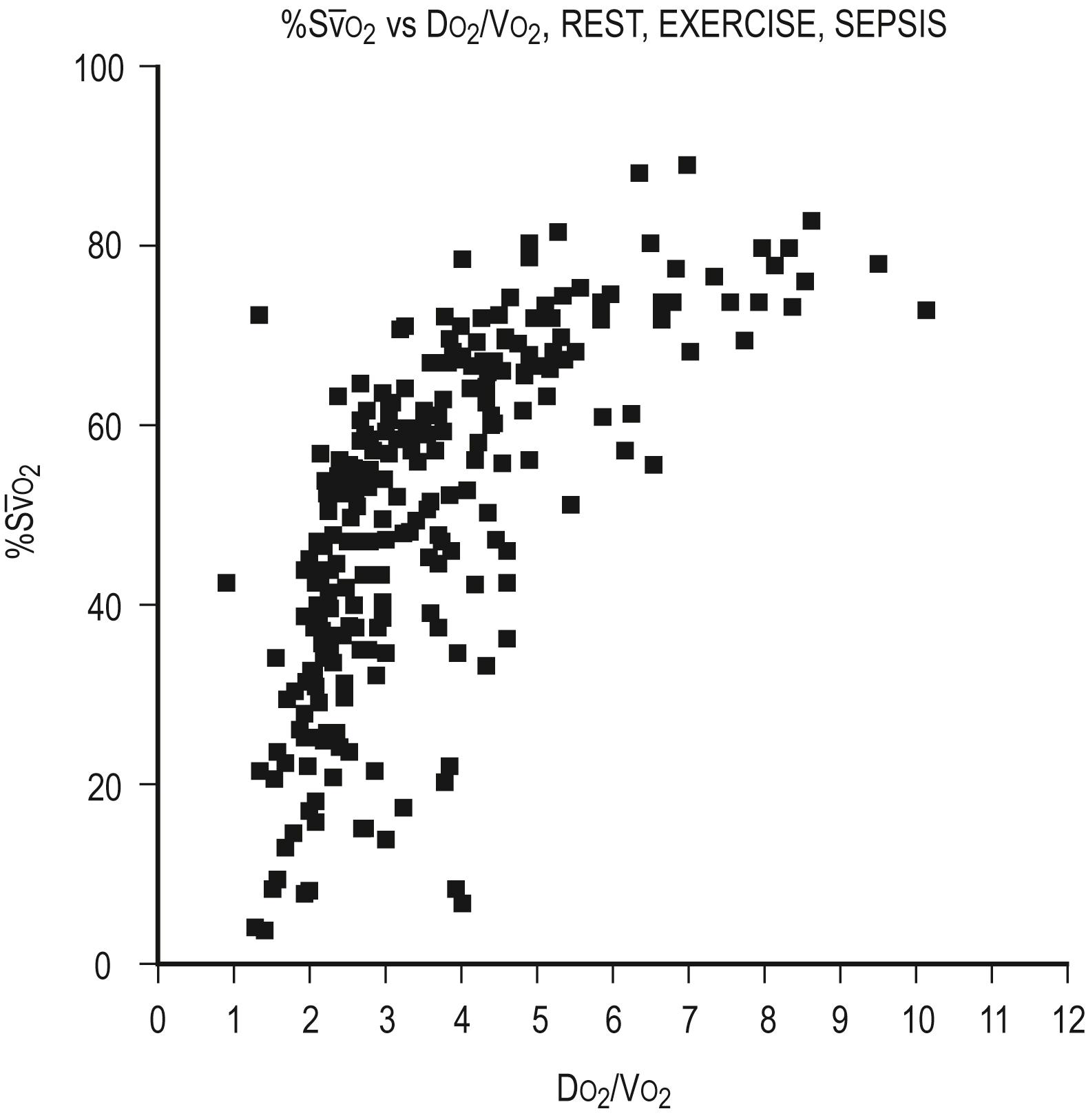
Typically, D O 2 is four to five times greater than the associated oxygen consumption ( V O 2 ). As D O 2 increases or V O 2 decreases, more oxygen remains in the venous blood. The result is an increase in the oxygen hemoglobin saturation in the mixed venous pulmonary artery blood ( S
O 2 ). In contrast, if the D O 2 decreases or V O 2 increases, relatively more oxygen is extracted from the blood and therefore less oxygen remains in the venous blood. A decrease in S
O 2 is the result. In general, the S
O 2 serves as an excellent monitor of oxygen kinetics because it specifically assesses the adequacy of D O 2 in relation to V O 2 ( D O 2 / V O 2 ratio). In other words, the amount of oxygen delivered to the tissues in relation to consumption determines the oxygen saturation of the mixed venous blood ( Fig. 7.5 ). Many pulmonary arterial catheters contain fiberoptic bundles that provide continuous mixed venous oximetry data. Such data provide a means for assessing the adequacy of D O 2 , rapid assessment of the response to interventions such as mechanical ventilation, and cost savings due to a diminished need for sequential blood gas monitoring. If a pulmonary artery catheter is unavailable, the central venous oxygen saturation ( Sc
O 2 ) may serve as a surrogate of the S
O 2 . Use of bedside echocardiography has largely replaced pulmonary artery catheter use for monitoring in children and commonly used to predict the pulmonary pressures though central venous oxygen saturation measurements still require blood gas samples.
