Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Endovascular abdominal aortic aneurysm repair (EVAR) for abdominal aortic aneurysms (AAAs) has gained wide acceptance since it was first reported in 1991. Randomized controlled trials have shown decreased short-term morbidity and mortality when compared with open controls. Increasing clinical experience coupled with refinements in device design, such as the development of lower-profile delivery systems and improvements in preoperative imaging for procedure planning and device sizing, have reduced the complications associated with EVAR. However, as with any therapy, the potential for complications exists. This chapter reviews the common short- and long-term complications of EVAR as well as their prevention and management options.
It is useful to classify complications of stent grafting according to their temporal occurrence. Early complications may be related to difficulty with percutaneous access, the passage of the device, failure at a seal zone, or accidental coverage of a side branch, such as the renal artery. Late complications are most often related to endoleaks but can include other entities such as limb occlusion, degeneration of the proximal neck, device fatigue, graft infection, and rupture.
In order for EVAR to be considered as an option for a patient, the access vessels (the femoral and iliac arteries) must be fully evaluated and deemed to be of adequate caliber and acceptable tortuosity to accommodate the device and the delivery system. Challenges related to the access vessels can result in significant perioperative morbidity and potential mortality.
As device delivery profiles have decreased in size, fully percutaneous endovascular aneurysm repair (P-EVAR) has gained popularity as a technique. Most authors report using percutaneous closure with larger-profile devices up to 24-French sheaths. A recent review of the Targeted Vascular data set from the American College of Surgeons National Surgical Quality Improvement Program database comparing P-EVAR with traditional cutdown demonstrated shorter operative time, shorter length of stay, and fewer wound complications associated with P-EVAR. On multivariate analysis, the only predictor of percutaneous access failure was performance of any concomitant procedure.
Major complications of the percutaneous approach stem from failure to adequately close the femoral arteriotomy. These complications include retroperitoneal hemorrhage and femoral artery pseudoaneurysm formation. In most cases, the failure is noted immediately and the femoral artery is repaired by open technique. One study examined 279 femoral arteries that were accessed percutaneously with an immediate failure rate of 6%. The midterm follow-up data on this group showed 3 of 156 (1.9%) having late complications, including one femoral artery dissection and two pseudoaneurysms.
Relative contraindications to percutaneous repair, which may be associated with a higher rate of complication, include circumferential or anterior calcifications of the femoral arteries, small access-vessel size, severe groin scarring, a high femoral bifurcation above the inguinal ligament, and large groin pannus. A high puncture site can be associated with hemorrhage on mobilization, whereas a low puncture site can be associated with vessel occlusion. Ultrasound guidance can be a useful adjunctive technique to find the optimal puncture site and avoid potential complications.
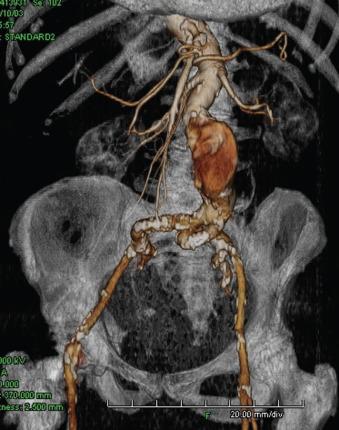
Iliac artery rupture during passage of the device can lead to significant blood loss; if not controlled expeditiously, it can be potentially lethal. Fortunately, with the quality of current computed tomography (CT) imaging, it is possible to anticipate the majority of access problems. Technologic improvements including smaller sizes and hydrophilic coating make endografting accessible to more patients. However, although the delivery profile of abdominal aortic endografts has been decreasing in size, the smallest endograft approved by the US Food and Drug Administration still requires a 14 French sheath. Typically the size requirements range from 18 to 25 French. As such, depending on the device, the iliac arteries should have a minimal luminal diameter of 7 to 9 mm. Apart from vessel diameter, other anatomic factors must be considered, such as the extent of calcification as well as tortuosity and the presence of previous stents ( Fig. 61.1 ). In general, if only one of these factors is marginal, transfemoral delivery can be attempted. However, if more than one factor is marginal, an alternative access such as an iliac conduit should be considered.
