Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() The revised (2007) TASC Inter-Society Consensus defines acute leg ischaemia as any sudden decrease in limb perfusion causing a potential threat to limb viability.
The revised (2007) TASC Inter-Society Consensus defines acute leg ischaemia as any sudden decrease in limb perfusion causing a potential threat to limb viability.
Symptoms of acute limb ischaemia (ALI) are usually present for less than 2 weeks. However, some overlap with chronic limb-threatening ischaemia (CLTI) is inevitable and increasingly common. The severity of ischaemia is best defined according to the modified Rutherford criteria ( Table 8.1 ), , using the following categories:
I Viable
IIa Marginally threatened (salvageable if promptly treated)
IIb Immediately threatened (salvageable if promptly revascularised)
III Irreversible
| Category | Prognosis | Capillary return | Motor deficit | Sensory loss | Doppler signals | |
|---|---|---|---|---|---|---|
| Arterial | Venous | |||||
| I Viable | Not immediatelythreatened | Intact | None | None | Audible | Audible |
| IIa Marginally threatened | Salvageable ifpromptly treated | Intact/slow | None | None or minimal (toes) | Inaudible∗ | Audible |
| IIb Immediately threatened | Salvageableif promptly revascularised | Slow/absent | Mild/moderate | More than toes | Inaudible | Audible |
| III Irreversible | Unsalvageable; major tissue loss and permanent nerve damage inevitable Requires primary amputation | Absent staining | Profound, paralysis (rigor∗) | Profound, anaesthetic | Inaudible | Inaudible |
The true incidence of ALI is unknown. Clinical presentation is variable, treatment is not standardised and often the incidence of ALI is reported together with that of CLTI in epidemiological studies. The Oxford Vascular Study found that the incidence of ALI was 10 per 100 000 per year between 2002 and 2012.
The COhorte des Patients ARTeriopathes (COPART; a prospective multicentre registry of patients from three academic hospitals in Southwest France) found that ALI was responsible for 9% of hospitalisations compared to CLTI (91%) amongst patients hospitalised for lower limb peripheral artery disease (PAD).
There is evidence that outcomes are improved when patients are managed by a vascular service providing 24-hour cover. ALI is associated with a high cost to the community because of the significant risk of amputation (10–30% at 30 days) and prolonged hospitalisation.
ALI is the result of occlusion of a native artery or vascular/endovascular prosthesis. Most commonly, embolism or in situ thrombosis can cause native arterial occlusion ( Box 8.1 ).
Atherosclerosis
Popliteal aneurysm
Bypass graft occlusion
Endovascular stent or stent graft occlusion
Iatrogenic (localised arterial dissection post endovascular intervention, for example, arterial closure device failure)
Thrombotic conditions
Atrial fibrillation
Mural thrombosis
Vegetations
Proximal aneurysms
Atherosclerotic plaque
Vasculitis
Dissection
Trauma (including iatrogenic)
External compression
Popliteal entrapment
Cystic adventitial disease
Iliac endofibrosis
Paradoxical embolism
Tumour embolism
Foreign body embolism, e.g., intravenous drug use
Acute compartment syndrome
Low cardiac output states, e.g., hypotension, shock, sepsis
Until about 30 years ago, embolic disease was the underlying cause of most cases of ALI. Emboli large enough to occlude major vessels usually arise in the heart. Rheumatic mitral valve disease was the most common cause, with large emboli forming in a dilated left atrium.