Four factors are manipulated in an attempt to improve the D O 2 / V O 2 ratio: cardiac output, hemoglobin concentration, Sa O 2 , and V O 2 . The result of various interventions designed to increase cardiac output, such as volume administration, infusion of inotropic agents, administration of afterload-reducing drugs, and correction of acid–base abnormalities, can be assessed by the effect on the S
O 2 . One of the most efficient ways to enhance D O 2 is to increase the oxygen-carrying capacity of the blood. For instance, an increase in hemoglobin from 7.5 g/dL to 15 g/dL will be associated with a twofold increase in D O 2 at constant cardiac output. However, blood viscosity is also increased with blood transfusion, which may result in a reduction in cardiac output. The Sa O 2 often can be enhanced through application of supplemental oxygen and mechanical ventilation.
Assessment of the “best PEEP” identifies the level at which D O 2 and S
O 2 are optimal without compromising compliance. Evaluation of the best PEEP should be performed in any patient requiring an Fi O 2 greater than 0.60 and can be determined by continuous monitoring of the S
O 2 as the PEEP is sequentially increased from 5 cmH 2 O to 15 cmH 2 O over a short period. The point at which the S
O 2 is maximal indicates optimal D O 2 . The use of PEEP with mechanical ventilation is limited, however, by the adverse effects observed on cardiac output, the effect of barotrauma, and the risk for ventilator-induced lung injury with application of peak inspiratory pressures greater than 30–40 cmH 2 O. Furthermore, oxygen consumption can be elevated secondary to sepsis, burns, agitation, seizures, hyperthermia, hyperthyroidism, and increased catecholamine production or infusion. A number of interventions may be applied to reduce V O 2 , such as sedation and mechanical ventilation. Paralysis may enhance the effectiveness of mechanical ventilation while simultaneously reducing V O 2 . In the appropriate setting, hypothermia may be induced with an associated reduction of 7% in V O 2 with each 1°C decrease in core temperature.
The ventilator must overcome the pressure generated by the elastic recoil of the lung at end inspiration plus the resistance to flow at the airway. To do so, most ventilators in the ICU are pneumatically powered by gas pressurized at 50 lb per square inch (psi). Microprocessor controls allow accurate management of proportional solenoid-driven valves, which carefully control infusion of a blend of air and oxygen into the ventilator circuit while simultaneously opening and closing an expiratory valve. Additional components of a ventilator include a bacterial filter, a pneumotachometer, a humidifier, a heater/thermostat, an oxygen analyzer, and a pressure manometer. A chamber for nebulizing drugs is usually incorporated into the inspiratory circuit. The V t is not usually measured directly. Rather, flow is assessed as a function of time, thereby allowing calculation of V t . The modes of ventilation are characterized by three variables that affect patient and ventilator synchrony or interaction: the parameter used to initiate or “trigger” a breath, the parameter used to “limit” the size of the breath, and the parameter used to terminate inspiration or “cycle” the breath ( Fig. 7.6 ).
