Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Most patients diagnosed with primary hyperparathyroidism are asymptomatic or are only mildly symptomatic, compared with the classic descriptions of patients with hyperparathyroidism.
Calcium levels are regulated by the actions of parathyroid hormone (PTH), vitamin D, and calcitonin on the intestines, bone, and kidneys.
Knowledge of parathyroid anatomy and embryology is key in finding diseased parathyroid glands in both their normal and abnormal locations.
An elevated calcium with an elevated PTH level is diagnostic of primary hyperparathyroidism. Similarly a high normal calcium with a nonsuppressed PTH level suggests primary hyperparathyroidism.
Single adenomas are seen in 80% to 85% of cases of primary hyperparathyroidism. Four gland hyperplasia accounts for another 10% to 15% of cases.
There are many localization studies that can be obtained before parathyroid surgery to aid in localization intraoperatively. A negative localization test is not a contraindication to surgery if the patient otherwise has clear-cut surgical indication.
The revised NIH consensus guidelines are used to help decide which patients are appropriate surgical candidates.
PTH has a very short half-life of 3 to 5 minutes, so intraoperative PTH measurement can provide an adjunct during surgery.
In the absence of definitive preoperative identification of a parathyroid adenoma, bilateral neck exploration remains the classic approach for parathyroid surgery. However, if during surgery parathyroid resection is associated with a drop in the intraoperative rapid PTH assay by 50% with entry into the normal range, surgical exploration may be halted.
Intraoperative nerve monitoring for the recurrent laryngeal nerve is a useful adjunct during parathyroid surgery.
Having at least two localizing studies prior to reoperative parathyroid surgery is beneficial.
The management of parathyroid disorders has evolved over time with the progressive knowledge that has improved the diagnosis, as well as preoperative and intraoperative management of the disorder.
British anatomist Sir Richard Owen is generally acknowledged as being the first to describe the existence of the parathyroid glands in 1852. The glands were discovered at autopsy of the Zoological Society of London's Indian rhinoceros. In 1877, Swedish medical student Ivar Sandstrom reported the existence of distinct glandular tissue adjacent to the thyroid in a dog, and in 1880 he reported the discovery of a similar organ in humans (glandulae parathyroideae). The earliest reports of clinical hyperparathyroidism (HPT) involved bone disease, or osteitis fibrosa cystica, as termed by von Recklinghausen, although the association with HPT was not discovered until later.
Halsted was motivated to find the parathyroid blood supply based on his experience with patients after thyroidectomy. Gley made the association with tetany after parathyroidectomy. Rasmussen and Craig isolated parathyroid hormone (PTH) in 1959. Berson and Yalo earned the Nobel prize for developing an assay to measure PTH levels in serum. Autoanalyzing systems that rapidly measure calcium levels nowadays allow patients with HPT to be diagnosed before they are symptomatic or develop complications.
Olsch performed the first successful parathyroidectomy in the United States in 1928 at Barnes Hospital of Washington University by removing a large parathyroid adenoma, precipitating a profound decline in serum calcium requiring massive doses of parathyroid extract and IV calcium to save the patient. One of the most famous patients to have suffered from HPT is Captain Charles Martell, who was a merchant marine. He had a total of seven operations and was ultimately found to have a mediastinal parathyroid adenoma. He unfortunately died soon after the successful operation from complications related to kidney stones.
Although laboratory values may vary somewhat, the normal range for calcium in the blood is between 8.5 and 10.2 mg/dL. Calcium exists in two forms, bound to protein (55%) or in a free ionized state (45%). The nonionized form is mostly bound to albumin so significant changes in albumin will change the total calcium level. The most common formula to obtain a corrected calcium level is that for every 1.0 mg/dL decrease in albumin there is a 0.8 mg/dL decrease in calcium. Ionized calcium ranges between 4.5 and 5.0 mg/dL in most laboratories and is inexpensive and easy to obtain. It is affected by blood pH, so both should be measured together. Ionized calcium will be decreased by 0.36 mmol/L for every one unit increase in pH.
PTH is secreted in response to low serum ionized calcium levels and is inhibited via a feedback mechanism when the serum ionized calcium level is high. Intact PTH is an 84–amino acid protein that is the biologically active form of the hormone. It has a half-life of only 3 to 5 minutes.
