Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Children present many unique challenges to the epilepsy surgeon necessitating the use of special techniques and equipment. A large portion of adult epilepsy surgery treats mesial temporal sclerosis (MTS), while in children, extratemporal epilepsy is more common and developmental lesions are frequently encountered. The scope of surgical treatment of pediatric epilepsy may involve mapping the site of seizure onset and surrounding essential brain functions, resection of a seizure focus, or disconnection of the majority or even the entirety of a hemisphere.
The incidence of epilepsy is higher in children than adults with about 5% of children experiencing a seizure before the age of 20. , The majority of these (80%) will never have another seizure, and therefore not meet the diagnosis of epilepsy. Among children with epilepsy, 20% will be refractory to medical therapy even with the numerous new medications available. Repetitive seizures and anticonvulsive medications present noxious stimuli that may inhibit brain development. Seizures may additionally hamper socialization and school integration, causing deleterious impacts beyond the physiologic. , However, brain plasticity could also benefit the child in recovering from resective or disconnective surgery. While these factors weigh significantly in the decision to pursue surgical treatment and the timing of such treatment, each child and family must assess their particular situation with the advice of the epilepsy team to help them weigh the risks of ongoing epilepsy, the risks of surgery, and the likelihood of seizure control with surgery.
All evaluations for resective epilepsy surgery focus on identifying a localized source of seizure onset. Noninvasive tools used to accomplish such localization include seizure semiology, neurologic exam (including neuropsychology), scalp electroencephalogram (EEG), and imaging. In the most straightforward of cases, all modalities of localization identify a single, safely-resectable source of seizure onset. In such cases, invasive mapping is unnecessary. In some cases, many of the nonoperative localization modalities give equivocal results or discordant localization. In these cases, operative mapping can reveal an otherwise obscure epileptic focus.
In addition to identifying a seizure focus, mapping can define the extent of a subtle or diffuse epileptic focus such as cortical dysplasia. Such mapping guides the extent of surgical resection.
Finally, mapping can localize neurologic function, defining the relationship between functional brain tissue and an epileptic focus. Such information predicts what if any neurologic deficit will be induced by resection, vital information for a family and care team in deciding whether to proceed with resection. Advances in functional imaging are increasingly able to localize neurologic function, often supplanting or supplementing invasive mapping.
Attempts at nonoperative localization of an epilepsy focus include an array of specialized testing and a team of trained personnel to administer and interpret them. History (particularly of seizure semiology), neurologic exam (including neuropsychology to elucidate subtle cognitive deficits), prolonged video electroencephalography, and advanced imaging are all vital elements of the epilepsy surgery workup.
The clinical semiology of a seizure provides the first clue to localizing its onset. Penfield described an assortment of mental, sensory, and physical aspects of seizures specific to various brain regions. These range from mental phenomena such as déjà vu and ill-defined epigastric rising to olfactory and gustatory auras to motor convulsions. The first manifestation of a given seizure most accurately reflects the area of onset, whereas seizure spread can lead to later involvement of other areas outside to true site of seizure onset.
Seizures of temporal lobe origin are the most likely to have auras. Those originating in the mesial temporal structures classically begin with an ill-defined epigastric sensation accompanied with panic or autonomic disturbance. Neocortical temporal seizures commonly have auditory, visual, or perceptual hallucinations. Head turning, posturing, automatisms, and behavioral arrest are common accompaniments of temporal seizures.
Frontal lobe seizures are often brief, occur in clusters, and may include complex motor posturing, versive eye movements, or vocalization. They can rapidly spread yielding early generalization, drop attacks, or early signs of temporal lobe activation.
Parietal lobe seizures often present with somatosensory symptoms but may have abdominal symptoms such as nausea or a gustatory sensation. Occipital seizures may be heralded by visual phenomena including scotoma.
The classic neuropsychology workup of an epilepsy patient consists of a variety of standardized tests and questionnaires that establish a profile of the patient’s cognitive, emotional, and behavioral abilities. This testing quantifies the patient’s abilities, deficits, and coping strategies. Such information can localize areas of dysfunctional cortex, which may be the site of seizure onset. Neuropsychology also predicts the deficits likely to be incurred by resection of a given focus and the impact that such deficits will have on the individual’s life.
In many emerging technologies such as functional magnetic resonance imaging, the neuropsychologist plays a vital role in administering verbal and functional tasks and assessing the patient’s cooperation with such tests. As these tests are very dependent on cooperation from the child while in the magnetic resonance imaging (MRI) machine, the neuropsychologist plays a vital role in extracting and interpreting information from an otherwise uncooperative patient. ,
Electrophysiologic data are acquired by placing electrodes on the scalp and recording electrical differences between them. Interictal EEG can reveal epileptiform discharges such as spike and sharp waves, providing clues to the lateralization and localization of seizure onset, but prolonged video EEG provides an electrophysiologic picture of the seizures themselves. Video EEG data can confirm that spells are seizures (as opposed to breath-holding or other nonepileptic spells) and in many instances begin to localize the onset of the seizure. Most centers want to record several typical seizures and will continue monitoring until this is accomplished.
