Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bone cement (polymethylmethacrylate [PMMA]) does not act as a glue to fix implants to bone. Rather, it serves as a grout to fill space with fixation achieved by mechanical interlock.
The ability to shape, form, and infiltrate PMMA as needed during surgery is an appealing aspect of the material. Long-term fixation and survival can be achieved with PMMA-cemented implant systems.
Shrinkage of the cement during curing is inevitable, which can contribute to gap formation at implant interfaces and porosity in the cement. The location and distribution of gaps/porosity can be modulated by creating a temperature differential between the components of the implant system.
The mechanical strength of cement–bone interfaces is dictated by the amount of mechanical interlock between cement and bone.
Morphological changes to the cement–bone interface are likely to occur after implantation and may diminish over time through resorption of the trabeculae that initially interlock with cement. This loss of interlock results in a weaker cement–bone interface.
Orthopedic bone cement (polymethylmethacrylate [PMMA]) is a versatile material used in total hip arthroplasty (THA) to fix implant components to bone, to fill cavitary defects, and as a carrier for local delivery of antibiotics. The basic chemical structure of orthopedic PMMA is the same as commercial acrylic, with common trade names such as Plexiglass, Lucite, and Perspex. What is particularly unique about orthopedic PMMA is that final processing of the material is performed in the operating room at time of application. As such, the surgeon has considerable input into the final manufacture, handling, and application of the PMMA before it sets in a final solid form. In general, PMMA does not act as a glue with a chemical bond to bone or implant. Rather, it serves as a grout to fill space with fixation achieved by mechanical interlock.
Bone cement is provided in packets, with a powder component consisting of methacrylate polymer and/or copolymer and a methylmethacrylate (MMA) monomer liquid component ( Table 4.1 ). The powder component consists of small spheres of polymer (1–100 microns in diameter), but also contains dibenzoyl peroxide (BPO) as an initiator for the free radical polymerization process and a radiopacifier such as zirconium dioxide or barium sulfate. A coloring agent (e.g., chlorophyllin) and antibiotics can also be added to the powder component. The liquid component is provided in ampules with the monomer along with an activator, stabilizer, and sometimes a coloring agent. Mixing the monomer and polymer powder without the initiator (usually N,N -dimethyl- p -toluidine [DMPT]) and activator would result in a slowly thickening cement, unsuitable for surgical use. The initiator system of BPO and DMPT initiates the formation of radicals at room temperature. These radicals break the C C double bond in the MMA and initiate polymerization. A large number of intertwined polymer chains develop quickly, resulting in molecular weights of 10 5 to 10 6 g/mol ( Fig. 4.1 ). The rapid increase in viscosity of the cement limits mobility of the chains and eventually slows the polymerization process. As a result, the monomer is not fully converted to polymer, leaving a 2% to 6% residual of monomer. Continuous polymerization can occur over the next several weeks and any residual monomer is eluted into the bloodstream and metabolized or exhaled.
| Powder | Liquid |
|---|---|
| Polymer | Monomer |
|
|
| Copolymer a | Activator |
|
|
| Initiator | Stabilizer (Radical Catcher) a |
|
|
| Dye a | Dye a |
|
|
| Radiopacifier a | |
|
|
| Antibiotics a | |
|
|
| Plasticizer a | |
|
|
| Other a | |
|
![Fig. 4.1, (A) When the initiator (dibenzoyl peroxide [BPO]) within the powder is mixed with the activator (mostly dimethyl-p-toluidine [DMPT]) in the liquid, benzoate radicals (R•) containing “unpaired electrons” are created. These radicals start the polymerization reaction by breaking up the C C bond of methylmethacrylate (MMA), generating new radicals within the MMA molecules. The highly reactive unpaired electrons of the MMA molecules combine with new MMA molecules to create large (10 5 –10 6 g/mol or more) polymethylmethacrylate (PMMA) chains. (B) When 2 PMMA chains containing a free radical combine, the overall quantity of free radicals decreases. The polymerization reaction ends when free radicals are depleted. Fig. 4.1, (A) When the initiator (dibenzoyl peroxide [BPO]) within the powder is mixed with the activator (mostly dimethyl-p-toluidine [DMPT]) in the liquid, benzoate radicals (R•) containing “unpaired electrons” are created. These radicals start the polymerization reaction by breaking up the C C bond of methylmethacrylate (MMA), generating new radicals within the MMA molecules. The highly reactive unpaired electrons of the MMA molecules combine with new MMA molecules to create large (10 5 –10 6 g/mol or more) polymethylmethacrylate (PMMA) chains. (B) When 2 PMMA chains containing a free radical combine, the overall quantity of free radicals decreases. The polymerization reaction ends when free radicals are depleted.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MaterialsinHipSurgeryPolymethylmethacrylate/0_3s20B9780323554640000040.jpg)