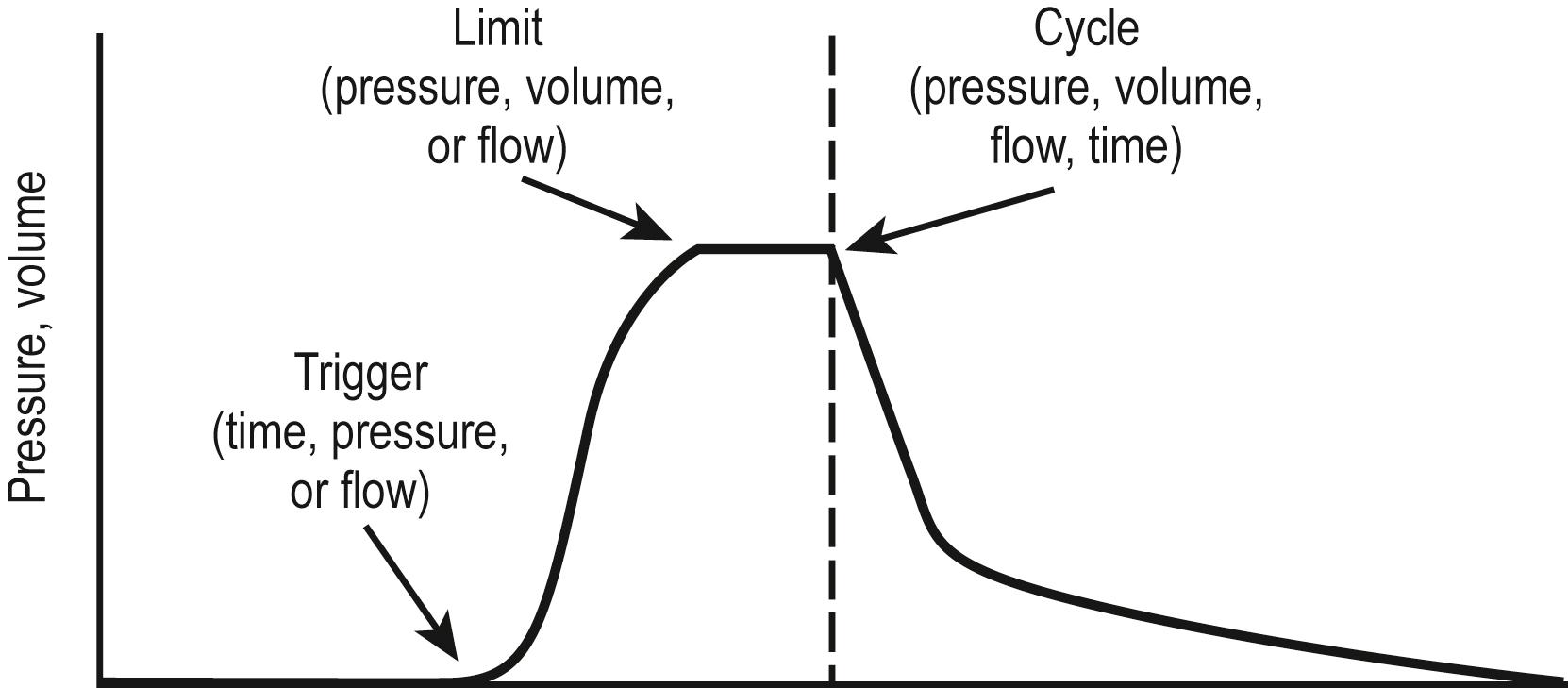
Gas flow in most ventilators is triggered by either time (controlled breath) or patient effort (assisted breath). Controlled ventilation modes are time triggered: the inspiratory phase is concluded once a desired volume, pressure, or flow is attained, but the expiratory time will be the difference between the inspiratory time and the preset respiratory cycle time. In the assist mode, the ventilator is pressure or flow triggered. With the former, a pressure generated by the patient of approximately −1 cmH 2 O will trigger the initiation of a breath. The sensitivity of the triggering device can be adjusted so that patient work is minimized. Other ventilators detect the reduction in constant ventilator tubing gas flow that is associated with patient initiation of a breath. Detection of this decrease in flow results in initiation of a positive-pressure breath.