Ictal video EEG will precisely localize the seizure focus in about a third of patients with temporal lobe epilepsy. Precise localization is even less common in those with extratemporal epilepsy where seizures tend to spread rapidly. Rapid spread from the orbitofrontal or posterior parietal cortex can falsely localize to the temporal lobe.
Beyond the detection of interictal spikes, or the delineation of ictal onset zone, newer signal processing methods for EEG analysis allow for even further clinical insight. High-frequency oscillations (HFOs) are focal increases in amplitude within frequency ranges between 80 and 500 Hz. Since their initial discovery in rats, they have been demonstrated as a good marker for the epileptogenic zone , independent of interictal spikes. Further, post-resection clinical outcome has been associated with the degree of removal of HFO-positive areas. While this a promising technology, clinical application remains to be fully implemented due to limitations in detection and specificity.
Dense-array EEG is a newer technology that holds promise for more precise seizure localization. The dense array consists of 256 electrodes held to the head by an elastic mesh, giving substantially more information and spatial resolution than the standard 32-electrode array. In recent studies, dense-array monitoring was shown to have a higher sensitivity and specificity for epileptogenic zone localization compared to both conventional EEG and imaging techniques.
Surgical epilepsy cases are sometimes divided into lesional and nonlesional. Classically lesional cases from well-circumscribed pathologies such as cavernoma, dysembryoplastic neuroectodermal tumors (DNETs), and ganglioglioma have a significantly better prognosis than nonlesional cases. The identification of a structural abnormality on MRI is a strong predictor of localization. When the scalp EEG confirms seizure onset from the lesion, localization is strongly suggested, and resection often proceeds without invasive mapping.
The distinction between lesional and nonlesional epilepsies has blurred a bit as improved imaging has revealed cortical dysplasias, focal sclerosis, or other pathologies previously noted only by histology. Voxel-based MRI postprocessing has been recently shown to help visualize blurred gray–white matter junctions or abnormal extension of cortical bands otherwise not recognizable on MRI. A negative MRI may still allow for further surgical planning. In a recent series of pediatric temporal lobectomies, half of those eventually shown to have histologically confirmed MTS had normal hippocampus on MRI. In such cases, other imaging such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetoencephalography (MEG) can help to further define the extent of the epileptic-onset zone and the functionality within and surrounding the malformation.
PET provides a tomographic image of brain glucose utilization, while SPECT images blood flow. Both can reveal areas of metabolic derangement (hypometabolism interictally and hypermetabolism during a seizure) that can represent a seizure focus. , MEG collects minute magnetic potentials generated by bands of synchronized neural currents, a technique that can reveal epileptic foci as well as functional circuits (e.g., motor units). In a recent case series, MEG was shown to compare favorably to invasive monitoring in differentiating between temporal and insular onset of seizures, in a largely image negative pediatric population. The specific role for these various modalities is not determined and considerable variability exists across programs.
Invasive mapping can identify a focus of seizure onset, define the extent of an epileptic zone, or localize neurologic functions. The intraoperative mapping needed is determined by the preoperative workup and must be determined on an individualized basis. In some patients, no definitive focus can be identified on preoperative studies, and invasive monitoring is needed to lateralize. This may occur in a child with classic temporal lobe epilepsy but evidence for bilateral onset on scalp EEG recordings.
In the setting of infiltrative low-grade tumors or focal cortical dysplasia, invasive EEG recording may be used to define the relationship of epilepsy onset to the lesion. This may guide the surgeon to resect epileptogenic tissue beyond the bounds of the imaging abnormality. Such infiltrative tumors and cortical dysplasias can have functional neurologic tissue within them. If these lesions are anatomically near motor or speech cortex, mapping of these functions can further define this relationship. It may be found that a lesion has displaced the motor fibers in such a way that resection can be accomplished while preserving function or an aberrant localization of speech function may be present precluding violation of a typically ineloquent area of cortex.
Direct cortical recording and stimulation mapping can be accomplished via intraoperative electrocorticography (ECoG) or via implantation of grid, strip, and/or depth electrodes for extraoperative study. The choice of technique depends on the information needed and the familiarity of the epilepsy team with the various techniques.
