Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Salivary gland malignancies are diverse and heterogeneous. Their behavior and resulting clinical management are highly dependent on their histologic type and, often, their grade.
High-grade histologic types include high-grade mucoepidermoid carcinoma, squamous cell carcinoma, undifferentiated carcinoma, high-grade adenocarcinoma not otherwise specified, solid-type (Grade III) adenoid cystic carcinoma, small cell carcinoma, salivary duct carcinoma, and any tumor with so-called high-grade transformation.
When lesions are resectable, treatment is primarily surgical, with or without adjuvant postoperative radiation.
Controversy exists regarding when to treat a node-negative neck; however, in general, histopathologic type, tumor stage, and evidence of perineural or bone invasion can guide management.
Indications for adjuvant postoperative radiation include advanced stage, close or positive margins, high-grade histologic types, perineural invasion, and evidence of local tissue invasion.
Malignant neoplasms of the major and minor salivary glands are rare and represent approximately 3% of all head and neck malignancies. The estimated incidence is only 0.9 per 100,000 in the United States, but the rate increases with age and peaks at ages 65 to 74 years. Less than 5% of all salivary gland tumors occur in the pediatric age group. Although salivary gland tumors in children were believed to be much more likely to be malignant than those in adults, a recent Danish study of 61 pediatric patients with salivary cancers demonstrated malignancy rates comparable to adults.
Of all salivary neoplasms, both benign and malignant, the vast majority occur in the parotid gland, and the fewest occur in the sublingual gland. An interesting inverse relationship exists between the overall incidence of neoplasms by site and the percentage that are malignant ( Table 85.1 ). In a review of 2410 cases of salivary gland tumors, 73% occurred in the parotid, and of those, only 15% were malignant. On the other hand, minor salivary gland tumors constituted only 14% of the total number of cases, but 46% were malignant. Similarly, submandibular gland neoplasms constituted 11% of the cases, and 37% were malignant; sublingual gland neoplasms constituted only 0.3%, and 86% were malignant.
| Site | Absolute Numbers | % Frequency | % Malignant |
|---|---|---|---|
| Parotid | 1756 | 72.9 | 14.7 |
| Submandibular | 257 | 10.7 | 37 |
| Sublingual | 7 | 0.3 | 85.7 |
| Minor glands | 336 | 14 | 46.4 |
| Unknown | 54 | 2.2 | — |
The frequency of the different histologic types of salivary gland malignancy also varies depending on the gland and site. A number of studies have found the most common primary malignancy in the salivary glands to be mucoepidermoid carcinoma. In one of the largest reviews of salivary gland neoplasms (2807 total), Spiro examined 1278 cases of malignant salivary gland tumors and reported that 34% were mucoepidermoid carcinoma ( Table 85.2 ). The next most common type was adenoid cystic carcinoma (AdCC; 22%), followed by adenocarcinoma (18%), a mixture of tumors that has been subdivided, as described below: malignant mixed tumor (13%); acinic cell carcinoma (ACC; 7%); and squamous cell carcinoma (SCC; 4%). When considering the types by anatomic site, mucoepidermoid carcinoma was the most frequent malignancy of the parotid, but AdCC was the most frequent of the submandibular and minor salivary glands. One exception to the latter was malignant minor salivary gland tumors that arise in the nasal cavity and paranasal sinuses, in which case adenocarcinoma was the most common type, as opposed to AdCC.
| Histologic Type | Number | % |
|---|---|---|
| Mucoepidermoid carcinoma | 439 | 34 |
| Adenoid cystic carcinoma | 281 | 22 |
| Adenocarcinoma not otherwise specified | 225 | 18 |
| Malignant mixed tumors (various histologic types, primarily salivary duct carcinoma) | 161 | 13 |
| Acinic cell carcinoma | 84 | 7 |
| Squamous cell carcinoma | 53 | 4 |
| Other | 35 | 3 |
| Total | 1278 |
This chapter discusses the evaluation of patients with these malignancies, histopathology of the more common types, and currently accepted treatment. Both the rarity of these malignancies and the wide variety of histologic types have made their study challenging. In fact, what is known about their clinical behavior and treatment outcomes is based almost entirely on retrospective studies.
The clinical presentation of malignant salivary gland neoplasms can range from that of indolent asymptomatic masses to rapidly growing painful masses with progressive facial nerve paralysis. In Spiro's review of 2807 salivary gland tumors, pain was a symptom in only 10% of malignant cases but was more frequently seen with malignant neoplasms than with benign ones. In general, episodic swelling and pain most often indicates salivary gland obstruction and inflammation, whereas constant pain is more worrisome for malignancy. However, sialadenitis can be secondary to obstruction by a salivary gland neoplasm; thus neoplasm must be considered in the evaluation of any salivary gland swelling with pain. Approximately 10% of parotid gland malignancies are seen initially with associated facial paralysis (sometimes mistaken for Bell palsy), and this portends a poor prognosis.
The parotid glands are the largest of the major salivary glands and are unique in that they are the only salivary glands that contain intraglandular lymph nodes. Both parotid glands can be divided into superficial and deep “lobes” by the plane in which the branches of the facial nerve course. These lobes are primarily defined for surgical treatment purposes, but anatomically they are also distinguished by the fact that the majority of the intraglandular lymph nodes are in the superficial lobe. The deep lobe is medial to the plane of the facial nerve and extends into the parapharyngeal space.
A thorough examination of the parotid gland includes palpation of the gland itself and of the neck, along with assessment of the overlying skin; bimanual palpation of the buccal space, which includes the Stensen duct; examination of the oropharynx and nasopharynx; and a thorough evaluation of facial nerve function and symmetry. The parapharyngeal space can be divided into a prestyloid (or anterolateral) compartment and a poststyloid (or posteromedial) compartment by a fascial layer that extends from the styloid process to the tensor veli palatini. Deep lobe parotid tumors can involve the prestyloid compartment of the parapharyngeal space and can present as a submucosal bulging mass in the oropharynx and/or nasopharynx that distorts the soft palate or obstructs the eustachian tube. If the tumor extends into the poststyloid compartment, cranial nerve (CN) neuropathies may manifest as a decreased gag reflex (CN IX, X), aspiration (CN IX, X), asymmetric palate elevation (CN X), hoarseness (CN X), dysphagia (CN X), weakness of the trapezius muscle (CN XI), or atrophy and/or paresis of the tongue (CN XII). If a parotid tumor extends posteromedially into the infratemporal fossa, trismus may also be associated.
The pair of submandibular glands, located in the submandibular triangle (Level Ib) of the neck and extending to the medial aspect of the mandible, represents the second largest of the major salivary glands. These glands are intimately associated with the lingual nerve, hypoglossal nerve, facial artery and vein, and overlying marginal mandibular branch of the facial nerve. They drain into the floor of the mouth via Wharton's duct.
Bimanual (intraoral and external) palpation of any submandibular gland tumor should be performed to assess the extent of the tumor and determine whether fixation to adjacent structures, such as the mandible or skin, is present. A careful neurologic examination should also be performed to assess nerve involvement. In particular, worrisome signs of malignancy include numbness of the tongue, which suggests lingual nerve involvement, weakness of the tongue, suggestive of hypoglossal nerve involvement, or weakness of the lower lip, which suggests facial nerve involvement. Careful examination of the neck is also important, because 25% to 28% of submandibular malignancies will have metastases to regional lymph nodes.