The magnitude of the breath is controlled or limited by one of three variables: pressure, volume, or flow. When a breath is volume, pressure, or flow controlled, it indicates that inspiration concludes once the limiting variable is reached. Pressure-controlled or pressure-limited modes are the most popular for all age groups, although volume-control ventilation may be of advantage in preterm newborns. In the pressure modes, the respiratory rate, the inspiratory gas flow, the PEEP level, the inspiratory/expiratory (I/E) ratio, and the P aw are determined. The ventilator infuses gas until the desired peak inspiratory pressure (PIP) is provided. Zero-flow conditions are realized at end inspiration during pressure-limited ventilation. Therefore, in this mode, PIP is frequently equivalent to end-inspiratory pressure (EIP) or plateau pressure.
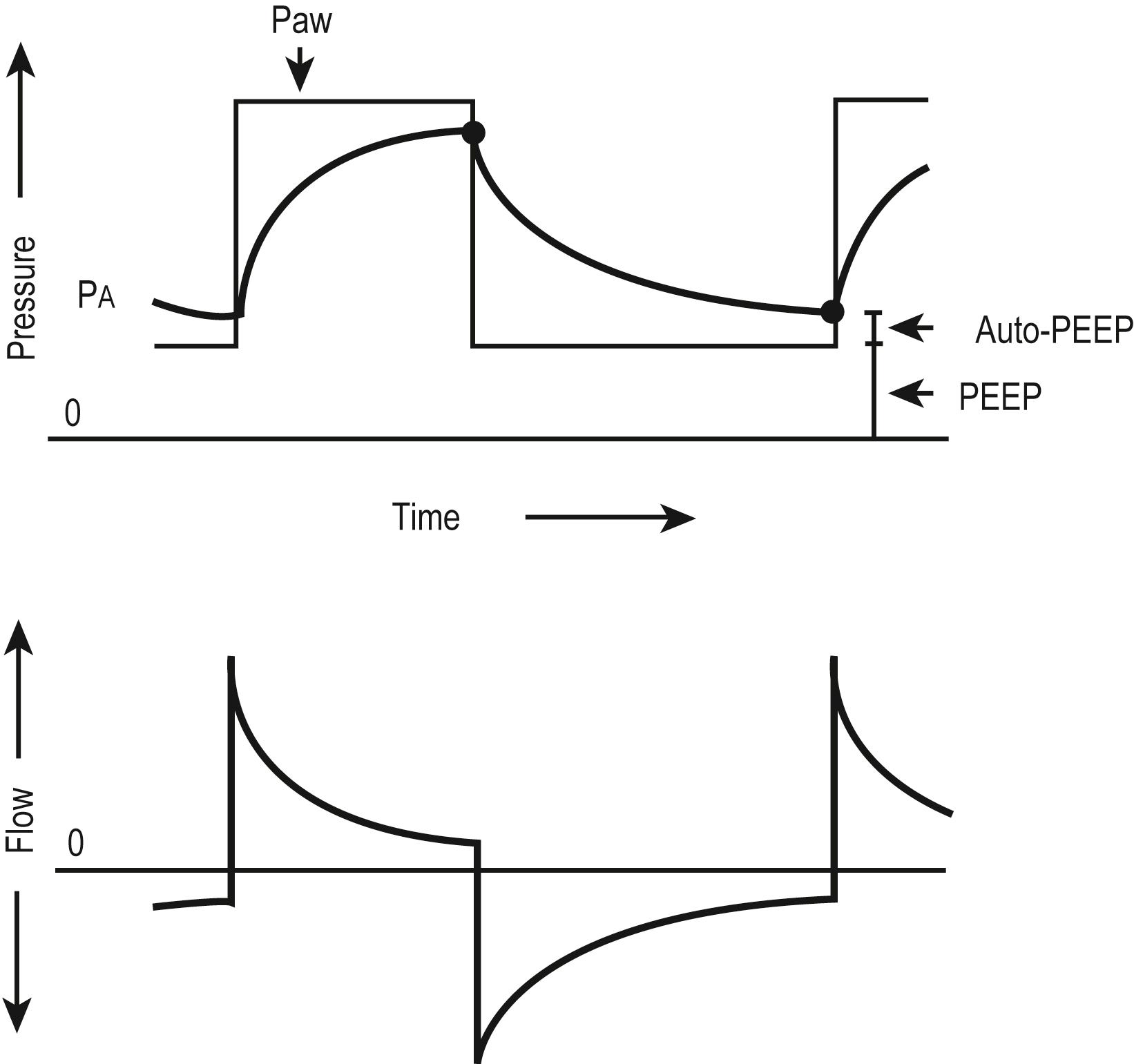
In many ventilators, the gas flow rate is fixed, although some ventilators allow manipulation of the flow rate and therefore the rate of positive-pressure development. Those with rapid flow rates will provide rapid ascent of pressure to the preset maximum, where it will remain for the duration of the inspiratory phase. This “square wave” pressure pattern results in decelerating flow during inspiration ( Fig. 7.7 ). Airway pressure is front loaded, which increases P aw , alveolar volume, and oxygenation without increasing PIP. However, one of the biggest advantages of pressure-controlled or pressure-limited ventilation is the ability to avoid lung overdistention and barotrauma/volutrauma. The disadvantage of pressure-controlled or pressure-limited ventilation is that the delivered volume varies with airway resistance and pulmonary compliance and may be reduced when short inspiratory times are applied ( Fig. 7.8 ). For this reason, both V t and
E must be monitored carefully.