Some centers approach poorly defined lesions and occult lesions on MRI with MEG, PET, and ECoG alone, avoiding grid placement entirely. While good results from this approach have been published, it is difficult to directly compare it to the liberal use of prolonged grid monitoring. At our center, both ECoG and extraoperative grid monitoring are a part of the armamentarium. The approach used is determined on a case-by-case basis depending on the information from the preoperative workup and what vital questions remain.
A significant amount of information about the epileptogenicity and function of an area of tissue can be obtained from intraoperative electrical monitoring. ECoG data are obtained by placing electrodes on the exposed brain and observing the EEG record for epileptiform discharges (spike and sharp waves). , Anesthetic considerations are important to avoid suppressing or altering the EEG record.
Some functional information can be obtained in the anesthetized patient. A distinct pattern of cortical activity (phase reversal) of evoked-somatosensory responses can localize the central sulcus. Direct stimulation of motor cortex can elicit motor responses that are visualized or recorded on EMG. Awake craniotomy in cooperative older children (sometimes as young as 10) can be used to map language cortex. Current is passed through a handheld bipolar stimulator to inhibit an area of cortex while the patient is performing speech tasks. Speech arrest induced during this procedure reflects an area of vital speech function. The interest in HFO activity as a marker for epileptogenic areas suggests that such approaches may have utility in guiding intraoperative decision making. Recently, the feasibility of HFO detection has been shown in anesthetized patients. Currently, intraoperative HFO detection is being trialed for numerous types of epilepsy cases in both awake and asleep settings. ,
Implanted electrodes allow for EEG monitoring and stimulation mapping outside of the operating room. Recordings include the awake, drowsy, and sleep states and ideally will include several of the patient’s seizures. Monitoring typically continues for 5 to 7 days but can be extended if additional information is needed. Very brief intracranial monitoring (24 or 48 hours) can be a useful technique in toddlers who would not tolerate prolonged implantation.
Intracranial monitoring electrodes are typically placed in the subdural space, but epidural placement is employed at times, and yields comparable information. , A typical grid consists of 64 electrodes on an 8 × 8 cm array, whereas scalp EEG typically employs 32 electrodes to cover the entire head.
Implanting electrodes additionally allows for prolonged functional mapping outside the operating room by stimulating through the same electrodes. By applying current across two neighboring electrodes, the intervening area of cortex (about a centimeter of tissue) is inhibited. Deficits observed can identify the function of the cortex and the deficits likely to be incurred by resection. Lower stimulation of motor cortex may produce muscle contraction rather than inhibition. Many children who cannot tolerate awake intraoperative mapping will cooperate with extraoperative mapping. Additionally, the mapping can take place during complex activities such as drawing, writing, or playing a musical instrument potentially identifying important integrative functions.