The pair of sublingual glands is located in the floor of the mouth, one on each side of the frenulum, lateral to the Wharton duct in the submucosal compartment. The drainage of these glands is either directly into the floor of the mouth via the ducts of Rivinus or indirectly via the largest duct (Bartholin duct) emptying into the Wharton ducts. As with submandibular gland tumors, bimanual palpation of the floor of the mouth is important to assess the extent and possible fixation of sublingual gland tumors to the mandible. Because the sublingual gland is intimately associated with the lingual and hypoglossal nerves, a careful neurologic examination is important, as previously stated. Although tumors in this area are usually painless, the vast majority (86%) of sublingual gland tumors are malignant.
Minor salivary gland tissue is plentiful, and estimates suggest that somewhere between 500 and 1000 glands are present along the upper aerodigestive tract. Although they are located in the submucosa throughout the oral cavity, oropharynx, nasal cavities, paranasal sinuses, pharynx, and larynx, the majority of them are located in the oral cavity, and the concentration is the highest in the submucosa of the hard palate. As such, the site most frequently involved with minor salivary gland malignancies is the hard palate. Compared with the major salivary glands, minor salivary glands have minimal capsular tissue, which makes local invasion of tumors into surrounding tissue common. Patients with malignancies of the minor salivary glands most often come to medical attention with a painless submucosal swelling, but fixation of the overlying mucosa and ulceration is often present. Approximately one quarter of patients complain of local pain, and pain or paresthesia/anesthesia is concerning for nerve invasion. Because minor salivary glands are distributed throughout the mucosalized surfaces of the head and neck, malignancies of these glands may manifest in diverse ways, depending on the location. Symptoms may include nasal airway obstruction, sinusitis, eustachian tube dysfunction, or hoarseness. A thorough head and neck evaluation that includes fiberoptic examination and cross-sectional imaging should be performed in all cases.
Magnetic resonance imaging (MRI) is the radiologic modality most often recommended to assess salivary gland neoplasms, if no contraindications to its use are present. Benign and malignant neoplasms of the parotid gland are well visualized on T1-weighted imaging (T1WI), because they are easily distinguished from the fatty parenchyma of the gland, which appears hyperintense. In general, benign epithelial neoplasms, such as pleomorphic adenomas, and low-grade malignancies have low T1- and high T2-weighted signal intensities ( Fig. 85.1A and B ). High-grade carcinomas tend to have low to intermediate signal intensities on both T1WI and T2-weighted imaging (T2WI); this will be discussed later in the chapter. The use of contrast material, such as gadolinium, and fat saturation of T1WI can provide additional information regarding the extent of a salivary gland malignancy (see Fig. 85.1C and D ). This is particularly useful in terms of assessing bone involvement and perineural spread. Bone marrow and cortex appear hypointense on fat-saturated images, and infiltrating tumor tissue appears hyperintense when enhanced with gadolinium. Enlarged foramina at the skull base and the presence of hyperintense enhanced tumor tissue are suggestive of perineural spread.
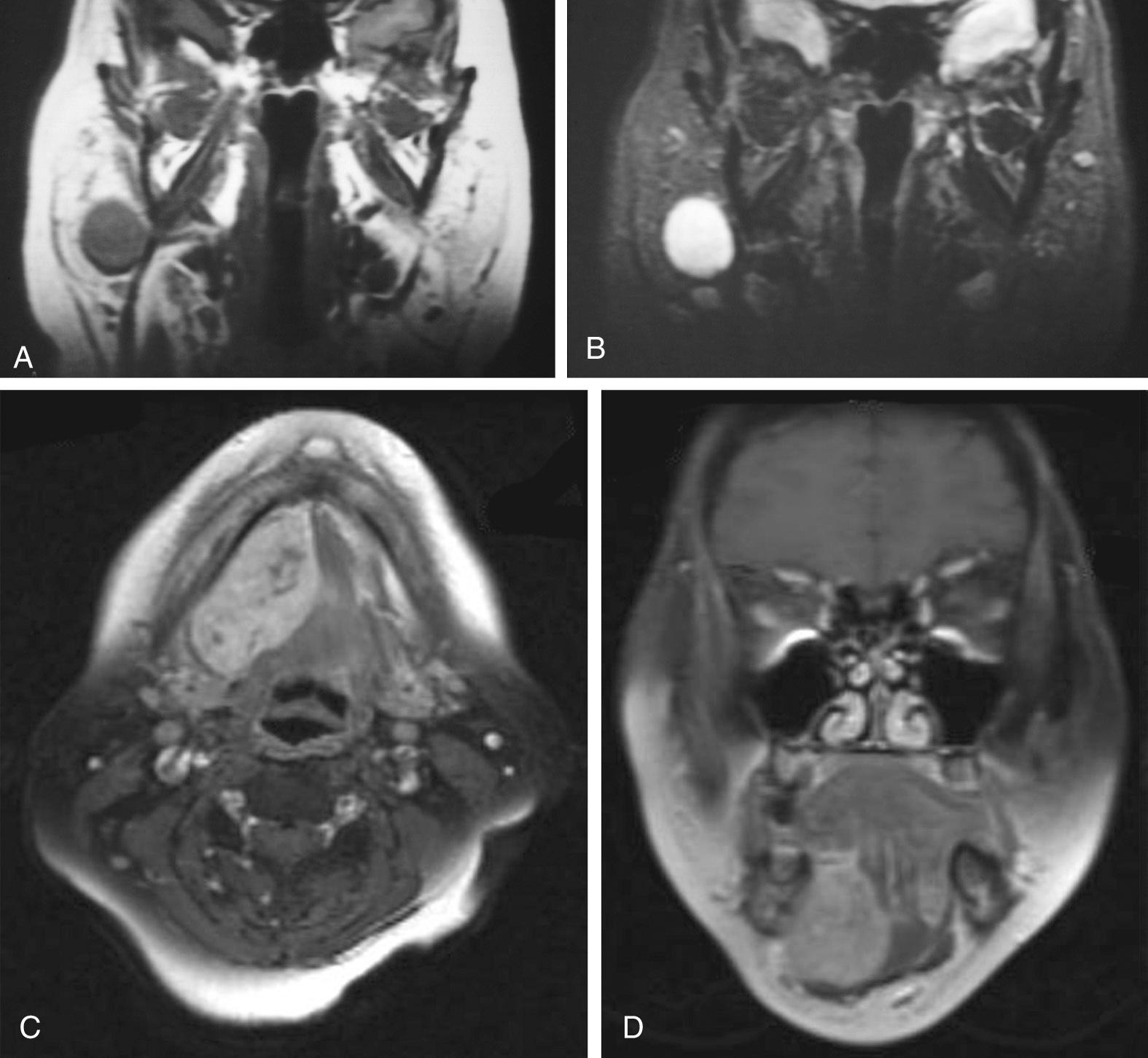
Some have proposed that T2WI is helpful in distinguishing benign from malignant salivary gland neoplasms. Som and Biller examined 35 parotid tumors by MRI and observed that benign tumors and low-grade malignancies had low T1WI and high T2WI signal intensities and clearly defined margins. High-grade malignancies, on the other hand, had low T1- and T2-weighted signal intensities and poorly defined margins. Freling and colleagues, however, did not make the same observations. They examined 116 patients with parotid masses, 30 of which were malignant tumors, and found no correlation between malignancy and signal intensity, heterogeneity, or radiographic margins on MRI. Malignant lesions could be discriminated from benign ones only when infiltration into adjacent structures was apparent. Among the malignant lesions, no correlation was found between tumor grade and MRI features; thus, MRI provides useful information about the extent of the primary, but histopathologic diagnosis is still required to distinguish benign from malignant processes.