Volume-controlled or volume-limited ventilation requires delineation of the V t , respiratory rate, and inspiratory gas flow. Gas will be inspired until the preset V t is attained. The volume will remain constant despite changes in pulmonary mechanics, although the resulting EIP and PIP may be altered. Flow-controlled or flow-limited ventilation is similar in many respects to volume-controlled or volume-limited ventilation. A flow pattern is predetermined, which effectively results in a fixed volume as the limiting component of inspiration.
The ventilator breath is concluded based on one of four variables: volume, time, pressure, or flow. With volume-cycled ventilation, inspiration is terminated when a prescribed volume is obtained. Likewise, with time-, pressure-, or flow-cycled ventilation, expiration begins after a certain period has passed, the airway pressure reaches a certain value, or when the flow has decreased to a predetermined level, respectively.
A factor that limits inspiration suggests that the chosen value limits the level of the variable during inspiration, but the inspiratory phase does not necessarily conclude once this value is attained. For instance, during pressure-limited ventilation, gas flow continues until a given pressure limit is attained. However, the inspiratory phase may continue beyond that point. The limitation controls only the magnitude of the breath but does not always determine the length of the inspiratory phase. In contrast, during pressure-controlled ventilation, both gas flow and the inspiratory phase terminate once the preset pressure is reached because pressure is used to limit the magnitude of the breath and the gas flow.
Finally, a newer mode of ventilation is neurally adjusted ventilator assist (NAVA), in which the electrical activity of the diaphragm (Edi), captured by an orogastric or nasogastric tube with small electrodes imbedded within and positioned at the lower esophagus, triggers synchronized support provided by the ventilator. The cycle changes based on initiation, size, and termination of Edi by the patient. An increase in NAVA level on the ventilator results in a complementary decrease in the effort by the patient, offloading patient effort on to the ventilator and vice versa. NAVA is primarily used in patients with spontaneous respiration and can be applied in a noninvasive way without an endotracheal tube, similar to CPAP (see below).
Controlled mechanical ventilation (CMV) is time triggered, flow limited, and volume or pressure cycled. Spontaneous breaths can be taken between the mandatory breaths. However, no additional gas is provided during spontaneous breaths. Therefore, the work of breathing is markedly increased in the spontaneously breathing patient. This mode of ventilation is no longer used.