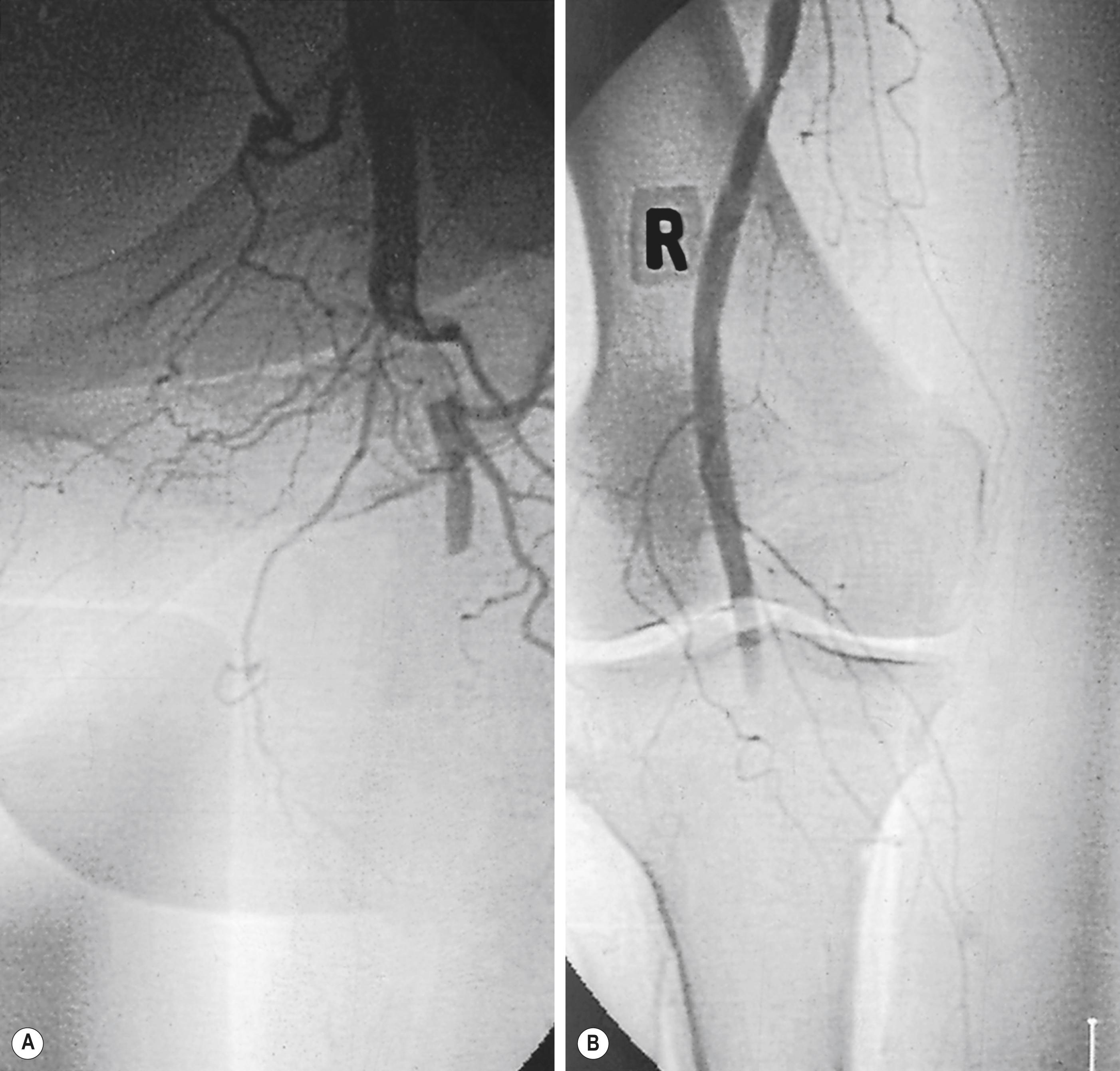
Atrial fibrillation caused by ischaemic heart disease is now the cardiac origin in 80% of embolic cases; mural thrombus following acute myocardial infarction causes most of the remainder. Less commonly, embolisation originates from mural thrombi of the aorta, aortic or popliteal aneurysms and iliac arteries. Large emboli typically lodge at an arterial bifurcation, particularly in the common femoral or popliteal arteries ( Fig. 8.1 ). Patients with cardiac embolism may also have co-existing PAD as a result of the underlying process of atherosclerosis. This increases the difficulty in establishing the cause of the ischaemia and in planning revascularisation. In 20% of patients with ALI, a source for the embolus cannot be found.
Less common sources of emboli include proximal aneurysms or atherosclerotic plaques, usually located in the thoracic or abdominal aorta, or arising from popliteal artery aneurysm. Whereas cardiac embolism usually consists entirely of platelet-rich thrombus, embolism from proximal arteries can include atherosclerotic plaques or cholesterol-rich emboli. This has a much worse prognosis than cardiac embolism because embolectomy is less effective – small particles of atheroembolism can pass to very distal vessels in the foot. This digital embolism can result in the ‘acute blue toe syndrome’. In this condition, the embolic source should be identified and treated if possible. Often, this is a proximal arterial plaque that has ruptured, and the emboli are a mixture of platelet-rich thrombus and cholesterol ( Fig. 8.2 ).
