Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Peripheral nerve injuries affect 2.8% of trauma patients.
One of the important features of the peripheral nervous system in comparison with the central nervous system is its capacity for recovery through both regeneration and remyelination of axons.
The ultimate goal of managing peripheral nerve injuries is to deliver the optimum treatment. This comes from proper determination of the injured nerve(s) as well as the type and severity of injury. This information can be obtained from history, physical examination, electrodiagnostic testing, and imaging studies performed in a timely fashion.
Each peripheral nerve is composed of fibers from more than one spinal nerve root; correspondingly, each spinal nerve contributes fibers to more than one peripheral nerve.
A preganglionic injury is injury proximal to the dorsal root ganglion. Spontaneous recovery is impossible, and early surgical intervention is indicated. On the other hand, with postganglionic injury, neurons may regenerate axons in the appropriate conditions.
The goal of surgical nerve repair is to maintain or restore nerve continuity. Tension-free coaptation is a must and may be done end to end or with intervening nerve grafts, as circumstances warrant.
Nerve transfers have emerged as favored options for many severe brachial plexus injuries.
Management of patient expectations is critical because recovery after nerve injury can take months to years and is often incomplete.
Nerve repair can be augmented with muscle/tendon transfers to maximize functional outcome.
A clear understanding of the peripheral nervous system's presentation, injury types, and response to trauma is crucial to provide efficient treatment. Apart from the entrapment neuropathy (see Chapter 62 ), acute peripheral nerve injury (APNI) mechanisms are briefly discussed in this chapter along with the management strategies and options. Acute peripheral nerve injury can result from a variety of conditions, including blunt or penetrating trauma, compression, and iatrogenic causes.
Stretch-related injuries are the most common type. Because of the extracellular matrix and connective tissue layers, peripheral nerves are elastic and also somewhat resilient to stretch. Injury occurs when traction forces surpass the capacity of nerve to stretch. Although the continuity is retained in most situations, sometimes the traction is great enough to lead to a complete loss of continuity. For instance, brachial plexus avulsion is an example of complete discontinuity of the nerve element. Another significant point of this type of injury is its association to other injuries such as limb fractures where the nerve is in the vicinity of the bone, as in the case of humeral fracture and radial nerve injury.
Laceration injury results from sharp objects and may constitute 30% of serious injuries. Even with lacerating mechanisms, continuity may be partially maintained and total transection is less frequent.
Compression is a third common cause of peripheral nerve injury (PNI). These injuries may present either relatively acutely as, for instance, Saturday night palsy due to radial nerve compression, or they may develop over a relatively long period as in entrapment neuropathies. Although nerve continuity is well maintained in this type of injury, complete loss of both motor and sensory function can happen.
Combined mechanisms of nerve injury are most frequent in trauma. Motor vehicle and sports-related injuries are mainly due to stretch/traction; however, both contusion and laceration may also occur. Missile injuries may cause nerve contusions or transection, but most often the nerve continuity is maintained, with the cavitation effect resulting in significant intraneural damage. Compression injuries may be iatrogenic, as in the case of body positioning during anesthesia; the mechanism of injury in this condition is usually a combination of contusion and ischemia. Penetration by sharp objects often results in nerve laceration, although soft tissue damage and resulting compartment hematomas and increased pressure can cause swelling with secondary compression.
The optimal management of PNI depends on the efficient identification of what nerve or nerves are involved, the site affected, the type of injury, and its severity. This requires a comprehensive understanding of the relevant anatomy and physiology, along with a focused history and physical examination. Supplementary electrodiagnostic and imaging studies are necessary to direct the clinical information toward the best management plan.
Each peripheral nerve carries fibers from more than one spinal nerve root. That intuitively means that each spinal nerve contributes to more than one peripheral nerve. Based on this information, the clinical picture of radiculopathy can often be differentiated from a PNI localization by neurologic findings. In the case of radiculopathy, the weakness is usually mild to moderate in a given muscle (as other spinal roots contribute), and the sensory alteration appears in a dermatomal distribution (collectively, a myodermatomal pattern). By obvious contrast, PNI usually present with relatively more significant paresis and more precise and discrete sensory changes (nerve territory) and muscle atrophy supplied purely by a given nerve.