| Mode | Trigger | Limit | Cycle | Comment |
|---|---|---|---|---|
| CMV | Time | Flow | Pressure/volume | No longer used |
| IMV | Time | Volume/pressure | Time Volume/pressure |
For no respiratory drive (neurologically impaired or paralyzed) |
| Work of breathing elevated in spontaneously breathing patient | ||||
| SIMV a | Pressure/flow | Volume/pressure | Time Volume/pressure |
Supports limited number of breaths |
| ACV a | Pressure/flow | Volume/pressure | Time Volume/pressure |
Supports all patient breaths Similar to IMV but patient controls breaths |
| Sedation for hyperventilation and backup rate for apnea | ||||
| PSV a | Pressure/flow | Pressure | Flow Time |
Supports all patient breaths Usually partially supported to allow for weaning |
| Time cycled when termination sensitivity for flow is off | ||||
| VSV a | Pressure/flow | Volume | Flow Time |
Similar to PSV but volume used for partial support |
| VAPSV | Pressure/flow | Pressure | Flow Time |
Maintains a desired tidal volume using both VSV and PSV |
| Dynamically maintains tidal volume | ||||
| PAV a | Patient | Pressure | Patient | Size of the breath is determined by patient effort |
Intermittent mandatory ventilation (IMV) is time triggered, volume or pressure limited, and either time, volume, or pressure cycled. A rate is set, as is a volume or pressure parameter. Additional inspired gas is provided by the ventilator to support spontaneous breathing when additional breaths are desired. The difference between CMV and IMV is that, in the IMV mode, inspired gases are provided to the patient during spontaneous breaths. IMV is useful in patients who do not have respiratory drive such as those who are neurologically impaired or pharmacologically paralyzed. Work of breathing is still elevated with this mode in the awake and spontaneously breathing patient.
In the synchronized intermittent mandatory ventilation (SIMV) mode, the ventilator synchronizes IMV breaths with the patient’s spontaneous breaths ( Fig. 7.9 ). Small, patient-initiated negative deflections in airway pressure (pressure triggered) or decreases in the constant ventilator gas flow (bias flow) passing through the exhalation valve (flow triggered) provide a signal to the ventilator that a patient breath has been initiated. Ventilated breaths are timed with the patient’s spontaneous respiration, but the number of supported breaths each minute is predetermined and remains constant. Additional constant inspired gas flow is provided for use during any other spontaneous breaths. Advances in neonatal ventilators have provided the means for detecting small alterations in bias flow. Therefore, flow-triggered SIMV can be applied to newborns, which appears to enhance ventilatory patterns and allows ventilation with reduced airway pressures and Fi O 2 . SIMV may be associated with a reduction in the duration of ventilation and the incidence of air leak in newborns in general, as well as in those premature infants with bronchopulmonary dysplasia (BPD) and intraventricular hemorrhage.
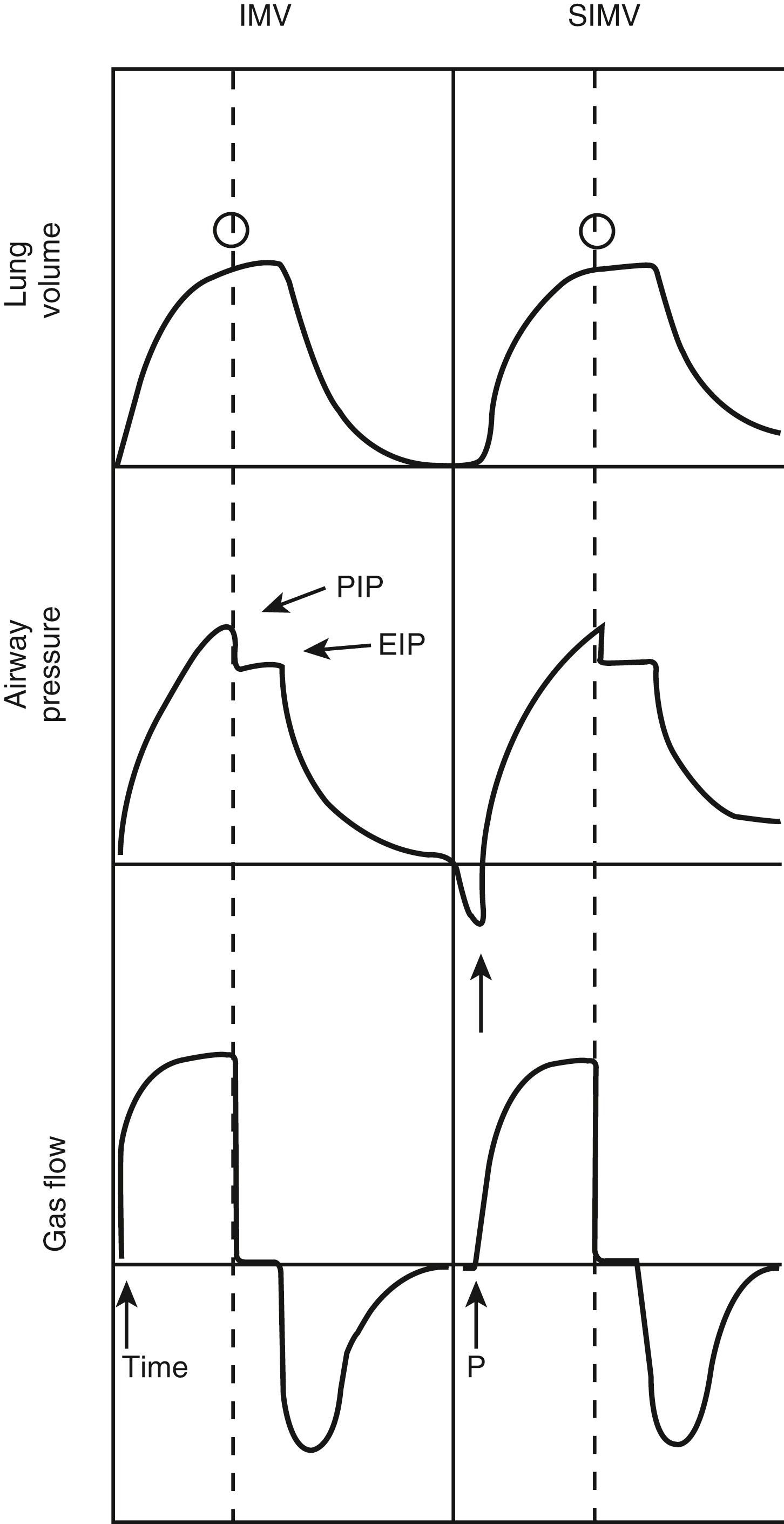