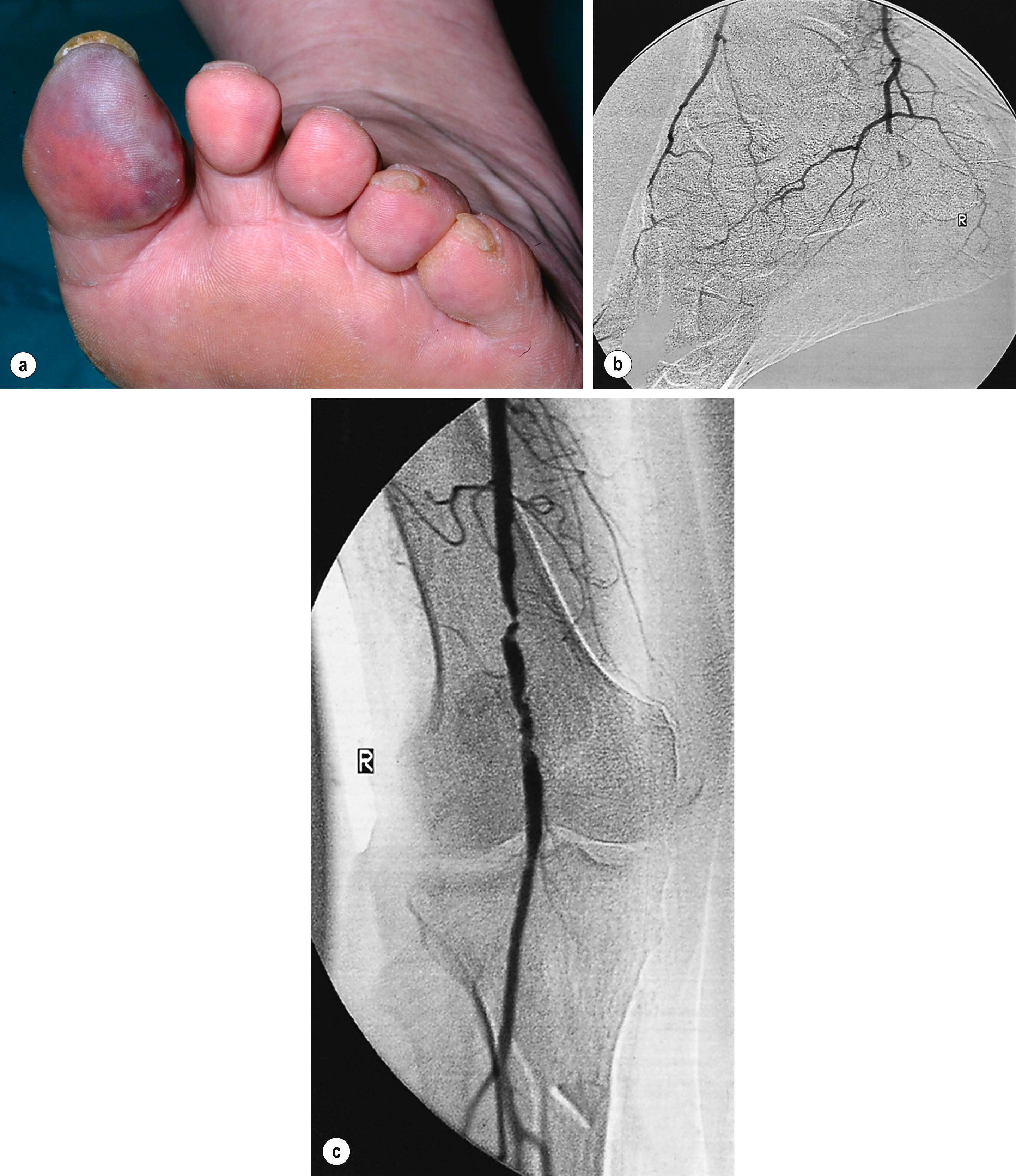
Embolisation of cholesterol-rich atheroma can occur spontaneously, but also follows intra-arterial manipulation following endovascular intervention, or occasionally surgery (causing ‘trash foot’). This can be disastrous, since both large and small arteries may be occluded, and it may not be possible to revascularise with either surgery or thrombolysis. This may result in limb or end-organ damage and is sometimes fatal ( Fig. 8.3 ).