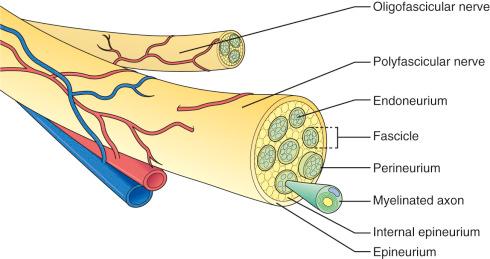
The microscopic anatomy of the peripheral nerve ( Fig. 61.1 ) consists of nerve fibers within the confines of connective tissue layers (endoneurium, perineurium and epineurium) and augmented by blood vessels, lymphatics, and nervi nervorum. Once a peripheral nerve is injured, a complex and exquisitely regulated sequence of events begins to remove the damaged tissue and initiate the reparative process. The nerve's response to injury involves the site of damage as well as the cell bodies in the spinal cord and dorsal root ganglia. Schwann cells, macrophages, inflammatory cells, and neurotrophic factors play critical roles in nerve degeneration and subsequent regeneration.
Successful peripheral nerve recovery and the duration of this process depend on the initial extent of injury. Two clinically helpful grading systems have been developed to assess the severity of injury, namely that of Seddon and Sunderland, that correlate the microscopic changes of the injury and patient manifestation ( Fig. 61.2 ).
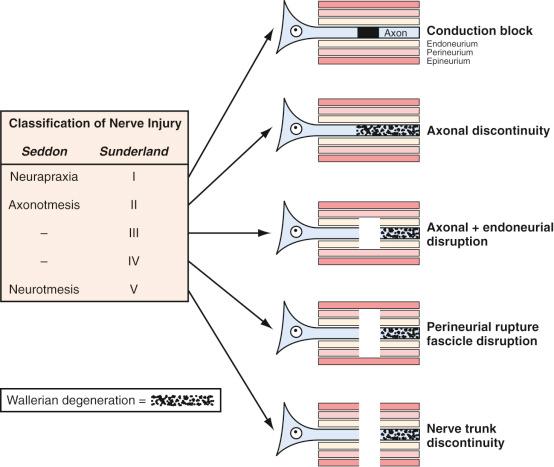
Seddon classified nerve injuries by severity into three broad grades: neurapraxia, axonotmesis, and neurotmesis. In neurapraxia, the mildest injury type, nerve and axonal continuity is maintained, and dysfunction is from a transient conduction block. Nonetheless, subtle changes in myelin structure have also been noticed in more severe forms of neurapraxia that take weeks to recover from. Axonotmesis, the second grade, involves complete disruption of both the nerve axon and surrounding myelin, whereas the perineurium and epineurium are preserved. Axon and myelin degeneration occur distal to the injury site, resulting in total denervation. Excellent recovery is expected in this grade, because the intact endoneurium and perineurium provide a precise track for sprouting axons to reinnervate their own target end organ in the process of regeneration. Neurotmesis is the most severe category and involves complete nerve disconnection. Functional loss is complete, and recovery is not expected without surgical intervention because of loss of surrounding mesenchymal guidance and scar formation.
Sunderland's grading system described five categories of nerve injury according to severity. A first-degree injury is equivalent to Seddon's neurapraxia, and a second-degree injury is equivalent to axonotmesis. Third-degree injury occurs when there is, in addition to axon interruption, partial or complete endoneurium damage. Spontaneous functional recovery is possible, but highly variable, and relies on the severity of endoneurium injury. Sunderland divides Seddon's neurotmesis into fourth- and fifth-degree injuries. In a fourth-degree injury, all components of the nerve are disrupted except the epineurium, so the nerve, while in physical continuity, has complete loss of internal continuity. Operative intervention is necessary to remove the scar segment (neuroma-in-continuity) and reconstruct the nerve gap. A fifth-degree injury entails complete disconnection of the nerve, and surgical treatment is warranted.