Two classes of conduits have been described: open and endovascular. Open conduits are typically performed through a retroperitoneal incision. It is the authors' preference to construct this conduit under combined spinal-epidural anesthesia. A 10-mm crimped Dacron graft is sewn in an end-to-side fashion to the distal common iliac artery and the graft is clamped distally. The graft can then be punctured and used analogously to a native vessel to allow for delivery of the device. After delivery, the graft can simply be ligated, or it can be tunneled down to the groin and anastomosed to the femoral artery to treat any significant iliac occlusive disease. An open conduit also maintains perfusion to the ipsilateral internal iliac artery ( Fig. 61.2 ).
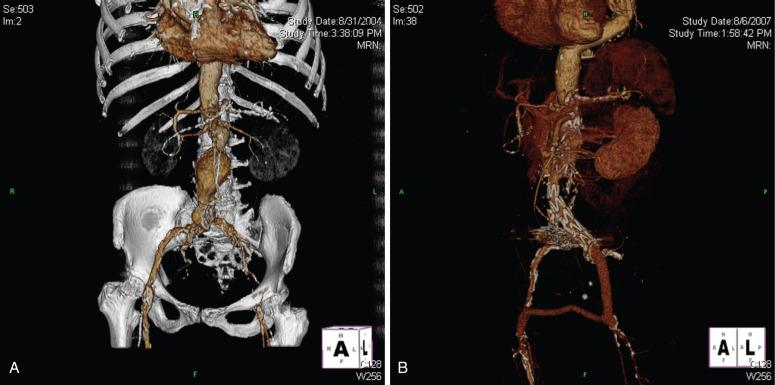
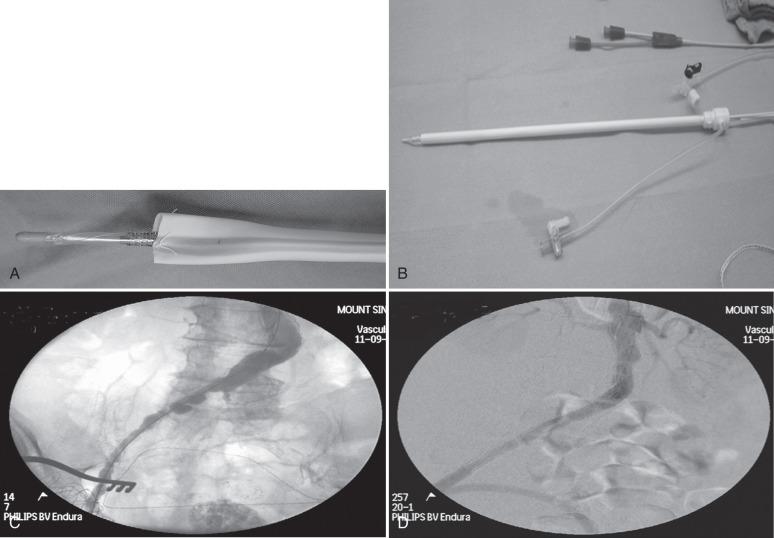
Endoluminal conduits consist of either a commercially available self-expanding stent-graft deployed in the iliac artery prior to delivery of the aortic stent graft or a balloon-expandable stent sewn to a thin-walled 8-mm polytetrafluoroethylene (PTFE) graft delivered through an open femoral arteriotomy. The PTFE graft is predilated at the tip to an appropriate diameter to match the diameter of the common iliac artery at the seal zone. The graft is sewn to an appropriately sized balloon-expandable metal stent and then crimped onto a balloon sized for the iliac seal zone ( Fig. 61.3A and B ). The balloon, stent, and PTFE graft are then back-loaded into a sheath (typically 16 to 18 French), and an angioplasty balloon (typically 6 mm by 2 cm) is used to form a tapered tip for the delivery system. The commercially available conduit is simpler to use, but the “homemade” conduit allows for surgical reconstruction of a diseased common femoral artery. Either type of endoluminal conduit can be placed using only local anesthesia if necessary. The endoluminal conduit is delivered transfemorally after judicious predilatation of the iliac arteries. Once the stent is in the common iliac artery, the sheath is withdrawn, the stent is expanded, and the iliac artery is dilated throughout its entire length down to the groin. At this point the endoluminal conduit can be accessed in a fashion similar to a native vessel to allow for delivery of the device (see Fig. 61.3C and D ). Upon completion of the procedure, the PTFE graft is anastomosed to the femoral artery. The benefit of an endoluminal conduit is that it obviates a retroperitoneal incision in a patient with a hostile abdomen. In addition, in patients with circumferential iliac calcification, the conduit does not have to be sutured to the artery. Disadvantages include the fact that the ipsilateral internal iliac artery must typically be covered. This may result in a higher risk of spinal cord injury in patients with extensive thoracic or thoracoabdominal aneurysms.