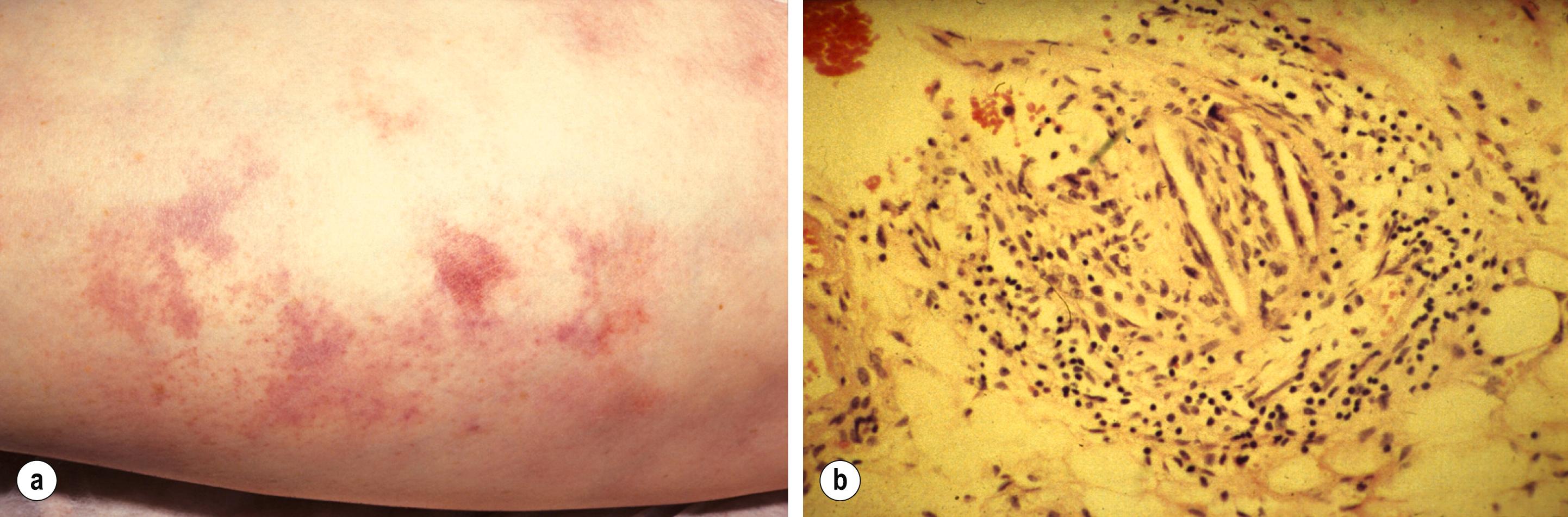
In-situ thrombosis in a native artery is now the most frequent cause of ALI, commonly in patients with a high atherosclerotic burden. It may be the result of rupture of an atherosclerotic plaque or critical flow arrest at the site of an atherosclerotic stenosis. The advancing age of the population and the commensurate increase in atherosclerosis have increased the proportion of ALI caused by thrombosis. Acute native vessel arterial occlusion may also be caused by surgery (e.g., knee replacement disrupting the geniculate collateral vessels formed around a popliteal occlusion), heart failure, thrombotic tendency (polycythaemia, dehydration, malignancy, etc.) or trauma.
Acute thrombosis of a popliteal aneurysm poses the highest risk to the leg. Typically, this occurs in elderly men in association with aneurysms elsewhere (50% have an aortic aneurysm) or generalised arterial ectasia. As they enlarge, popliteal artery aneurysms can fill with lamellar thrombus, which may cause either acute thrombosis or distal embolisation that occludes the tibial vessels. The latter will place the leg in extreme jeopardy, with up to 30% risk of limb loss. It is much more common for a popliteal artery aneurysm to cause distal embolisation than to rupture.
The increasing use of both open and endovascular techniques to revascularise ischaemic limbs means that surgeons often have to deal with acute thrombosis of bypass grafts and arteries that have previously undergone endovascular treatment. Grafts occlude for a variety of reasons. Within 1 month of insertion, graft occlusion is usually the result of technical problems at the time of surgery or poor distal run-off. Graft occlusion within 1 year of placement is often caused by myointimal hyperplasia at an anastomosis or the development of stenoses within a vein graft. Occlusion after 1 year is usually caused by progression of distal atherosclerosis. Prosthetic grafts have a higher occlusion rate than autogenous vein grafts (see Chapter 7Chapter 3Chapter 7 ).