The process of polymerization of bone cement has been described as occurring over 4 stages: mixing, waiting, working, and hardening. However, the process is a continuum, starting with the mix of the powder/liquid components and ending with the final hardening to a solid. The delineation of these 4 phases is subjective but is useful for relative comparison among different cements and for consideration of important factors such as effects of temperature and humidity. During the mixing phase, the polymer beads are wetted by the monomer liquid and the cement maintains a relatively liquid behavior. During the waiting phase, the polymer beads swell and polymerization continues, with the cement taking on a sticky texture. At this point, the cement sticks to a surgical glove when contacted. The working phase is the time during which the cement can be applied to the bone. The cement has a doughy texture owing to the polymer chain propagation, can easily be manipulated, and some heat generation is noticeable. At the end of the doughy phase, the cement becomes less malleable and does not recombine easily into a mass after separation. During the setting or hardening phase, chain growth is finished and the cement reaches a maximum temperature.
Working curves for the cement provided by the manufacturer show the 4 phases in time as a function of ambient temperature ( Fig. 4.2 ). Cement polymerizes more quickly with higher ambient temperature and with higher ambient humidity. In addition, the storage temperature and equilibrium time in the operating room for bone cement can also influence the rate of polymerization. For example, the set time for SmartSet HV (DePuy CMW, Blackpool, UK) as determined using ASTM F451-08 , was 8 minutes and 54 seconds for cement stored and mixed at 20°C. For cement stored at 28°C, but then moved to a 20°C operating room, set time decreased to 6 minutes and 21 seconds after a wait of 15 minutes before mixing. Waiting for 1 hour after storage at 28°C resulted in an increase in set time back to 8 minutes and 40 seconds.
![Fig. 4.2, The temperature-working curve of high-viscosity (Palacos R, Heraeus Medical GmbH, Wehrheim, Germany [top] ), medium-viscosity (Surgical Simplex P, Stryker, Mahwah, NJ [middle] ), and low-viscosity cement (CMW 3, DePuy CMW, Blackpool, UK [bottom] ). I, Mixing phase. II, Waiting phase. III, Application phase. IV, Setting phase. The green arrow shows the duration of the application phase at an ambient temperature of 19°C. Fig. 4.2, The temperature-working curve of high-viscosity (Palacos R, Heraeus Medical GmbH, Wehrheim, Germany [top] ), medium-viscosity (Surgical Simplex P, Stryker, Mahwah, NJ [middle] ), and low-viscosity cement (CMW 3, DePuy CMW, Blackpool, UK [bottom] ). I, Mixing phase. II, Waiting phase. III, Application phase. IV, Setting phase. The green arrow shows the duration of the application phase at an ambient temperature of 19°C.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MaterialsinHipSurgeryPolymethylmethacrylate/1_3s20B9780323554640000040.jpg)
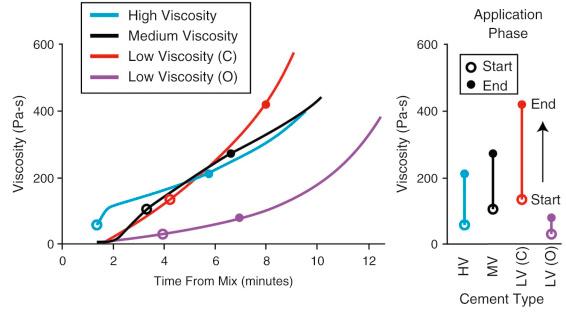
In addition to the effects of ambient and storage temperature, the handling properties of commercially available cements vary considerably because the constituents and polymerization chemistry are different for each cement brand. Therefore experience should be gained in working with new cements in a variety of conditions prior to use in the operating room. In the literature, 3 general classes of cement have been identified to describe the handling characteristics: low-viscosity, medium-viscosity, and high-viscosity types. Low-viscosity cements mix easily and remain in a low-viscosity state during an extended waiting phase. This allows for easy transfer to any application device. During the application phase, viscosity can increase rapidly. In contrast, high-viscosity cements have a short waiting phase and reach a doughy state (that does not stick to a surgical glove) rather quickly. As a consequence, high-viscosity cements do not flow easily, making application with a syringe more difficult. Prechilling the cement components in this case can help extend the waiting phase and maintain the cement in a lower-viscosity state for vacuum mixing. High-viscosity cements are easy to manipulate manually in the application phase, can be pressed into the bone surface, and are not sticky to handle. Medium-viscosity cements can be easily vacuum mixed, like low-viscosity cements, but reach a higher viscosity during the application phase, much like a high-viscosity cement.