Note that histologic changes are mild or absent in first-degree injuries in which the mechanism is conduction block only. In all other grades of nerve injury, because the axon is disconnected, distal to the site of damage, wallerian degeneration occurs. In wallerian degeneration, the initial histologic change encompasses fragmentation of both axons and myelin, which begins within hours of injury. In this process, both neurotubules and neurofilaments become disorganized, and the axonal contour becomes irregular, owing to varicose swellings. By 48 to 96 hours after injury, axonal continuity is disrupted and conduction of impulses ceases. Myelin disintegration is slightly delayed as compared to axonal dissolution but is well advanced by 36 to 48 hours.
Schwann cells play a pivotal role in wallerian degeneration. They become active within 24 hours of injury, demonstrating nuclear and cytoplasmic enlargement as well as an increased mitotic rate. These cells divide quickly to form dedifferentiated daughter Schwann cells that up-regulate gene expression for a multitude of molecules to participate in the degeneration and regeneration process. Primarily Schwann cells assist to remove the degenerated axonal and myelin debris, a role they then pass on to macrophages, which are recruited to the injured nerve by cytokines released from Schwann and other cells. Both Schwann cells and macrophages work together to phagocytose and clear the site of injury, a process that in the peripheral nerve usually lasts from 1 to a few weeks. During the initial stages, the endoneurial tubes swell in response to the trauma, but after the first 2 weeks they become smaller in caliber. By 5 to 8 weeks, the degenerative process is usually complete, and so-called bands of Bungner, composed of Schwann cells within an endoneurial sheath, are all that remain, which are in place to receive and guide regenerating axons.
In some third-degree and almost all fourth-degree injuries, the local reaction is more considerable as there is greater intra- and extrafascicular damage. Due to endoneurium elasticity, the severed fibers' ends retract. Vasa nervorum injury leads to hemorrhage and edema, which lead to a vigorous inflammation. This response triggers fibroblast proliferation and forms a fibrous scar that, along with bundles of regenerating axons and proliferating Schwann cells, causes a fusiform swelling of the injured site (neuroma-in-continuity). Thus the entire nerve trunk, which is in continuity, is permanently enlarged, and effective regeneration of axons distally is aborted.
The pathologic changes sustained by the proximal portion and neuronal cell body rely on the distance of the site of injury from the cell body. The nucleus migrates to the periphery of the cell, and certain cytoplasmic elements (like Nissl granules and endoplasmic reticulum) undergo chromatolysis; the structural change corresponds to a molecular change with down-regulation of maintenance and structural protein genes and up-regulation of those related to regeneration. Neuron cell survival depends on the Schwann cells and trophic molecules ( Box 61.1 ) present in the immediate environment.
Neurotrophic factors such as nerve growth factor (NGF), brain-derived neurotrophic factor, ciliary neurotrophic factor, and many others have been identified. They are important in nerve cell maintenance and survival, as well as for aiding in the regenerative and repair processes.
NGF is released from peripheral nerve target organs and transferred to the nerve cell body via retrograde axonal transport. The amount of NGF and other trophic factors reaching the cell body as a result of axonal disruption occurrence decreases soon after the injury, and critical deprivation may even trigger cell death.
Immediately after injury, the amounts of NGF and NGF messenger RNA are increased in glial and surrounding cells to replace sources lost from target organs. The invading macrophages stimulate NGF production via interleukin-1 release (the macrophage is involved with both phagocytosis and regeneration). Moreover, Schwann cells produce NGF at the site of injury. NGF binds to specific tyrosine kinase receptors transmitting a signal to regulate gene activation.
NGF receptor concentration on the Schwann cells increases also. The NGF binds to these receptors on the Schwann cells and assists in the regrowth of axon sprouts. The NGF taken up by the axon is then transferred retrograde from the growth site to the cell body, providing a continued stimulus for growth and a guide for the advancing axon.
Early after nerve damage, until the distal axons are totally degenerated, motor and sensory nerve potentials still can be observed in the distal segment. Denervation changes in the muscles happen with even further delay. Therefore electrodiagnostic studies, used to predict severity of the lesion and to guide treatment recommendations, should be deferred until 3 to 4 weeks after injury.