Iliac limb occlusions are observed after aorto-bifemoral bypass surgery if limbs become kinked or have poor outflow. Endovascular stent grafts used to repair abdominal aortic aneurysms (endovascular aneurysm repair [EVAR]) have similar modes of failure. Occlusion is more common when the iliac limb of the stent graft is extended into the external iliac artery. Occlusion may occur any time after implantation in up to 5% of patients.
A special group of iatrogenic occlusion of external iliac and femoral vessels is related to the maldeployment or failure of arterial closure devices. These achieve closure of arteries after endovascular intervention using percutaneously delivered sutures or plugs. Inadvertently, they can cause arrest of flow through direct closure or stenosis of the artery, or by dissection of the vessel. Presentation is usually in the early post-intervention period but may be delayed.
Spontaneous native arterial thrombosis occasionally occurs without an underlying flow-limiting stenosis and these patients should be investigated for an intrinsic clotting abnormality/thrombophilia, for example, antiphospholipid syndrome, activated protein C deficiency or malignancy.
Occasionally, acute arterial occlusion may be caused by arterial dissection, trauma, extrinsic compression or illicit drug use (cocaine and ‘crack’ cocaine). In a young patient with acute popliteal artery occlusion, either popliteal entrapment or cystic adventitial disease should be considered (see Chapter 2 on chronic limb ischaemia).
A number of factors may have changed the presentation of ALI. The incidence of co-existing PAD is increasing, meaning that revascularisation can be more challenging in the acute setting. Medical therapy has improved and many patients are now treated with cardioprotective drugs such as antiplatelet therapy and statins if they have risk factors for cardiovascular disease. Antiplatelet therapy probably reduces the risk of deterioration and the need for limb revascularisation in those patients with established PAD. Recent evidence suggests that low-dose (2.5 mg) rivaroxaban twice a day, in combination with low-dose (100 mg) aspirin may significantly reduce the risk of ALI when compared to aspirin alone. Similarly, patients with atrial fibrillation are often established on anticoagulation to reduce their risk of embolic complications. However, despite adequate anticoagulation, some patients do still present with ALI.
A randomised double-blind placebo-controlled trial evaluating Vorapaxar used ALI as one of the endpoints. The investigators demonstrated that, in selected patients with symptomatic PAD without atrial fibrillation, Vorapaxar reduced the incidence of ALI regardless of the cause. With further research, this protease-activated receptor 1 antagonist could be used to reduce the risk of ALI in patients with known PAD, however it has not yet been widely adopted.
The severity of ischaemia at presentation is the most important factor affecting outcome of the leg. , Complete occlusion of a proximal artery in the absence of preformed collateral vessels (as in cardiac embolism) results in the classical clinical presentation of pain, paralysis, paraesthesia, pallor, pulselessness and a perishingly cold leg (poikilothermia). In reality, all six of these signs are rarely encountered. The pain is severe and frequently resistant to analgesia. Calf pain and tenderness with a tense muscle compartment indicates severe muscle ischaemia or necrosis and often irreversible ischaemia. Sensorimotor deficit including muscle paralysis and paraesthesia is indicative of muscle and nerve ischaemia with the potential for salvage only if treated promptly. Initially, the leg is white with empty veins but after 6–12 hours vasodilatation occurs, probably caused by hypoxia of the smooth muscle. The capillaries then fill with stagnant deoxygenated blood, resulting in a mottled appearance that blanches on digital pressure ( Fig. 8.4 ). If flow is not restored rapidly, the arteries distal to the occlusion fill with propagated thrombus and the capillaries rupture, resulting in a fixed blue staining of the skin that is a sign of irreversible ischaemia. These features are typical of an acute arterial occlusion in the absence of existing collaterals and suggest an embolic cause.