While the working curves provide some guidance as to the 4 handling phases of an individual cement, ambiguity exists as to the actual cement viscosity characteristics during each phase. In addition, the low-viscosity, medium-viscosity, and high-viscosity monikers can sometimes be misleading with regard to the time-viscosity characteristics of the cement ( Fig. 4.3 ). As an example, CMW3 (DePuy, New Brunswick, NJ) and Osteopal (Heraeus Medical, Hanau, Germany) have both been described as low-viscosity cements and both have very low viscosity after initial mix. However, the viscosity of CMW3 rises much more quickly compared to Osteopal. In fact, the viscosity for CMW3 at the start of the application phase (based on working curves) is higher than a medium-viscosity (Simplex, Stryker, Mahwah, NJ) and high-viscosity (Palacos, Zimmer, Warsaw, IN) cement. The viscosity at the end of the working phase is also highest for CMW3. The viscosity of the cement at the time of application and during pressurization will influence the degree of infiltration into bone and wettability and entrapped air during metal implant insertion.
PMMA cement without a radiopacifier is difficult to discern on clinical radiograph; thus, small ceramic particles of barium sulfate (BaSO 4 , 9–13 w/w%) and zirconium dioxide (ZrO 2 , 9–15 w/w%) are added to the powder mixture to improve radiographic visualization. These particles are distributed through the PMMA powder component and remain so throughout the hardened cement, although BaSO 4 has some tendency to aggregate and form clumps. ZrO 2 is more radiodense than BaSO 4 and generally results in greater radiopacity. The greatest radiodensity in standard cements used for THA are achieved with ZrO 2 with 15% weight fraction. Cements used for vertebroplasty have an even higher fraction of radiopacifier.
Some evidence exists that the radiopacifier agents cause increased osteoclastic bone resorption based on cell culture studies and animal models. In a rat model assessing the effects of PMMA, BaSO 4 , and ZrO 2 particles on the bone–implant interface, all 3 particle forms increased bone resorption compared to a control case with no particles. However, only the BaSO 4 case reached a significant difference compared to control. Failure analysis of retrieved THA femoral components show that ZrO 2 particles can damage the metal implant surface, can be torn out of the PMMA, and can accumulate at the cement–bone interface along with PMMA debris. Further, these particles are of nanoscale size and are capable of contributing to osteolysis. To date, no clinical trial has been conducted comparing cement with and without standard radiopacifier or alternative water-soluble nonionic contrast agents ; thus, it remains unclear whether there exists an increased clinical risk of loosening due to the presence of a radiopacifier.
Many manufacturers offer versions of PMMA cements with antibiotics mixed into the powder component. Gentamicin (0.5–1 g) and tobramycin (1 g) are the most common, although vancomycin (1–4 g) and erythromycin (0.73 g) plus colistin (0.24 g) are also available depending on the country of use. Antibiotic elution occurs via diffusion from the surface of the cured cement adjacent to the bone. Elution studies show that the antimicrobial concentration of the drugs is highest immediately after application and decreases substantially over the first few days. The cumulative antimicrobial activity also depends on whether the cement is vacuum mixed or mixed in atmospheric conditions and on the type of cement. Whether vacuum mixing improves antibiotic elution is product dependent, but it appears that high-viscosity cements have improved elution characteristics when vacuum mixed. This is thought to be due to increased local porosity of the high-viscosity cements.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here