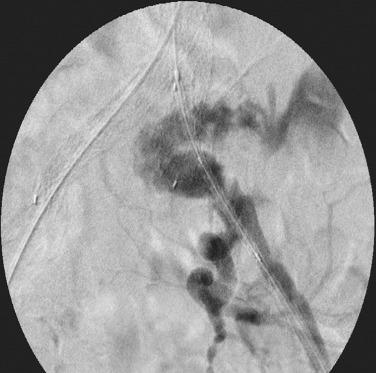
Despite adequate planning, access vessel rupture can occur ( Fig. 61.4 ). Two conditions are necessary to allow for a safe outcome. First, prompt recognition of the rupture is mandatory. Any unexplained hypotension noted intraoperatively should be investigated with a retrograde injection of contrast through the iliac artery. Second, it is absolutely essential to maintain wire access. This allows for placement of a compliant balloon for aortic occlusion to control hemorrhage. The compliant balloon can be inflated in the common iliac artery just proximal to the site of rupture to allow for continued perfusion of the contralateral iliac artery. At this point, a decision can be made as to how to repair the iliac rupture. If the device has already been delivered, an extension limb or a commercially available stent graft can be deployed over the site of rupture. There are several types available (Viabahn, VBX, Fluency and Atrium) and the delivery systems range in size from 6 to 12 French. If the rupture is not amenable to repair with a stent-graft, then a retroperitoneal incision can be made in a controlled fashion and either direct repair of the artery or placement of an open iliac conduit can be performed at this time.
A small-caliber calcified distal aorta may present access problems as well, especially when a bifurcated device is planned. A narrow lumen in the distal aorta might not allow full deployment of the main device, making access of the contralateral gate difficult. If possible, partial deployment of the device with constraint of the iliac limb can be performed. Then, after cannulation of the contralateral gate, the device can be fully deployed. This will hopefully prevent jailing out the contralateral gate. Once the aneurysm is excluded, judicious postdilatation of the iliac limbs should be performed to eliminate any stenoses. An alternative strategy is to use an aorto-uniiliac device.
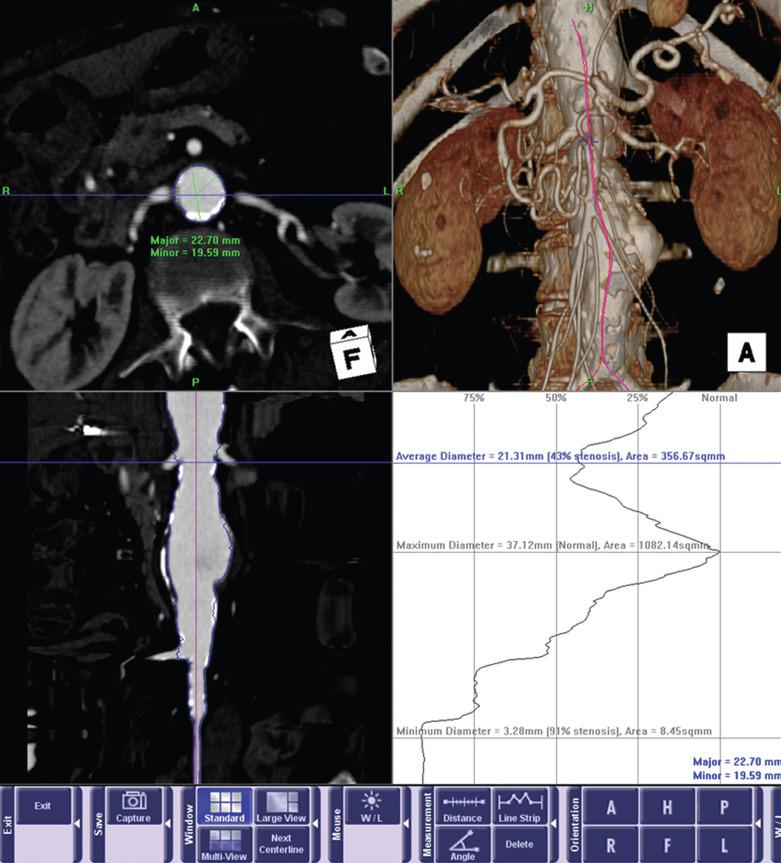
Although access vessel issues are responsible for the majority of acute complications, the long-term success of EVAR is ultimately dependent on the seal zone at the proximal neck. A recent study by AbuRahma and colleagues found that aortic neck length of less than 10 mm correlated with an increased rate of both early and late type I endoleaks. In addition to length, three other anatomic characteristics of the proximal neck determine suitability: angulation, shape, and the extent of mural thrombus ( Fig. 61.5 ). In general, long, straight necks with minimal thrombus are ideal. Preoperative assessment with three-dimensional CT angiography is essential in assessing these characteristics. Center-line reformatted images can provide an accurate assessment of neck length, shaded surface reconstructions can give an accurate view of the angulation and shape of the neck, and orthogonal reconstructions allow for accurate diameter measurements and assessment of the extent of mural thrombus. If one anatomic factor is unfavorable, one can consider attempting EVAR. However, if multiple factors are unfavorable, the likelihood of long-term failure increases significantly.
The choice of device may contribute to the success or failure of the proximal seal zone. Advances in device design such as suprarenal fixation, increased graft flexibility, and an increased range of sizes have allowed endografts to be used in a broader range of patients. However, there are no definitive data proving superiority of any one graft over another. The clinician should base his or her choice of device on several factors, such as diameter, conformability, trackability, and precision of deployment. In addition, the importance of operator familiarity with the device cannot be overemphasized.
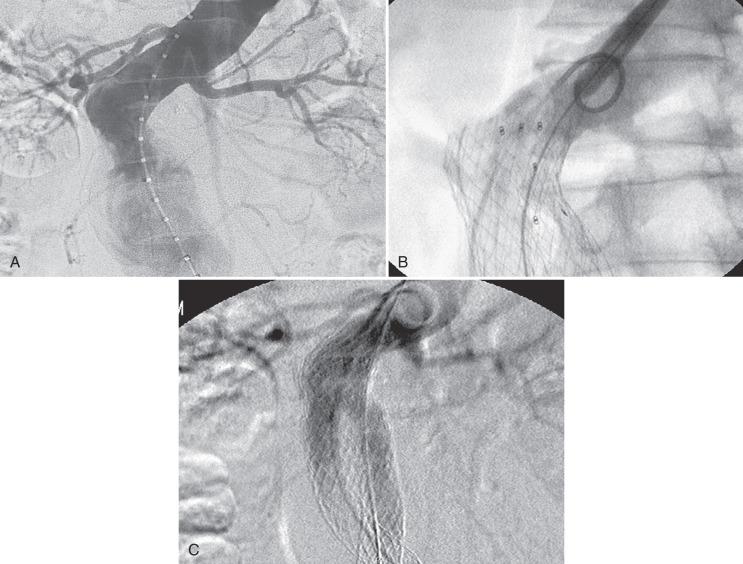
Accurate sizing and placement of the device is essential. Angiography from multiple projections should be performed to identify the true origin of the lowest renal artery. Three-dimensional reconstruction technology can allow for optimal determination of gantry angulation prior to the procedure. The entire length of suitable neck should be used to allow for durable fixation of the stent-graft. If a proximal type I endoleak is present after placement of the main device, several maneuvers may be helpful. It is the authors' practice to complete deployment of the contralateral limb before addressing the proximal leak. This provides additional column strength to the device before any salvage maneuvers are undertaken. At this point, the cause of the endoleak must be determined. If the device is too low, then placement of a proximal extension cuff is generally the first step. Occasionally the device abuts the lowest renal artery but does not cover the entire length of neck on the opposite wall due to poor device apposition from neck angulation. In this case, a second cuff may be helpful, as it may position itself in a different fashion once the main body is in place ( Fig. 61.6 ).