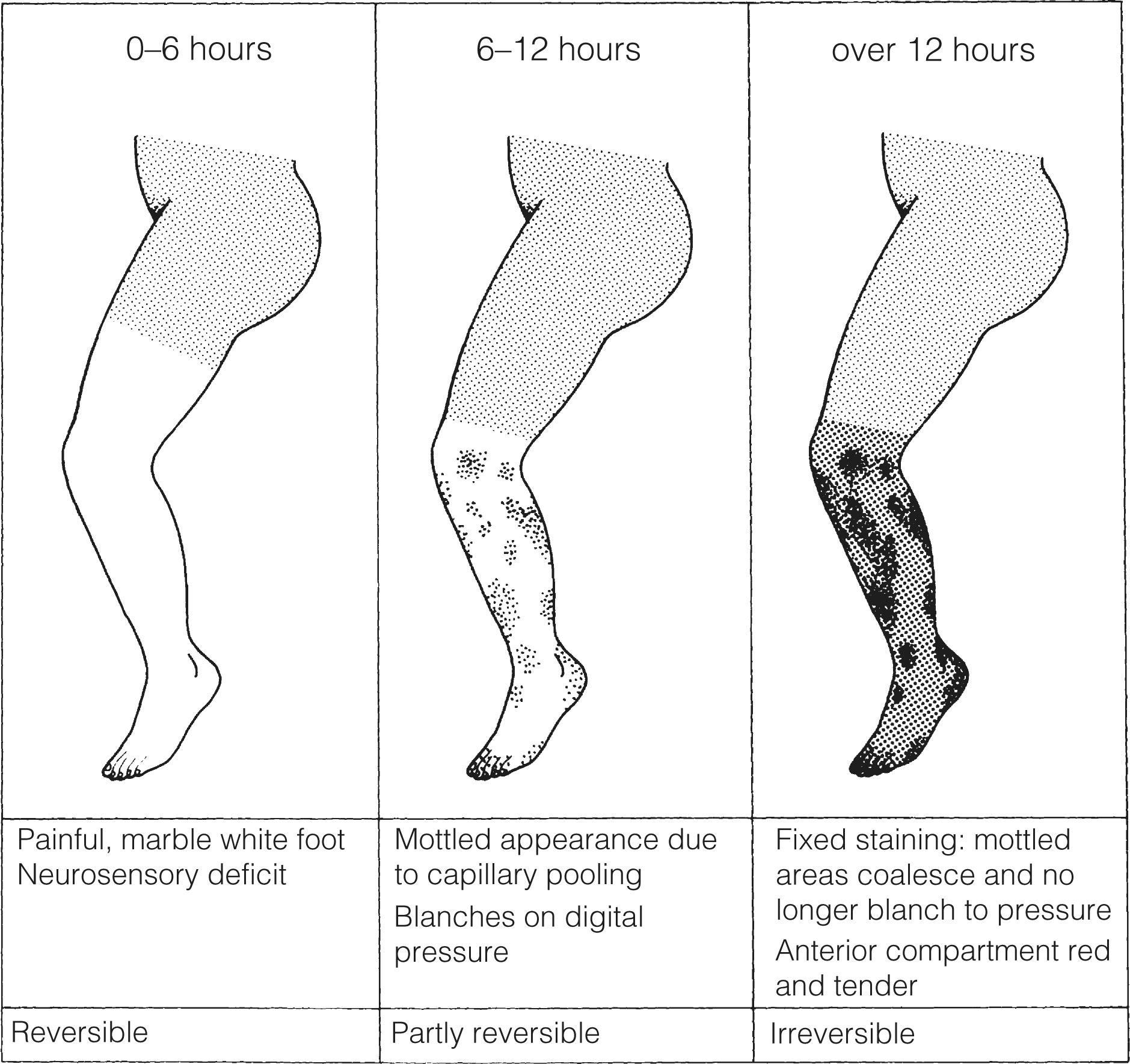
When arterial occlusion occurs as part of a chronic process where collaterals have developed (in patients with pre-existing PAD), typically the leg is less severely ischaemic. Patients with peripheral atherosclerosis deteriorate in a stepwise fashion as thrombosis supervenes on an existing arterial plaque. Patients often report a sudden change in symptoms, which progress over a few days: the foot often has a dusky hue with slow capillary return. Previous claudication or absent pulses in the contralateral foot help make a clinical diagnosis of in situ thrombosis. Palpation of a mass in either popliteal fossa suggests thrombosis of a popliteal aneurysm. Young patients (<50 years), those with an atypical history (e.g., severe back pain associated with aortic dissection) or recent endovascular intervention raise the possibility of non-atherosclerotic/embolic ALI.