Sequences of structural changes take place in the denervated muscle if neural regeneration does not occur. Within 2 months, atrophy is seen as a mean 70% reduction in the cross-sectional area. Sodium channels regress toward embryonic forms, and acetylcholine receptors redistribute to cover the entire muscle surface. This oversensitivity to acetylcholine manifests clinically as spontaneous uncoordinated muscle activity called fasciculation and by electromyography (EMG) known as fibrillation .
Determining the time and location of motor and sensory alteration is critical for an accurate assessment of the acute nerve injury. An instantaneous neurologic deficit concomitant with the injury, for example, signifies direct peripheral nerve involvement at the site of injury. In sharp contrast, a delayed neurologic deficit denotes subsequent nerve damage, suggesting a compressive etiology. Therefore in cases of progressively worsening neurologic deficit, an enlarging lesion in the vicinity of the nerve (such as pressure from a cast or splint or a hematoma) must be anticipated and urgently addressed. Moreover, some neurologic deficits develop over a relatively long period of time, as in bony callus after elbow trauma resulting in a tardy ulnar palsy.
Knowing the exact mechanism of injury is of great value when evaluating for the possibility of recovery and can guide treatment. For example, in cases of low- or no-impact trauma such as a fall from a standing position, neurapraxia will be the likely result. Conversely, the high impact and forces in motor vehicle accidents usually result in more severe nerve damage such as axonotmesis or neurotmesis.
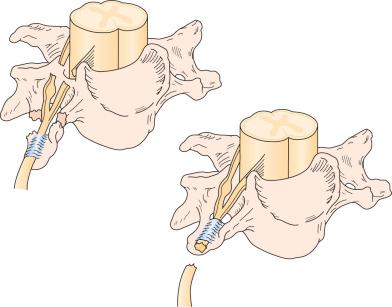
Nerve injuries to the brachial plexus can also be divided into preganglionic or postganglionic ( Figs. 61.2 and 61.3 ), with discerning surgical implications. A preganglionic injury indicates injury proximal to the dorsal root ganglion with permanent paralysis and complete sensory loss of the territory innervated by the avulsed roots. Moreover, there is no possibility of spontaneous recovery in the involved spinal nerve root. A postganglionic injury, however, retains continuity of the cell body within the ventral horn of the spinal cord with its peripheral axon, so that there is the potential for regeneration in the appropriate circumstances.
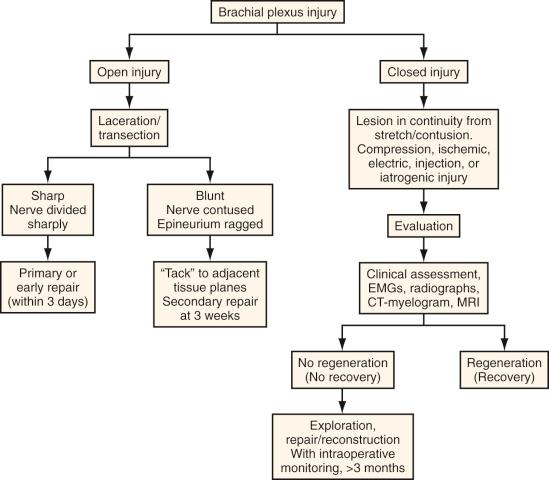
Acute nerve injuries can be further divided into open or closed. Open injuries include sharp lacerations and missile wounds, whereas the closed type includes traction or compressive injuries. Present guidelines recommend early or immediate repair (days) of clean, sharp nerve lacerations and the delayed (weeks) repair of dirty nerve lacerations. Missile wounds with vascular injury should be explored acutely. Early intervention is not recommended in closed injuries because of the potential for neurapraxic or other lower-grade injury. Spontaneous regeneration, when it happens, provides superior functional outcomes when compared to surgical reconstruction. Based on these considerations, the plan of surgical management of peripheral nerve and brachial plexus injuries is illustrated in Fig. 61.4 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here