Computed tomography (CT) with intravenous contrast is also widely used to evaluate salivary gland masses, primarily because of the speed with which images are acquired. CT is particularly useful for evaluating cortical bone erosion from adjacent tumors. CT is much better than MRI in visualizing small salivary duct calculi, and as such it is particularly helpful in assessing salivary gland masses, if sialolithiasis is considered to be a component of the etiology. Highly cellular tumors can be visualized in the parotid gland because the normal parotid tissue has a high fat content, which results in a lower radiodensity on CT imaging compared with neoplasms. However, in general, MRI is superior to CT for providing soft tissue detail and delineating the extent of salivary gland masses.
Positron emission CT (PET-CT) is increasingly used in pretreatment planning of cytologically confirmed high-grade salivary neoplasms. It is highly effective for the evaluation of distant metastatic disease. Its role is limited in the evaluation of the primary neoplasms because of the background activity from physiologic excretion of 18 F-fluorodeoxyglucose (FDG) by the salivary glands. Despite this interference, a recent meta-analysis demonstrated that the prevalence of incidental focal uptake in the parotid is 1% among PET scans, and most revealed benign neoplasms without a correlation between SUV and malignant histology.
Pretreatment diagnosis of salivary gland neoplasms has been made possible by the use of fine-needle aspiration (FNA) biopsies. This diagnostic tool was established in the mid- to late 1960s and has become a mainstay in the workup of salivary gland neoplasms. It is an extremely safe procedure that is well tolerated by patients and is considered to have no significant risk of tumor seeding to the surrounding tissue.
Although FNA biopsies of salivary neoplasms have proven to be extremely useful for preoperative planning, the interpretation of these biopsies can be difficult and can result in diagnostic ambiguity and inaccuracy. A few entities have classic, pathognomonic features on FNA biopsy. However, the variety of different tumor types and their widely overlapping histology usually necessitate the acquisition of biopsy or resection tissue for definitive diagnosis. Also, and most importantly, many malignant salivary gland tumors can only be diagnosed as such when the growth pattern (infiltration beyond the capsule into soft tissue, perineural invasion) is taken into consideration. In these cases, tumors can only be definitively diagnosed as malignant on surgical specimens that adequately demonstrate the tumor architecture and the tumor's relationship to peripheral tissue. This is particularly true for low-grade malignant neoplasms, such as polymorphous low-grade adenocarcinoma (PLGA), myoepithelial carcinoma, and basal cell adenocarcinoma, which usually have bland-looking tumor cells and lack necrosis or abundant mitoses. Among tumors of the head and neck, FNA biopsies of major salivary gland tumors are considered to have the highest rate of error. By most reports, the sensitivity of FNA biopsies to diagnose a malignant neoplasm is much lower than the specificity. In other words, it is more common to misdiagnose a malignant tumor as benign than the reverse. A 5-year review of data from 6249 participant responses from the College of American Pathologists Interlaboratory Comparison Program in nongynecologic cytology revealed that FNA biopsies had a 68% sensitivity for diagnosing a salivary gland neoplasm as malignant, corresponding to a false-negative rate of 32%. The greatest number of false-negative diagnoses occurred in cases of lymphoma (57%), followed by ACC (49%), low-grade mucoepidermoid carcinoma (43%), and AdCC (33%). In cases of benign neoplasms from the same study, the specificity of FNA biopsies was 91%, corresponding to a false-positive rate of 8%. The greatest false-positive rates were in cases of basal cell adenoma (53%), which were most often diagnosed as AdCC. When diagnosed as malignant, pleomorphic adenomas were most often misinterpreted as AdCC, and Warthin tumors were misdiagnosed as lymphoma. Recent studies have demonstrated improved sensitivity (73%) and specificity (87%) for the diagnosis of salivary malignancy using ultrasound-guided FNA, with 6% false-negative and 10% false-positive rates. To improve FNA diagnosis for salivary gland lesions (and based on the success of the Bethesda criteria for thyroid FNA), a new classification system has been developed termed the “Milan system”. This will become more commonly used across clinical practices and may become standard for salivary gland tumor FNA specimens.
Ultrasound-guided core tissue biopsy has the best diagnostic performance and should be considered as second line to a non-diagnostic FNA. However, there is the risk of facial nerve injury and, further, there have been reports of tumor seeding decades after core biopsy, which makes this option controversial.
Although the utility and ease of FNA biopsies have resulted in a decline in the use of intraoperative frozen sections, several indications for frozen section studies still exist, as reviewed by Seethala and colleagues and by Westra. These include assessment of the extent of tumor spread to local/regional tissues, such as the nerves and lymph nodes, assessment of surgical resection margins, and confirmation or establishment of the diagnosis in cases where the preoperative FNA biopsy was not diagnostic or was equivocal. In more recent years, many surgeons have used frozen sections to clarify tumor typing after somewhat nonspecific diagnoses on FNA and, furthermore, to make an intraoperative assessment of the extent of resection needed for the primary lesion and for information about the need for neck dissection.
Clinical staging of salivary gland carcinomas is important for prognosis and treatment decisions. The tumor/node/metastasis (TNM) staging classification for major salivary gland carcinomas established by the American Joint Committee on Cancer (AJCC) is the classification most commonly used in the United States. Recent AJCC 8th edition updates that became standard in 2018 ( Tables 85.3 and 85.4 ) now formally include gross extraparenchymal extension from primary tumors, which has to be established on clinical and macroscopic grounds (histologic extraparenchymal extension alone is not sufficient evidence). The updates also now formally utilize extranodal extension (ENE) in cervical nodal metastases because of its profound effect on prognosis in head and neck carcinomas, including those primary to the salivary glands. The presence of ENE increases the nodal stage by one compared to the prior 2010 TNM classification. ENE can be defined clinically by invasion into skin and/or muscle, fixation to adjacent structures, or weakness/paralysis of surrounding nerves. Radiographic evidence alone is not sufficient for diagnosis of ENE. The pathologic definition of ENE includes the presence of tumor invading through the capsule into surrounding connective tissue, regardless of stromal reaction. Extent of ENE may be important but is not defined well enough to be differentially utilized in staging. Minor salivary gland carcinomas are staged according to the anatomic site of origin (e.g., oral cavity, sinonasal tract, larynx).