Calcium homeostasis is affected by the interplay between PTH, calcium, and vitamin D on the gastrointestinal tract, bone, and kidneys. The target end organs of PTH are the kidneys, intestines, and bones. In the kidneys, PTH increases the rate of conversion of 25-hydroxyvitamin D 3 (calcifediol) to 1,25-dihydroxyvitamin D 3 (calcitriol), increases calcium reabsorption, and decreases phosphorus reabsorption in the tubules. Calcium and phosphorus reabsorption are increased in the intestines, whereas the effects on bone are via a PTH receptor on the osteoblasts resulting in increased production of cAMP, which in turn stimulates the osteoclasts.
Calcitonin is secreted by the parafollicular C cells in the thyroid and has a smaller role in calcium regulation. It is stimulated by high calcium levels and inhibits bone resorption; however, it does not alter calcium levels significantly. This is illustrated in medullary thyroid carcinoma. In this condition very high levels of calcitonin are present, yet these patients do not have hypocalcemia.
Several endogenous substances—including peptides, steroid hormones, and amines—influence PTH release. However, calcium represents the most potent regulator of PTH secretion. Even minor alterations within the physiologic plasma calcium range can induce considerable secretory responses: reduction of ionized plasma calcium by 0.04 mmol/L may elevate serum PTH by 100% or more. The rapid effect of extracellular calcium on PTH release suggests that calcium directly interferes with the release process, but the nature of this interference has been only partly clarified.
Intact PTH 1-84 is rapidly cleared from the human circulation and has a half-life of only a few minutes. PTH clearance occurs in the liver and kidney. Clinical analysis with immunometric PTH assays usually discriminates hypercalcemic patients with HPT from patients with other causes of hypercalcemia. Nonparathyroid tumors that produce intact PTH are exceptionally rare. These include ovarian and small cell carcinomas and thymoma ( Boxes 123.1 and 123.2 ).
Parathyroid hormone–related protein secretion by lung, esophagus, head and neck, renal cell, ovary, bladder, and pancreatic cancers; thymic carcinoma; islet cell carcinoma; carcinoid; and sclerosing hepatic carcinoma
Ectopic parathyroid hormone secretion by small cell lung cancer, small cell ovarian carcinoma, squamous cell lung carcinoma, ovarian adenocarcinoma, thymoma, papillary thyroid carcinoma, hepatocellular carcinoma, and undifferentiated neuroendocrine tumor
Ectopic 1,25-dihydroxyvitamin D production by B-cell lymphoma, Hodgkin disease, and lymphomatoid granulomatosis
Lytic bone metastases caused by multiple myeloma, lymphomas, breast cancer, and invasive sarcoma
Tumor production of other cytokines by T-cell lymphomas/leukemias, non-Hodgkin lymphoma, and other hematologic malignancies
Parathyroid hormone–secreting ovarian dermoid cyst or uterine fibroid
Thyrotoxicosis
Pheochromocytoma
Addison disease
Islet cell pancreatic tumors
VIPoma
Sarcoidosis
Wegener granulomatosis
Berylliosis
Silicone-induced and paraffin-induced granulomatosis
Eosinophilic granuloma
Tuberculosis (focal, disseminated, mycobacterium avium complex in AIDS)
Histoplasmosis
Coccidioidomycosis
Candidiasis
Leprosy
Cat-scratch disease
Vitamin D excess (oral or topical)
Vitamin A excess
Thiazide diuretics
Lithium
Estrogens and antiestrogens
Androgens
Aminophylline, theophylline
Ganciclovir
Recombinant growth hormone treatment of AIDS patients
Foscarnet
8-Chloro-cyclic adenosine monophosphate
Familial hypocalciuric hypercalcemia
Immobilization with or without Paget disease of bone
End-stage liver failure
Total parenteral nutrition
Milk-alkali syndrome
Hypophosphatasia
Systemic lupus erythematosus
Juvenile rheumatoid arthritis
Recent hepatitis B vaccination
Gaucher disease with acute pneumonia
Aluminum intoxication (long-term hemodialysis)
Manganese intoxication
Primary oxalosis
AIDS, Acquired immunodeficiency syndrome; VIPoma, vasoactive intestinal polypeptide–secreting tumor.
Normal parathyroid glands are small, 30 to 50 mg, and yellow to brown in color. Their shape can be described as oval or beanlike. The glands appear different with age: darker in younger patients and more yellow in older patients, which is affected by the fat content which increases with age.