Patients presenting with ALI are often in poor general health, which contributes to the observed high mortality rate from associated cardiovascular disease. For some frail elderly patients, ALI may be a terminal event. Decisions about care must take into account the patient’s pre-morbid frailty, fitness, comorbidities, as well as the projected quality of life associated with intervention. Most importantly, careful discussions with the patient (and relatives, particularly if the patient has cognitive impairment) is essential and should be documented clearly in the case notes. All patients with ALI should be urgently assessed by a vascular specialist in a vascular centre that offers the full range of open and endovascular interventions, ideally in a hybrid setting or operating theatre with a C arm.
Dehydration, cardiac failure, hypoxia and pain should all be managed in the standard way. Intravenous unfractionated heparin (5000 IU or 70–100 IU/kg) should be given immediately, followed by systemic heparinisation titrated to a target activated partial thromboplastin time, APTT). The aim of heparinisation is to restrict propagation of thrombus and provide an anti-inflammatory effect, whilst allowing the intrinsic fibrinolytic pathway to break down the thrombus. Whilst there is evidence that heparinisation improves the prognosis, there are no data to suggest that unfractionated heparin is superior to other methods of anticoagulation. In reality, many patients do not achieve a stable target APTT and in many units low-molecular-weight heparins have replaced unfractionated heparin because of their more reliable effect on anticoagulation. Anticoagulation should be delayed if urgent revascularisation is planned and the patient is likely to need epidural anaesthesia. The short half-life of unfractionated heparin is helpful in this situation. There is no role for systemic thrombolysis in the management of ALI.
Venous blood should be taken for full blood count, urea, electrolytes, glucose and coagulation screen. There are no good data to support the use of biochemical markers of ischaemia, however, many units use creatine kinase (CK), which is a marker of skeletal muscle damage as a result of rhabdomyolisis and ischaemia. Whilst not validated for routine use, an elevated CK has been associated with a significantly increased risk of major amputation (56.2% vs. 4.6% if normal CK), however, no biomarker should be used to decide whether to offer revascularisation or primary amputation.
An electrocardiogram (ECG) and chest radiograph may be of value in diagnosing and managing cardiac arrhythmias and heart failure. If a primary thrombotic tendency is suspected, investigation of this should be delayed as some of the diagnostic tests are inaccurate in the presence of fresh thrombus.
Diagnostic imaging is recommended to guide treatment, unless it poses a significant delay to treatment, or if the need for primary amputation is obvious. The recent European Society for Vascular and Endovascular Surgery (ESVS) guidelines on acute limb ischaemia recommend computed tomography angiography (CTA) in the first instance. Duplex ultrasound or contrast-enhanced magnetic resonance angiography (MRA) are alternatives, depending on availability. In practice, MRA is rarely used in the acute setting because of its long acquisition time and often limited immediate availability. Concerns about the use of contrast-enhanced imaging in the context of acute renal failure may be considered a relative problem when facing this life- and limb-threatening condition. Anatomical coverage of a standard lower limb CTA should extend from the renal arteries to the feet, with a second acquisition for the crural arteries if required.
The clinical assessment of the severity of limb ischaemia will help to decide the most appropriate form of therapy ( Fig. 8.5 ).