| P rimary T umor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor is ≤2 cm in greatest dimension without extraparenchymal extension a |
| T2 | Tumor is >2 cm but not >4 cm in greatest dimension without extraparenchymal extension a |
| T3 | Tumor is >4 cm in greatest dimension and/or having extraparenchymal extension a |
| T4a | Moderately advanced disease Tumor invades skin, mandible, ear canal, and/or facial nerve |
| T4b | Very advanced disease Tumor invades skull base and/or pterygoid plates and/or encases carotid artery |
| R egional L ymph N odes C linical N ( c N) |
|
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node ≤3 cm in greatest dimension and ENE(−) |
| N2a | Metastasis in a single ipsilateral node >3 cm, not >6 cm in greatest dimension and ENE(−) |
| N2b | Metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension and ENE(−) |
| N2c | Metastases in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension and ENE(−) |
| N3a | Metastasis in a lymph node >6 cm in greatest dimension and ENE(−) |
| N3b | Metastasis in any node(s) with clinically overt ENE(+) |
| R egional L ymph N odes P athologic N ( p N) |
|
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node ≤3 cm in greatest dimension and ENE(−) |
| N2a | Metastasis in a single ipsilateral or contralateral lymph node ≤3 cm and ENE(+); or A single ipsilateral node >3 cm, not >6 cm in greatest dimension and ENE(−) |
| N2b | Metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension and ENE(−) |
| N2c | Metastases in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension and ENE(−) |
| N3a | Metastasis in a lymph node >6 cm in greatest dimension and ENE(−) |
| N3b | Metastasis in a single ipsilateral node >3 cm in greatest dimension and ENE(+); or Multiple ipsilateral, contralateral, or bilateral nodes any and ENE(+) in any node; or Single contralateral node of any size and ENE(+) |
| D istant M etastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis (no pathologic M 0 ; use clinical M to complete stage group for surgically resected patient staging) |
| M1 | Distant metastasis |
Midline nodes are considered ipsilateral nodes.
ENE detected on histopathologic examination is designated as ENEmi (microscopic ENE ≤2 mm) or ENEma (major ENE >2 mm) Both ENEmi and ENEma qualify as ENE(+) for definition of pN. Note: A designation of “U” or “L” may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L)
a Extraparenchymal extension is clinical or macroscopic evidence of invasion of soft tissues. Microscopic evidence alone does not constitute extraparenchymal extension for classification purposes.
| 0 | Tis | N0 | M0 |
|---|---|---|---|
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| III | T3 | N0 | M0 |
| T1 | N1 | M0 | |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| IVA | T4a | N0 | M0 |
| T4a | N1 | M0 | |
| T0 | N2 | M0 | |
| T1 | N2 | M0 | |
| T2 | N2 | M0 | |
| T3 | N2 | M0 | |
| T4a | N2 | M0 | |
| IVB | T4b | Any N | M0 |
| Any T | N3 | M0 | |
| IVC | Any T | Any N | M1 |
Salivary gland malignancies are remarkably diverse and heterogeneous; their behavior and resulting clinical management are highly dependent on their histologic type and frequently on their grade. Therefore, knowledge of the types of tumors and their pathologic classification is critical for the clinician to provide proper treatment. Table 85.5 lists most of the critical pathology-related issues that clinicians need to consider. The following section is intended to provide a succinct but sufficient discussion of the pathology of these tumors, which includes a brief discussion of molecular alterations, an increasingly important aspect of salivary gland pathology.
| Tumor Type | Unique Pathologic Issue(s) |
|---|---|
| Mucoepidermoid carcinoma | Grade |
| Adenoid cystic carcinoma | Grade |
| Carcinoma ex pleomorphic adenoma | Specific histologic type of carcinoma; Grade; extent of invasion |
| Myoepithelial carcinoma | Grade |
| Basal cell adenocarcinoma | Grade |
| Adenocarcinoma not otherwise specified | Grade |
| Acinic cell carcinoma | Grade |
| Small cell carcinoma | Primary vs. metastasis |
| Squamous cell carcinoma | Primary vs. metastasis |
| Lymphoma | De novo vs. secondary; necessity of tissue biopsy after FNA diagnosis; specific histologic type |
| All carcinomas | High-grade transformation (Y/N) |
First, it is important to consider the normal histology of the glands, because most tumors differentiate into the same cell types that are present in the normal gland. Salivary glands contain acini composed of either serous or mucous cells or a mixture of both. Serous cells are rounded or polygonal in shape and characteristically have abundant blue cytoplasmic granules, which are periodic acid–Schiff (PAS) positive. Mucous cells consist almost entirely of lightly basophilic intracytoplasmic mucus. The fluid secreted by the parotid gland is almost exclusively serous, whereas that from the sublingual gland is almost exclusively mucous, and that from the submandibular gland is a mixture of serous and mucous. The striated and interlobular ducts have cuboidal to columnar lining cells with abundant eosinophilic cytoplasm; they form tubular structures within the glands ( Fig. 85.2 ). Both the acini and ducts have supportive myoepithelial cells along their periphery. The parotid glands normally contain, on average, 10 to 20 intraglandular and periglandular lymph nodes, a feature of great importance, because many parotid masses represent metastases to these lymph nodes from primary skin or other cancers of the head and neck. These nodes have an otherwise typical appearance to any other nodal tissue in the body. The submandibular and sublingual glands have no intraglandular lymph nodes.
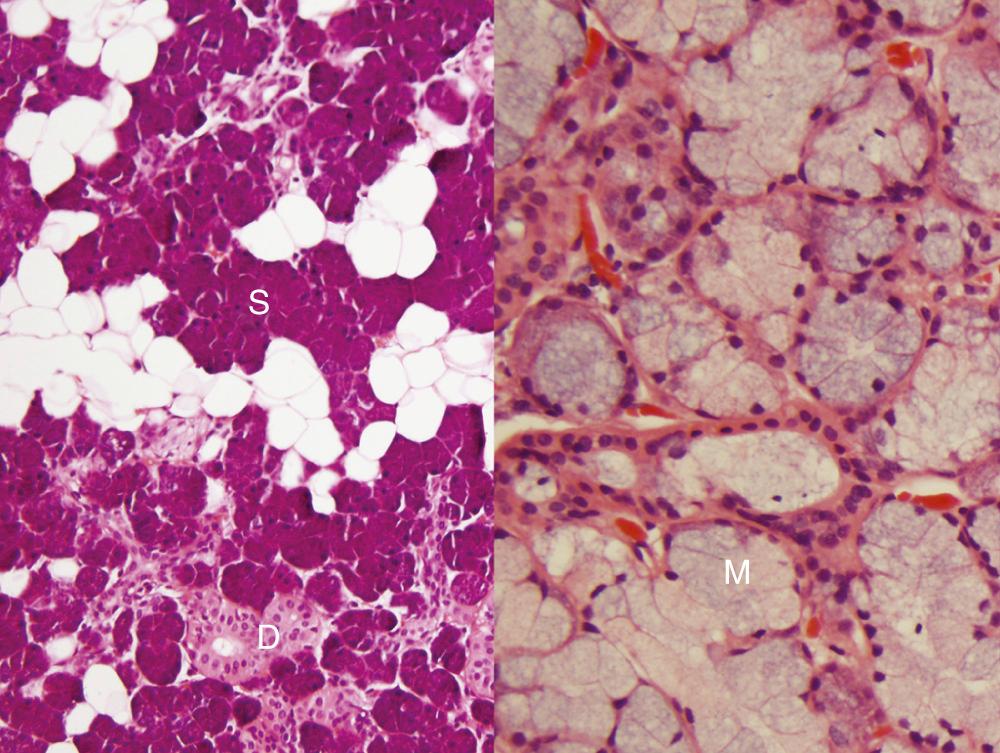
Many neoplasms of the salivary gland can be roughly classified on the basis of the type of normal salivary gland cell toward which they differentiate. Neoplasms can differentiate toward the acinar, ductal, or myoepithelial cells. However, practically speaking, most of them have dual differentiation—specifically, most salivary gland neoplasms have some myoepithelial differentiation. Also, most of the benign neoplasms have a malignant counterpart, such as pleomorphic adenoma and carcinoma ex pleomorphic adenoma, basal cell adenoma and basal cell adenocarcinoma, and myoepithelioma and myoepithelial carcinoma. The number of different malignant epithelial tumors in the World Health Organization (WHO) classification has increased greatly over the past 50 years and now includes 22 entities. The 2017 iteration improved the classification of undifferentiated and neuroendocrine carcinomas, omitted grade from the name of neoplasms such as polymorphous (low-grade) adenocarcinoma, and converted low-grade salivary ductal carcinoma to intraductal carcinoma. Mammary analogue secretory carcinoma is now recognized as just secretory carcinoma. Sialoblastoma is now classified as a “borderline tumor” given its unspecific and uncertain behavior. Metastasizing (or “malignant”) pleomorphic adenoma is now lumped together with its benign counterpart. It is important to note that the incidence of particular histologic types is dependent on the site. For example, PLGA virtually never occurs in the major glands, whereas ACC is extremely uncommon outside the parotid gland.