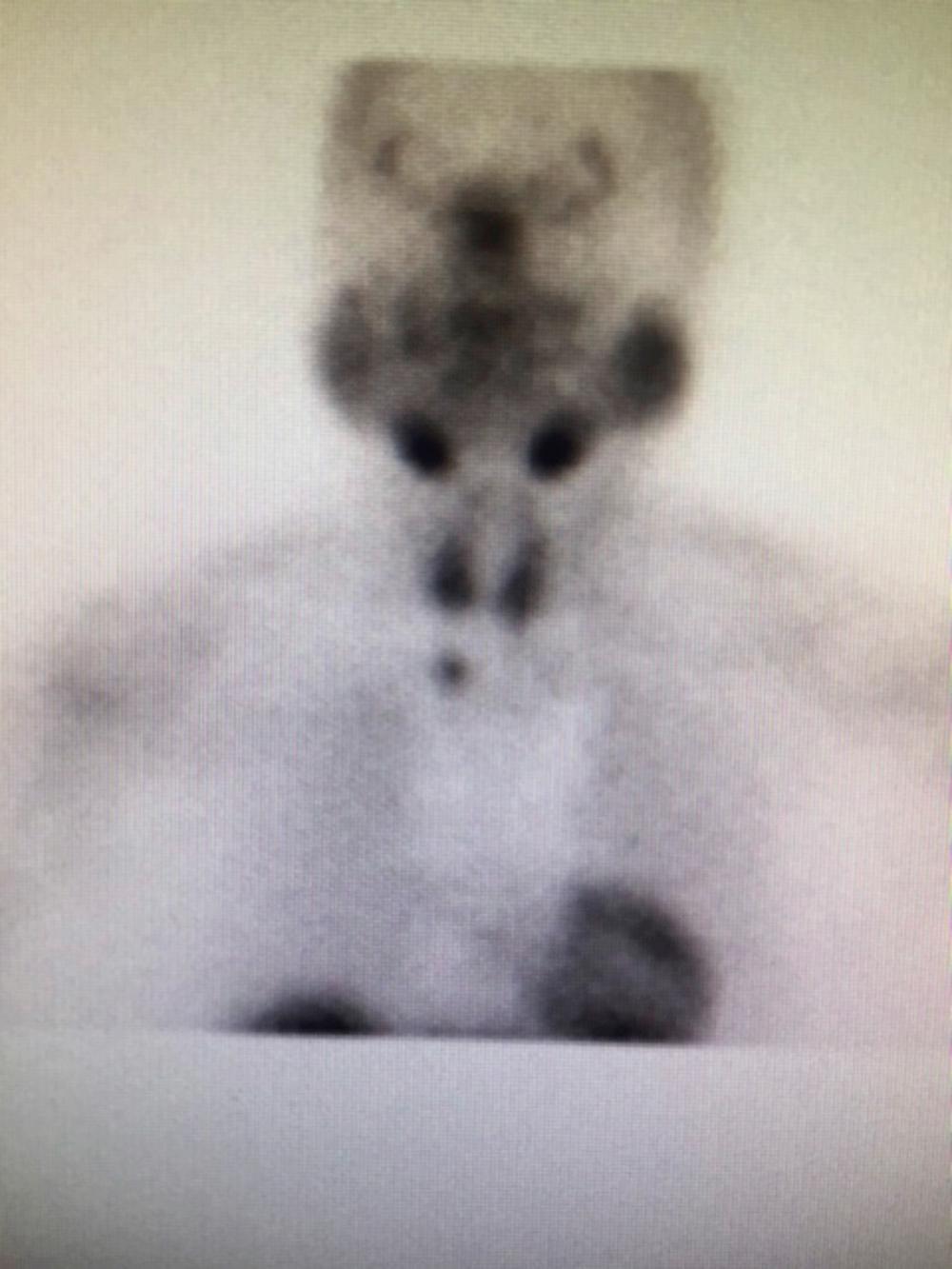
Most people have four parathyroid glands. There are two superior and two inferior glands. Multiple autopsy studies have found four glands 84% to 87% of the time, and three glands in 3% to 6% of patients. Supernumerary glands can be found, with studies showing up to 12 glands. These account for a small proportion of cases of persistent HPT after surgery. Studies with a large series of patients showed that a supernumerary gland is the cause of disease in only 0.7% (15 out of 2015 patients). Most of these fifth-gland tumors were located in the mediastinum ( Fig. 123.1 ). In a similar series of 762 patients with primary HPT, the supernumerary gland was close to the thymus and accounted for 0.8% of patients.