In the spontaneously breathing patient, brain stem reflexes dependent on cerebrospinal fluid levels of CO 2 and pH can be harnessed to determine the appropriate breathing rate. As in SIMV, with assist-control ventilation (ACV) the assisted breaths can be either pressure triggered or flow triggered. The triggering-mechanism sensitivity can be set in most ventilators. In contrast to SIMV, the ventilator supports all patient-initiated breaths. This mode is similar to IMV but allows the patient inherently to control the ventilation and minimizes patient work of breathing in adults and neonates. Occasionally, patients may hyperventilate, such as when they are agitated or have neurologic injury. Heavy sedation may be required if agitation is present. A minimal ventilator rate below the patient’s assist rate should be established in case of apnea.
Pressure support ventilation (PSV) is a pressure- or flow-triggered, pressure-limited, and flow-cycled mode of ventilation. It is similar in concept to ACV, in that mechanical support is provided for each spontaneous breath and the patient determines the ventilator rate. During each breath, inspiratory flow is applied until a predetermined pressure is attained. As the end of inspiration approaches, flow decreases to a level below a specified value (2–6 L/min) or a percentage of peak inspiratory flow (at 25%). At this point, inspiration terminates. Although it may apply full support, PSV is frequently used to support the patient partially by assigning a pressure limit for each breath that is less than that required for full support. For example, in the spontaneously breathing patient, PSV can be sequentially decreased from full support to a PSV 5–10 cmH 2 O above PEEP, allowing weaning while providing partial support with each breath. Thus, V t during PSV may depend on patient effort. PSV provides two advantages during ventilation of spontaneously breathing patients: it provides excellent support and decreases the work of breathing associated with ventilation; it lowers PIP and P aw while higher V t and cardiac output levels may be observed.
Pressure-triggered SIMV and PSV can be applied to newborns. Inspiration is terminated when the peak airway flow decreases to a set percentage between 5% and 25%. This flow cut-off for inspiration, known as the termination sensitivity, can be adjusted. The higher the termination sensitivity value, the shorter is the inspiratory time. The termination sensitivity function also may be disabled, at which point ventilation is time cycled instead of flow cycled. There is a reduction in work of breathing and sedation requirements when SIMV with pressure support is applied to newborns.
Volume support ventilation (VSV) is similar to PSV except that a volume, rather than a pressure, is assigned to provide partial support. Automation with VSV is enhanced because there is less need for manual changes to maintain stable tidal and minute volume during weaning. Both VSV and PSV are equally effective at weaning infants and children from the ventilator.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here