Given the rarity of salivary gland neoplasms and the complexity of the potential histologic subtypes, second opinion surgical pathology review can be beneficial. In a study of 814 cases of head and neck cancer in which surgical pathology was reviewed at a tertiary center, 97 cases involved salivary gland neoplasms; in this study, the diagnosis was changed on secondary review in 9% of cases. Of these nine cases, the revised pathology had an impact on recommended treatment in 88% and an effect on prognosis in 67%. This highlights the importance of accurate pathologic assessment in salivary gland neoplasms.
Mucoepidermoid carcinoma (MEC) is the most common salivary gland malignancy. The majority of cases occur in the major salivary glands, but MEC can also arise from minor salivary glands in the oral cavity, particularly in the hard palate, and from buccal mucosa, lip, and retromolar trigone. Rarely, they can also arise intraosseously in the mandible and maxilla, but MECs of this location are considered odontogenic in origin and have a less aggressive clinical behavior. Clinically, MECs are slightly more common in women and have a mean age of occurrence at approximately 45 years. They can also occur in children. In fact, they are the most common pediatric salivary gland carcinoma. Patients usually come to medical attention with a painless, slow-growing mass.
Generally, MECs are not distinct. They usually have both solid and cystic components, often with mucinous material within the cysts. This is what sometimes imparts a bluish color to them, which can mimic the appearance of a mucocele in the oral cavity. Microscopically, their hallmark is the presence of three cell types: mucous, squamoid (or epidermoid), and intermediate ( Fig. 85.3A ). The architecture is usually a mixture of cystic (see Fig. 85.3B ) and solid elements, the latter with sheets (see Fig. 85.3C ), nests, or duct-like structures. The mucous cells have abundant, light blue mucin in their cytoplasm, and nuclei are displaced to the periphery. The mucin is usually obvious, but in cases where it is scant, special stains such as PAS, mucicarmine, or Alcian blue can be used to highlight it. The squamoid cells are large with abundant pink cytoplasm, and although they look somewhat squamoid in appearance, they are not truly squamous. True keratinization in MEC is rare, and if present, it should prompt consideration of the tumor as adenosquamous carcinoma. Intermediate cells typically have more modest amounts of pink or clear cytoplasm. The proportion of cell types varies quite a bit among tumors: intermediate cells usually predominate, and mucous cells usually line cystic spaces. Cytologic atypia varies from minimal to quite prominent. Immunohistochemistry (IHC) is of limited utility in the diagnosis.
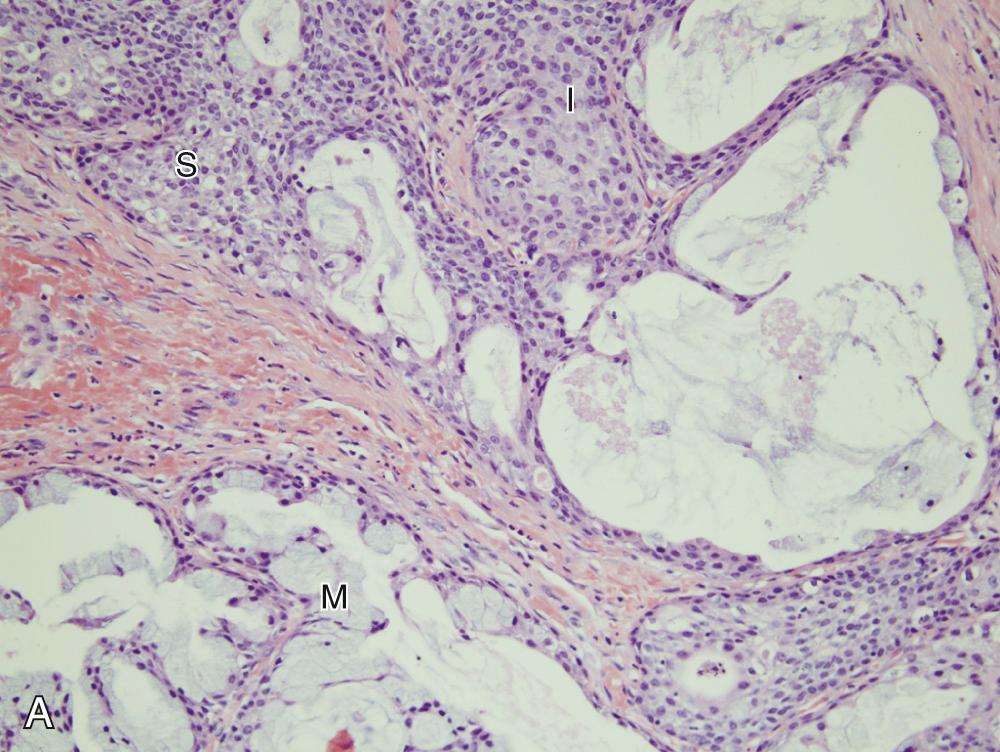
FNA yields a mixture of cell types. At a minimum, both glandular and squamoid components must be present to make the diagnosis. The mucous cells have abundant vacuolated cytoplasm, intermediate cells are relatively round and small with little cytoplasm and nuclei with open chromatin, and squamoid cells have moderate amounts of dense, homogeneous cytoplasm. These typically appear orange on Papanicolaou staining.
The differential diagnosis, particularly in tumors that arise along mucosal sites, includes necrotizing sialometaplasia, an uncommon nonneoplastic lesion of the hard palate with reactive changes in minor salivary glands and, more importantly, adenosquamous carcinoma—an aggressive variant of SCC. Adenosquamous carcinomas are high-grade, have definitive squamous differentiation, usually with keratinization (unlike MEC), and often have surface mucosal squamous dysplasia, a feature that MEC lacks.