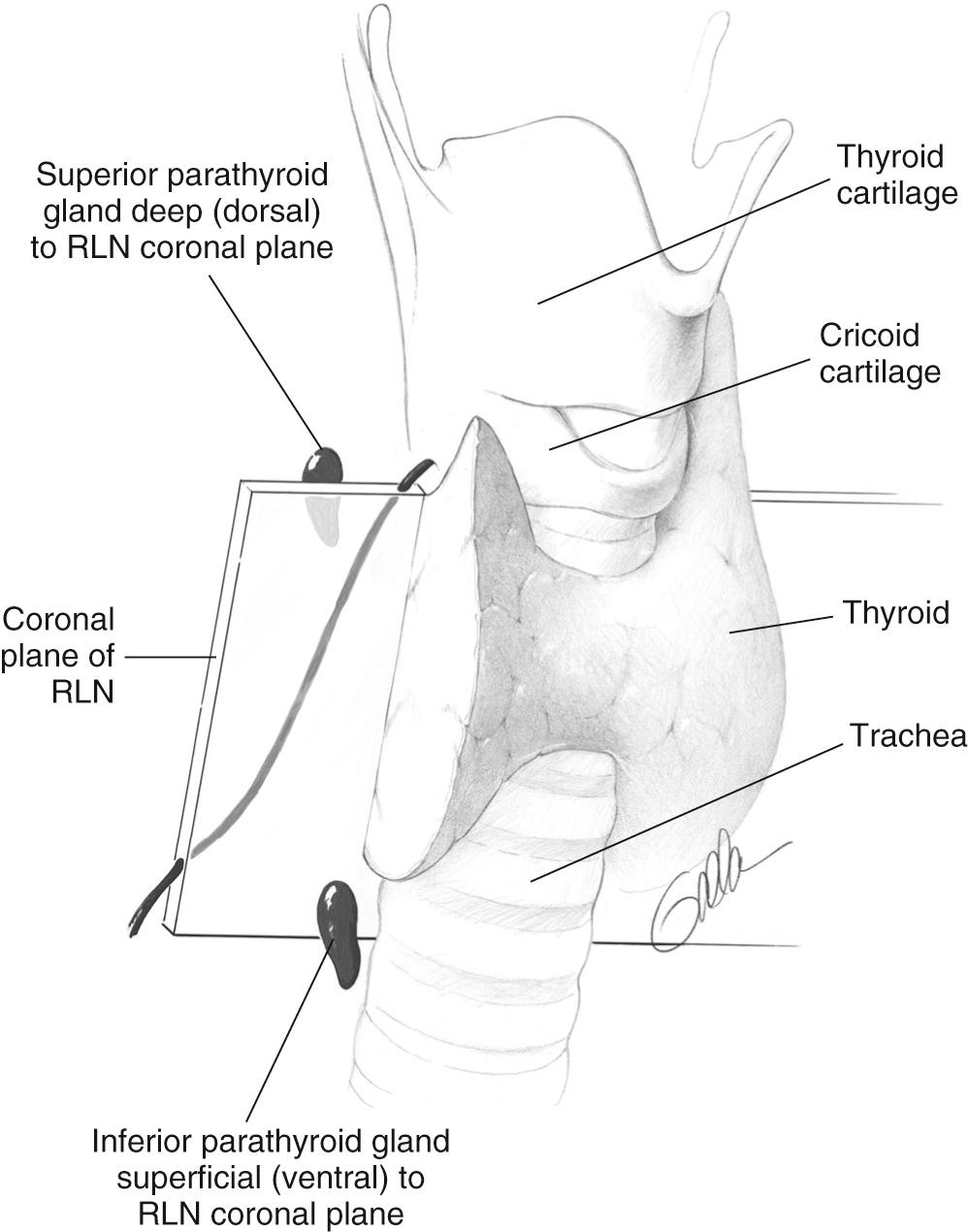
Discerning an abnormal gland from a normal gland relates to the experience of the surgeon. The glands are normally found posterior to the thyroid. The location relative to the recurrent laryngeal nerve (RLN) is usually constant, so the superior glands are usually dorsal (deep) to the RLN, while the inferior glands are ventral (superficial) to the nerve ( Fig. 123.2 ).