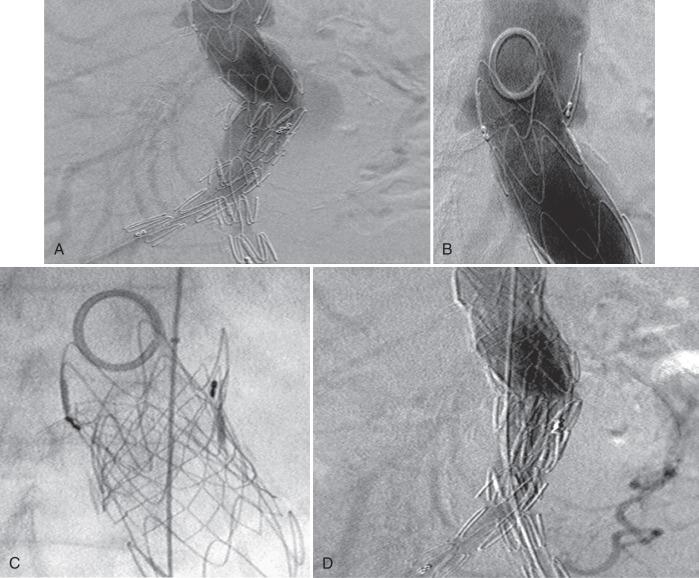
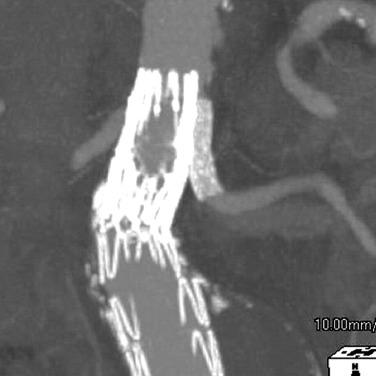
If a proximal endoleak persists despite coverage of the entire available neck, then the mechanism of the endoleak must be determined. Occasionally the device may be correctly sized and positioned but fails to seal because of conformability issues. Should this be the case, a large balloon-expandable stent may help seal the endoleak. Care should be taken when inflating the stent so as not to overdilate the aortic neck ( Fig. 61.7 ). It should be noted that the durability of a balloon-expandable stent deployed inside a commercially approved device has not been rigorously studied.
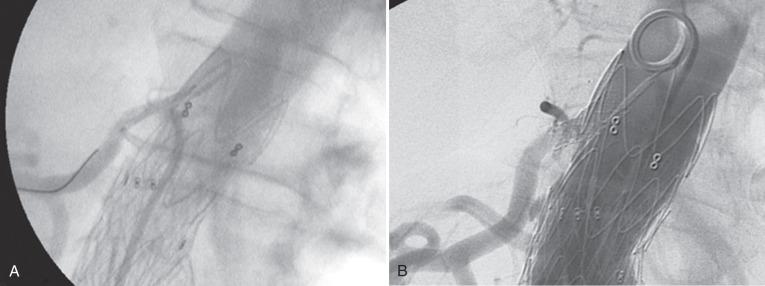
Accidental coverage of a renal artery can be a challenging complication. Other authors have described pulling the devices downward using either an aortic occlusion balloon or a wire and a catheter pulled over the device bifurcation and grasped from both femoral arteries. These maneuvers can be challenging to perform with any amount of control, particularly if the device has active fixation. Should there be any residual renal lumen, it is the preference of the authors to simply stent the renal arteries to maintain patency. As with most endovascular interventions, the key is to establish wire access. Brachial access can be particularly helpful because the stent grafts cover the inferior aspect of the renal ostium. Low-profile systems are preferable ( Fig. 61.8 ). Long-term salvage of renal function after accidental renal artery coverage is possible.
In addition, other authors describe using the “snorkel” or “chimney” technique as a means to prevent occlusion of the renal artery during endograft deployment. In this method, a brachial approach is used to deploy a stent in the renal artery prior to endograft placement. The stent ends up running parallel to the endograft to provide inflow to the renal artery ( Fig. 61.9 ). This maneuver requires a skilled practitioner but has been shown to have good results in small series.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here