Grading of MEC is important and correlates strongly with clinical behavior, although reproducibility and consistency are major issues and no particular system has been universally accepted. Clinical staging is as important as histologic grade, so the two should be considered in tandem. In general, low-grade lesions have a prominent cystic component and abundant well-differentiated mucous cells with little cytologic atypia and low mitotic activity. High-grade lesions are more solid with squamoid and intermediate cells predominating. They also have cytologic atypia, mitotic activity, necrosis, and infiltrative growth. A number of different grading schemes (well-reviewed by Luna and also by Seethala ) have been reported over the years. The most used grading system, originally designed by Auclair and colleagues, uses a three-tiered score based on a number of histologic features ( Table 85.6 ). The original grading system was initially criticized for a tendency to “undergrade” the tumors, when others demonstrated that a significant number of low-grade MECs developed progressive disease. The later modification by Brandwein and colleagues (see Table 85.6 ) refined the grading system such that none of the tumors that were classified as low-grade in their study went on to progressive disease. Low-grade MEC, as strictly defined by their criteria, rarely ever metastasizes or results in death of the patient. Intermediate-grade MEC is the most challenging for the clinician, because it has the least agreement among pathologists and also has a variable clinical behavior, which may depend greatly on the grading system used. Therapeutic decisions in such tumors often rely on other clinicopathologic features.
| Parameter (Auclair) | Point Value |
|---|---|
| Cystic component <20% | +2 |
| Neural invasion | +2 |
| ≥4 mitoses/10 hpf | +3 |
| Necrosis | +3 |
| Anaplasia | +4 |
| Grade | Point Score |
| Low (1) | 0–4 |
| Intermediate (2) | 5–6 |
| High (3) | ≥7 |
| Parameter (Brandwein) | Point Value |
| Cystic component <25% | +2 |
| Tumor front invades in small nests and islands | +2 |
| Pronounced nuclear atypia | +2 |
| Lymphatic and/or vascular invasion | +3 |
| Neural invasion | +3 |
| Necrosis | +3 |
| ≥4 mitoses/10 hpf | +3 |
| Bony invasion | +3 |
| Grade | Point Score |
| Low (1) | 0 |
| Intermediate (2) | 2–3 |
| High (3) | ≥4 |
In addition to grade, location of the primary tumor is potentially important in predicting clinical behavior. Several studies have shown that low-grade MECs of the submandibular gland recur and metastasize more frequently than those of the parotid or minor salivary glands. Whether this represents truly different biology, it merits the aggressive and thorough resection of any submandibular gland primary malignancy, especially for known MEC. Other prognostic markers are certainly needed. The consistent finding of translocation t(11;19)(q21;p13) seen in greater than half MECs, which results in a fusion of the CRTC1 and MAML2 genes, can be used for confirmation of the diagnosis. This is useful primarily in low- or intermediate-grade tumors since most high-grade MEC are negative. This translocation is associated with a better prognosis but not clearly independent of tumor grade.
AdCC is one of the more common and certainly more recognizable salivary gland tumors, notorious for its infiltrative growth and a slow, progressive behavior with recurrences and spread over a protracted course of many years. These tumors essentially occur with an even distribution across all salivary gland sites, although the total number of minor salivary gland cases outnumber those of major salivary glands, when all sites are considered together. These malignancies occur with an equal incidence in men and women over a wide age range with the peak incidence between 50 and 60 years of age.
Generally speaking, AdCCs are solid, light tan, firm, and well-circumscribed but unencapsulated tumors. Microscopically, three growth patterns have been described: tubular, cribriform, and solid. The tubular pattern consists of small tubules sitting in a pink, hyalinized, and hypocellular stroma ( Fig. 85.4A ). The solid pattern has only rounded lobules of tumor cells with few, or no, gland-like structures and without a defined architecture (see Fig. 85.4B ). The classic and most easily recognized pattern is cribriform, similar to Swiss cheese. Nests of cells are arranged around gland-like spaces that consist of PAS-positive blue or pink material (see Fig. 85.4C ). The central spaces look like glandular lumina but are actually an extracellular matrix that contains reduplicated basement membrane (or ground substance) material produced by the tumor cells, rather than true epithelial mucin. The cells in AdCC are markedly basaloid in appearance, with little cytoplasm and round-to-oval nuclei that are dark and hyperchromatic without nucleoli (see Fig. 85.4A ). They are quite uniform in size and show little mitotic activity, except for the solid type, in which mitotic activity can range from scant to extremely prominent. A rim of inconspicuous myoepithelial cells with clear cytoplasm is also usually present, particularly in the tubular pattern. Scattered, inconspicuous true ducts are present as well.
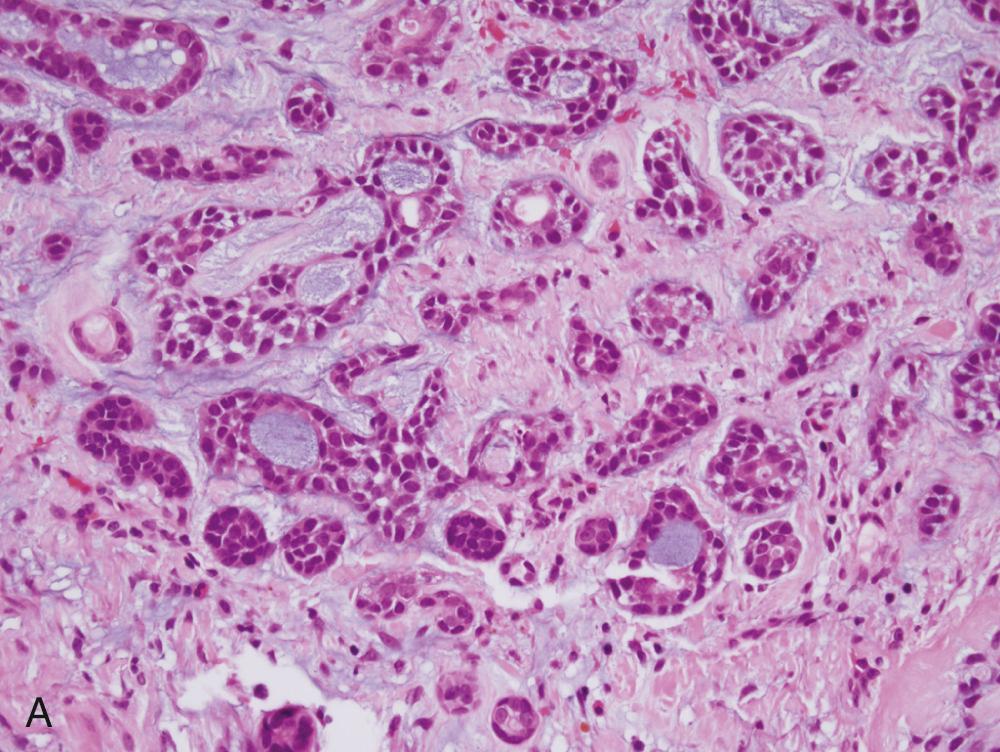
FNA frequently yields characteristic results in AdCC. Aspirates have tumor cells with scant cytoplasm and round, regular nuclei in sheets and clusters. These features by themselves are not characteristic and actually are so bland that they might suggest a benign lesion. However, the finding of well-defined, round “cylinders” and/or spheres of acellular stroma accompanying these cells is typical, but not 100% specific, for AdCC. The cells often “cling” to these rounded structures (see Fig. 85.4D ).