Most superior glands are found near the tracheoesophageal groove, just cranial to the point where the inferior thyroid artery and the RLN cross and are very intimate with, but not typically within the thyroid capsule. The superior parathyroid glands, which are intimately associated with the posterior capsule of the superior thyroid pole, are usually covered by an extension of the pretracheal fascia that envelopes the thyroid gland and connects it to the hypopharynx, esophagus, and the carotid sheath. The relationship of these superior parathyroid glands with the pretracheal fascia is such that the glands themselves are allowed freedom of movement under this “pseudocapsule.” This feature discriminates parathyroid glands from thyroid nodules that cannot move freely as the true capsule of the thyroid gland envelopes these nodules.
When enlarged, the superior parathyroid glands may also be found in the retroesophageal or paraesophageal space. The color of the glands is similar to the esophagus, so maintaining a nonbloody field and being cognizant of this anatomic variant will help in identifying a missing superior parathyroid gland.
The inferior parathyroid glands tend to have a more variable location, though most are found near the lower pole of the thyroid gland. As many as 28% of these glands are found within the thymus in the thyrothymic ligament or within the anterior superior mediastinal thymic gland. Intrathyroidal parathyroid glands are uncommon, reported in the 1% to 3% range, and are more common with superior glands.
For surgeons, knowledge of embryology is key to understanding normal and abnormal parathyroid anatomy.
The superior parathyroid glands develop from the fourth pharyngeal pouch, and the inferior parathyroid glands come from the third pharyngeal pouch along with the thymus ( Fig. 123.3 ).
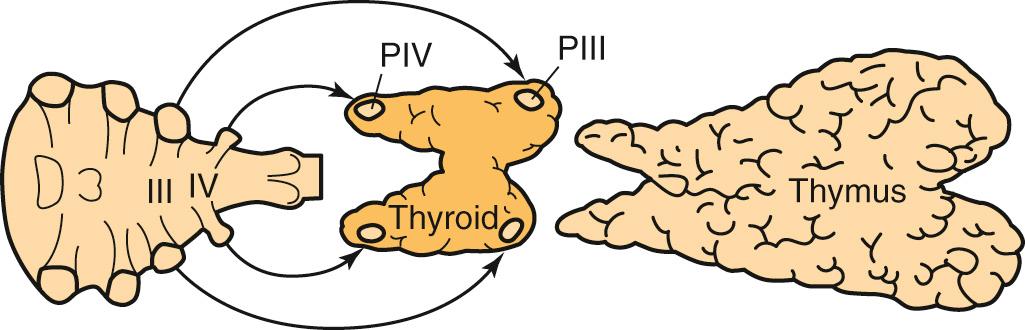
The inferior parathyroid glands may then end up in the anterior superior mediastinum, where a third of all missed parathyroid tumors may be found. Other ectopic locations include the carotid sheath, retroesophageal, and the submandibular region.
The superior glands are not as likely to be ectopic but may descend into the tracheal esophageal groove at or below the mid or inferior thyroid pole.
The blood supply for both the superior and inferior parathyroid glands is from the inferior thyroid artery, which branches from the thyrocervical trunk. However, ligation of the inferior thyroid artery during thyroid surgery may not always compromise the blood supply to the parathyroid glands. Abundant arterial anastomoses supply the parathyroid glands and include anastomoses with thyroid arteries and dominant arteries of the larynx, pharynx, esophagus, and trachea. A transient hypoparathyroidism from ischemia may occur in up to 20% of patients after total thyroidectomy. It is treated with oral and IV calcium supplementation as appropriate. Fortunately this effect is transient and usually resolves in a few weeks postoperatively. Ten percent of the inferior parathyroid glands derived their dominant arterial supply from a branch of the superior thyroid artery.
Parathyroid adenomas are monoclonal or oligoclonal neoplasms; the mechanism of propagation is believed to be clonal expansion of cells that have an altered sensitivity to calcium. Genetic rearrangements of CCND1 or parathyroid adenomatosis 1 oncogene, also known as cyclin D1, have been identified . This protooncogene is located in the vicinity of the regulatory region of the gene for PTH production.