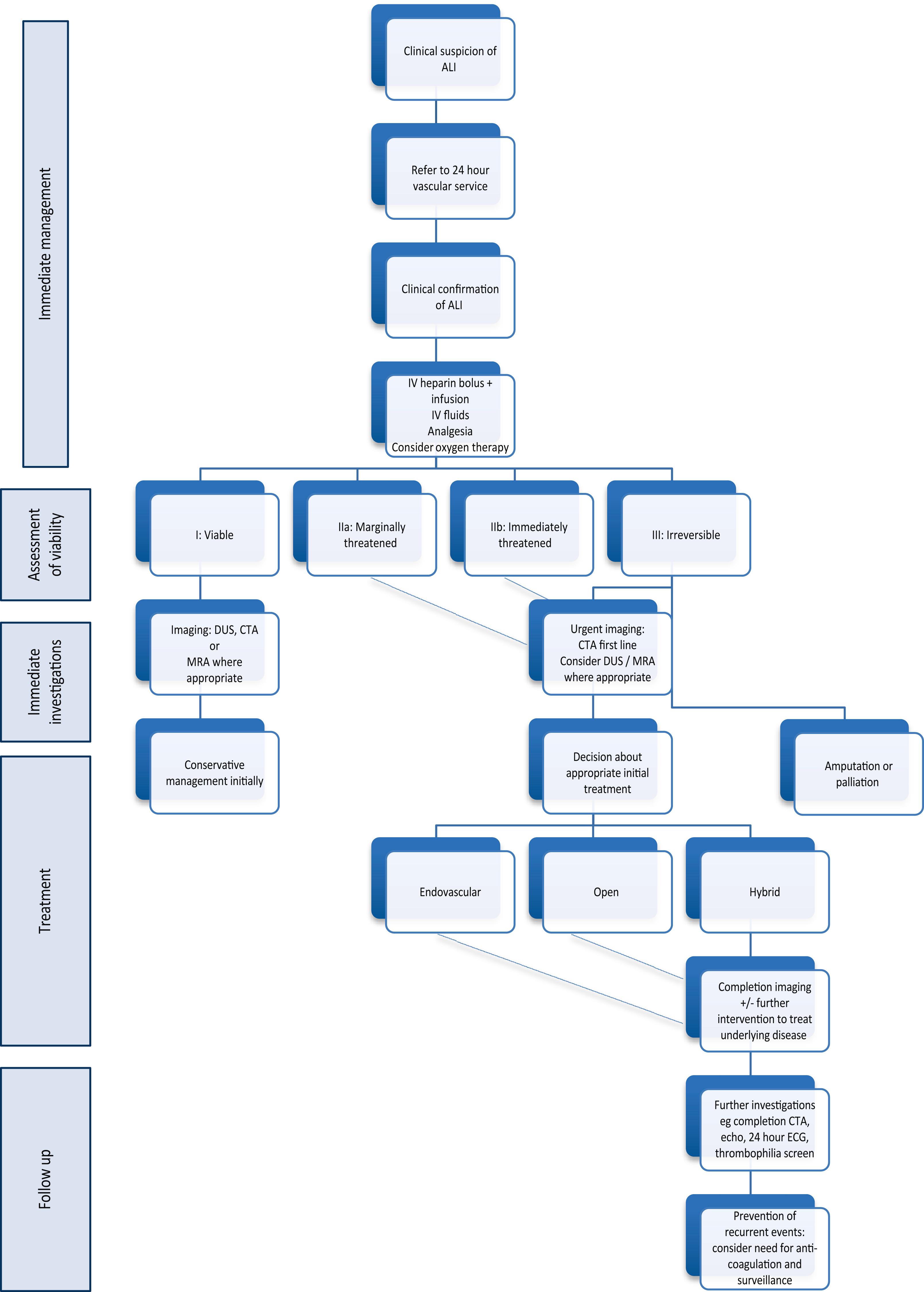
A small number of patients will present in a moribund state or with irreversible leg ischaemia (muscle paralysis, tense swollen fascial compartments, fixed skin staining) and terminal care should be considered. For the irreversibly ischaemic leg, revascularisation is, by definition, inappropriate and may be dangerous, owing to the risk of ischaemia-reperfusion injury and subsequent systemic inflammatory response syndrome. This includes the patient who develops ALI while being treated for another condition, usually as an inpatient on an elderly care ward. Prognosis is particularly dismal in this group. Surviving patients should be resuscitated and stabilised before considering primary amputation.
The acute white leg with sensorimotor deficit requires urgent intervention to prevent limb loss. Although the differentiation between thrombosis and embolus can be difficult, it is in this group of patients that embolism is more likely. An acute white leg with no prior history of claudication, normal contralateral pulses and a probable embolic source, such as atrial fibrillation, would indicate that embolisation is the most likely cause. Urgent revascularisation is indicated in these patients, after resuscitation. Traditionally, patients with Rutherford IIb ischaemia have been managed preferentially with an open approach given the requirement for swift restoration of blood flow, however, this dogma has been challenged recently with improvements in the ability to restore flow rapidly using a variety of advanced endovascular tehcniques. Operative approaches may include surgical thromboembolectomy, bypass, percutaneous catheter-directed thrombolysis (CDT), mechanical thrombectomy, thrombus aspiration (with or without CDT) and hybrid procedures such as thromboendarterectomy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here