Histologic grading has yielded conflicting results in the literature for predicting prognosis. Clinical stage, on the other hand, holds a great deal of prognostic information and, as such, should be considered as much or more than grade in clinical management. Also, as with MEC, AdCC of the submandibular gland has a much more aggressive clinical course than AdCC of other sites. The most accurate prognostic information has been garnered when tumors are not only graded by the predominant pattern—tubular, cribriform, or solid—but are more specifically categorized as Grade I, tubular with or without some cribriform areas and without any solid areas; Grade II, cribriform with minimal tubular areas and with less than 30% solid areas; and Grade III, any mixture of patterns but with greater than 30% solid areas. A frequent histologic feature of AdCC is perineural invasion, which is identified in approximately 70% to 75% of cases. Although somewhat inconsistent in the literature, many have correlated this finding with a worse prognosis, particularly when it involves a major nerve trunk. It is also well accepted that this perineural invasive tumor is probably the source of recurrent tumor, even after seemingly complete surgical resection of the primary tumors.
Another distinguishing feature of AdCC is that metastases tend to be distant and most often to the lungs. Although the exact incidence is somewhat controversial, AdCC only uncommonly metastasizes to regional lymph nodes and these are mostly with solid (Grade III) tumors. Distant metastases of AdCC can remain indolent for many years and, as such, should not necessarily preclude surgical resection of the primary tumor. It is important to assess the pace of the disease progression. Distant metastasis to bone, however, is often associated with a particularly poor outcome, with a 5-year survival of about 30%.
The diagnosis of AdCC is generally straightforward, from routine hematoxylin-eosin examination. However, it can be difficult on small biopsies, because the pathologist may not be able to fully appreciate the architecture and infiltrative growth. The differential diagnosis includes other salivary gland neoplasms, such as polymorphous adenocarcinoma (PAC), epithelial myoepithelial carcinoma, basaloid SCC, or high-grade neuroendocrine carcinoma. Although beyond the scope of this text, IHC is not usually necessary but can occasionally be helpful in differentiating among these tumor types. AdCC has been found to have a relatively consistent molecular translocation, t(6;9), which involves the MYB and NFIB genes. The literature suggests that this rearrangement is present in as many as two-thirds of cases. Although not prognostic or useful for routine clinical practice, it is occasionally useful diagnostically and may have treatment or management significance in the future.
PAC, formerly known as PLGA, is a unique, almost exclusively low-grade neoplasm first recognized as a distinct entity in the mid-1980s. PACs arise, with only rare exceptions, from the minor salivary glands. In most series, they are the second most common minor salivary gland carcinoma. The most common site is the palate, particularly at the junction of the hard and soft palates. Other sites include the upper lip, buccal mucosa, and base of tongue. Some recent reports suggest these extrapalatal sites to be higher risk for recurrence. PAC arising in major salivary glands is exceedingly rare. PAC is twice as common in women and tends to appear in the fourth to sixth decades as a slow-growing mass that may have been present for years. Such masses are often asymptomatic. On gross examination, they are circumscribed, unencapsulated, pale yellow or tan masses that range from 1 to 3 cm. Microscopically, the architectural features are quite variable, as the name would suggest. They are well-circumscribed but unencapsulated and may appear as solid nests in lobules, cribriform gland-like structures, or duct-like arrangements. A common appearance within the tumor is concentric whorling of the nests around each other in a single-file arrangement. This has been termed the “eye of the storm” pattern ( Fig. 85.5A ). Stromal hyalinization with a slate-gray coloration is also characteristic. Tumor cells are quite regular with moderate eosinophilic cytoplasm and characteristic round to oval, extremely regular nuclei with open chromatin (see Fig. 85.5B ). Little mitotic activity is apparent, and no necrosis is present. The periphery of the tumor shows infiltrative growth, and most cases show perineural invasion (see Fig. 85.5C ).
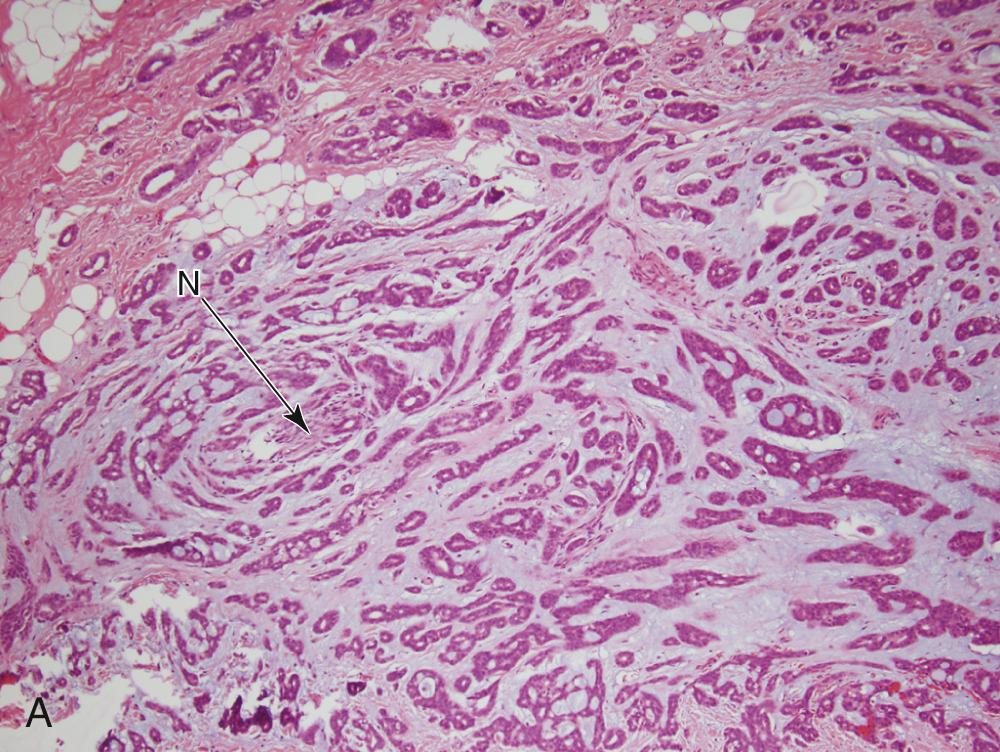
The differential diagnosis includes pleomorphic adenoma and AdCC. The myxochondroid areas of pleomorphic adenoma are not seen in PAC, and although minor salivary gland pleomorphic adenomas are unencapsulated, they will not show the infiltrative growth pattern of PAC and will not have perineural invasion. One particular immunostain that may be useful in difficult cases is worth mentioning here. Curiously, it has been recognized that almost all pleomorphic adenomas will be positive for glial fibrillary acidic protein, whereas almost all PAC are negative. AdCC is important to differentiate from PAC. This is done primarily by cytologic features, because AdCCs have basaloid cells with dark chromatin and little cytoplasm, whereas PAC cells have moderate eosinophilic cytoplasm and nuclei with open chromatin. Dual p40 and p63 positivity in AdCC versus p40-negative, p63-positive status in PAC has also been reported to be diagnostically useful.