In the case of sporadic parathyroid adenomas, it has been proposed that the etiology may be loss of suppressor gene function on chromosome 1p.
Another process for development of these neoplasms is felt to be due to changes in expression of a tumor suppressor gene where a mutation affects both alleles on the gene and both copies of the tumor suppressor gene are inactivated. HPT is a part of the multiple endocrine neoplasia type 1 (MEN1) syndrome. Somatic mutations in both gene copies of the MEN1 tumor suppressor gene are seen in 20% of patients with primary HPT. Familial hypercalcemic hypocalceuria (FHH) and neonatal severe HPT (NSHPT) are linked to point mutations in the calcium-sensing receptor gene that causes reduced activity. There is also evidence to support exposure to ionizing radiation as another cause for the development of HPT. Common syndromic HPT entities are described in a later section.
The gross appearance of parathyroid adenomas varies, but generally they are oval or bean shaped, reddish brown in color, and soft in consistency. Adenomas may be bilobed or multilobulated in conformation. In 70% of adenomas, a rim of normal parathyroid tissue may be found around the hypercellular portion of the replaced normal gland. However, the absence of this characteristic does not exclude the presence of a parathyroid adenoma. Under light microscopy, adenomas appear similar to normal parathyroid glands and exhibit a thin fibrous capsule with a cellular framework arranged in nests and cords invested by a rich capillary network. Other growth patterns include follicular, pseudopapillary, and acinar patterns.
Chief cells are the major parenchymal cells present in parathyroid glands. Oxyphil cells are also present; although fewer and larger, they have more mitochondria and thus are more likely to concentrate technetium 99m (Tc99m). They take up eosinophilic stain more than the chief cells. Besides the classic adenomas, a few subtypes have been described.
Oncocytic adenomas are composed primarily of oxyphil cells. They are mostly nonfunctional and are likely to be seen in older women. Lipoadenomas are composed of primarily chief cells with a few oxyphil cells and have a large amount of adipose tissue. They can be functional. Water clear cell adenomas and large clear cell adenomas have also been described in case reports.
Due to the difficulty in diagnosing parathyroid cancer, some lesions are described as atypical adenomas. Although they appear atypical, they lack the more concerning features of invasion seen in malignancy.
Although primary HPT is the most common cause of hypercalcemia, there are other disorders such as certain malignancies and granulomatous disease that also cause hypercalcemia.
Some patients may present with profoundly high calcium levels, also known as hypercalcemic crisis. Urgent treatment is needed to prevent arrhythmias and worsening neurologic status, even coma. Initial management includes hydration using normal saline to increase intravascular volume. Caution should be used in patients with a history of cardiac disease so as not to fluid overload the patient. All medications contributing to the hypercalcemic state must be discontinued. These include calcium supplements and thiazide diuretics. The next line of treatment should be a loop diuretic, which will inhibit renal calcium absorption.
Bisphosphonates inhibit osteoclast activity and will cause a profound drop in calcium level. Other medications include glucocorticoids and calcitonin. Glucocorticoids lower the calcium level by inhibiting vitamin D, inhibiting osteoclasts, and decreasing intestinal absorption and renal excretion of calcium. The additive effect of calcitonin and glucocorticoids on osteoclasts will also lower the calcium level.
The incidence of primary HPT is 1/1000 and is more common in women than men, with a predilection for postmenopausal women. The diagnosis of HPT is a biochemical diagnosis based on calcium and PTH level. In most cases, both calcium and PTH levels are elevated, although normocalcemic primary HPT is currently an accepted entity.
Normocalcemic primary HPT is a recently acknowledged entity where total and ionized serum calcium concentrations are normal but PTH levels are consistently elevated in the absence of secondary cause of HPT. This entity was officially recognized at the Third International Workshop on the Management of Asymptomatic Primary HPT. Before making the diagnosis of normocalcemic primary HPT, a thorough search for causes of secondary HPT should be performed, especially vitamin D deficiency. Many patients in whom normocalcemic PHPT is suspected may actually have hypercalcemic primary HPT with coexisting vitamin D deficiency. Renal leak hypercalciuria is another entity that should be ruled out; it can be done simply by giving a trial course of thiazide. In general, normocalcemic primary HPT is considered a nascent form of HPT. Normocalcemic primary HPT should be under regular monitoring for progression of the disease, with surgery considered for development of symptoms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here