Grid electrodes consist of a broad array of contacts that can cover a large area of cortex ( Fig. 71.1 ). They can be particularly helpful in defining an epileptic zone within a lobe or defining the relationship of a lesion to seizure onset. Convexity sites are particularly amenable to grid placement. Extraoperative language and motor mapping are typically performed through grids as they give ample coverage of the hand and face motor area as well as the typical language sites.
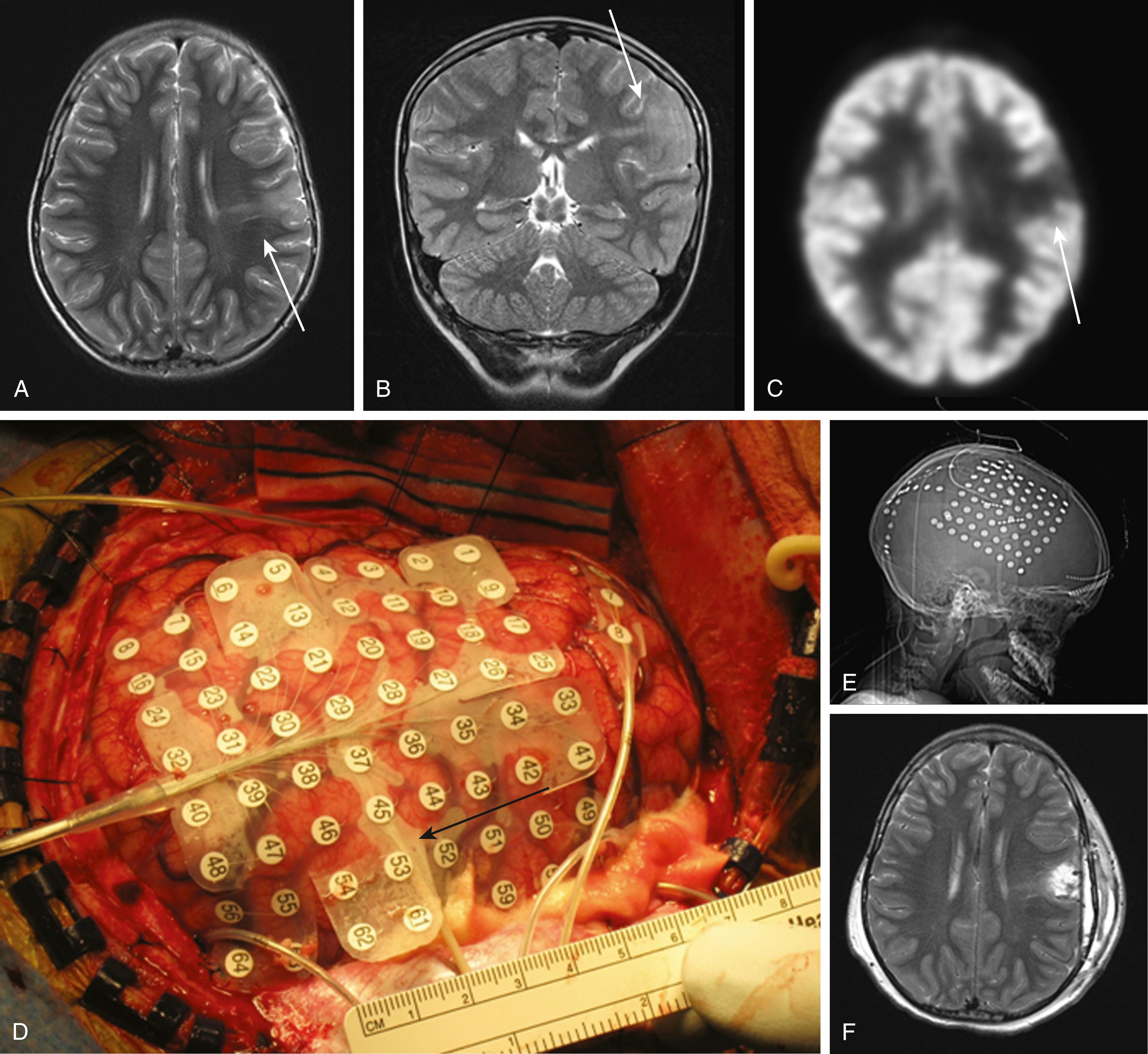
Strip electrodes consist of a single or double row of electrodes spaced along a flexible strip that can be safely passed along the subdural space. Coverage of the interhemispheric cortex, orbitofrontal cortex, and mesial temporal lobe—all locations poorly monitored by scalp recordings—can be achieved by passing strip electrodes. , A strip passed along the undersurface of the temporal lobe will typically place the distal electrode just at the parahippocampal gyrus, providing good monitoring of the mesial temporal structures. Placement of strip electrodes requires only a burr hole, allowing for limited monitoring of distant sites, including the contralateral hemisphere without requiring a craniotomy.
Distant and mesial temporal recordings can also be obtained with depth electrodes. These electrodes line a narrow probe that passes directly into the region of interest. Some centers also place depth electrodes directly within or along the deep margins of a lesion infiltrating the white matter (e.g., cortical dysplasia) to evaluate the depth of resection necessary. , These various electrodes are often used in combination to monitor all areas of interest.
Electrode implantation procedures require two separate operations (occasionally more) with a prolonged hospitalization between. The most common complications are cerebrospinal fluid (CSF) leakage, fever, and infection. Minor CSF leak is common while electrodes are in place, but rarely problematic. While low-grade fevers are common and wound infection much less so, suspicion should remain high to recognize and treat infections promptly.
Cerebral edema combined with the mass effect of the implants can cause elevated intracranial pressure. This occurs more commonly with more electrodes implanted. Mannitol and steroids typically will control intracranial pressure, but on rare occasions, grids may need to be removed.
Strip electrodes have fewer complications than grids, 1% overall compared to 3% to 4%. Strips also appear to be safer than depths. Isolated cases of permanent neurologic deficit and death have been reported. The risk of complications, as well as the additional hospitalization time, need to be balanced against the information gained by invasive monitoring.
Stereotactic EEG (SEEG) was first developed in France by Talairach and Bancaud as an alternative to subdural grid placement. Although initially popular in Europe, the method has recently gained wider acceptance in the United States, however efficacy for pediatric epilepsy remains an active area of clinical investigation. In adults, SEEG has been shown to be an efficacious, and low morbidity, alternative to subdural grid monitoring, with an added benefit of accessibility to deep seizure foci and a far less invasive upfront procedure. Further, SEEG has been postulated to be superior for the detection of deep seizure networks. For these reasons, SEEG placement is an attractive option for pediatric cases, which present unique challenges.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here