PAC is a very low-grade malignancy, and histologic grading is not applicable. Conservative resection is the treatment of choice. Tumor recurs locally in 10% to 15% of patients. Because lymph node metastases are distinctly uncommon (literature rates of ∼10% or less), neck dissection is only recommended with significant clinical adenopathy or needle-proven metastasis. Distant metastases are equally as uncommon, and patients have an excellent long-term prognosis. In fact, few patients have been documented to die of this tumor and only after prolonged periods. As with all salivary gland carcinomas, rare high-grade transformation can occur with the development of an extremely high-grade carcinoma in a background of otherwise typical PAC. These patients have poor prognosis.
ACC, as the name implies, is a tumor with cells that show differentiation toward cells of the normal salivary gland acini (see Fig. 85.2 ). ACC is uncommon and comprises 1% to 3% of all tumors and approximately 10% of all malignant tumors. More than 90% occur in the parotid and the rest are scattered among minor salivary gland sites and, rarely, the submandibular gland. Occurring over a wide age range, from children to the elderly, tumors are relatively evenly distributed in the second through seventh decades with a peak in the third decade. Of note, these tumors are the second most common childhood salivary gland malignancy. They come to medical attention as a slowly growing mass, which is only occasionally painful and is rarely associated with facial palsy.
In general, ACC is found as a single, usually circumscribed, rubbery, solid mass; up to one-third show cystic degeneration. Microscopically, tumors are highly variable, a feature that has traditionally incited angst in the diagnosing pathologist. The four principal histologic patterns are (1) solid/lobular, (2) microcystic, (3) papillary-cystic, and (4) follicular. Small tumors can be easily missed, because the acinar cells are so well differentiated that they blend into the surrounding normal gland. Two features are classic, however. The first is the characteristic acinic cell, which has blue cytoplasm with abundant serous-type granules and a small, round, centrally placed nucleus ( Fig. 85.6A ). PAS stains will be strongly positive in these cells. A number of other cell types are seen, including pink, clear, and vacuolated cells, such that most tumors are a mixture of different cell types (see Fig. 85.6A ). The second classic feature is a dense lymphoid infiltrate with germinal centers (see Fig. 85.6B ). The periphery of the tumors may or may not be infiltrative but is often “pushing” in nature (see Fig. 85.6C ). Although it could be assumed that lack of an infiltrative border might signal a benign lesion, ACC has no benign equivalent (i.e., there is no acinic cell adenoma). “High-grade transformation” has been described in as many as 20% of ACCs and consists of tumors with areas of classic, “conventional” ACC and an intermixed undifferentiated carcinoma in sheets with large pleomorphic cells, brisk mitotic activity (<2 per 10 hpf), and often, necrosis.
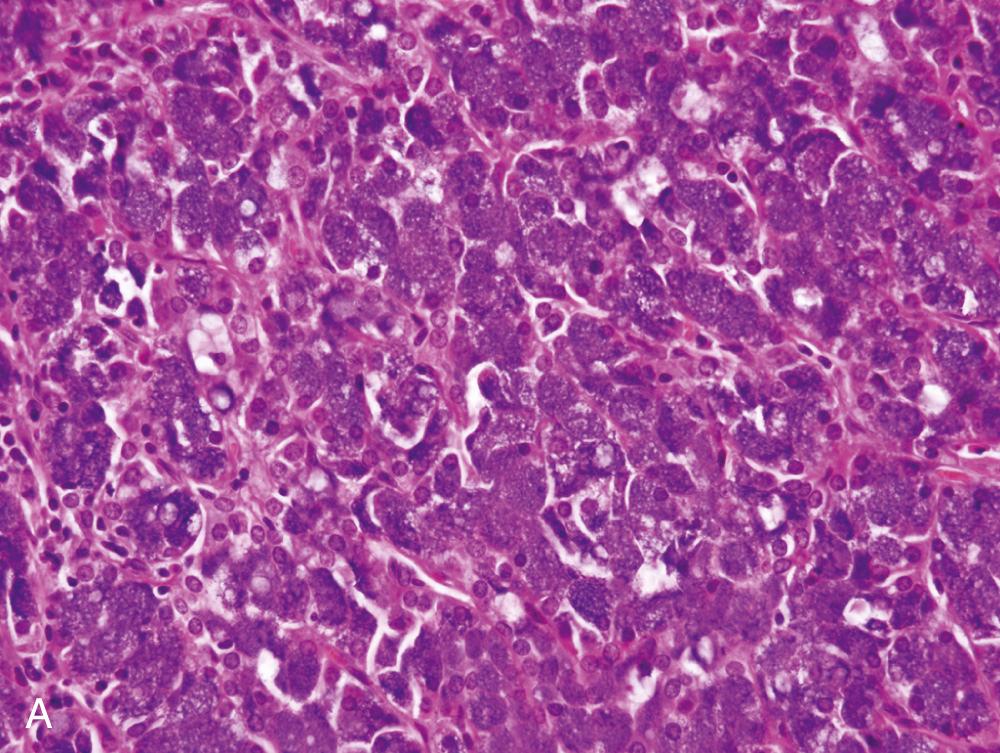
The differential diagnosis includes normal parotid gland. However, ACC will not have the exquisitely lobular architecture, intralesional fatty tissue, or the presence of ducts that normal salivary gland tissue will always have. For the pink and clear cell types, tumors such as oncocytic carcinoma, secretory carcinoma, and clear cell carcinoma not otherwise specified (NOS) must be considered, and the papillary variant of ACC must be differentiated from cystadenocarcinoma. The finding of focal cells with blue, serous-type cytoplasmic granules will confirm the diagnosis of ACC. IHC has relatively limited utility but is very helpful to distinguish ACC from secretory carcinoma. DOG1 is a newer marker of acinic cells and intercalated duct epithelium and shows a classic membrane pattern in cells of ACC. Secretory carcinoma is positive for S-100 and mammaglobin, whereas ACC is negative or patchy for these markers.
FNA biopsy of ACC is difficult because of its frequent resemblance to normal salivary gland tissue. The diagnosis rests on finding a cellular specimen with sheets and clusters of large acinar cells with abundant granular cytoplasm and central round, regular nuclei. There must be a lack of normal ductal epithelial cells and adipose tissue (both of these latter findings are from normal salivary gland tissue), but a prominent background component of lymphocytes is often present as they are recruited by the tumor cells in most cases of ACC.
Surgical resection with negative margins is the most important therapy; still, tumors will recur in approximately one-third of cases. Although classically regarded as low-grade malignancies, overall, 10% to 15% of these tumors will metastasize locally to regional lymph nodes or distantly to the lung and bones. ACCs are also notorious for the timing of recurrence, as they spread years beyond the primary presentation and have a protracted clinical course. Survival curves do not flatten out until after a decade. Survival is approximately 80% at 5 years and 70% at 10 years. Grading of these tumors has been classically thought of as correlating poorly with behavior; however, some more recent studies that have divided tumors into low- and high-grade types based on mitotic activity and necrosis have shown that the majority of high-grade tumors (also termed “with high-grade transformation”) recur and metastasize, whereas 10% or less of the bland-appearing low-grade tumors recur.
Malignant mixed tumor is the broad term used to encompass three different salivary gland malignancies: (1) carcinoma ex pleomorphic adenoma; (2) true malignant mixed tumor, or carcinosarcoma; and (3) metastasizing pleomorphic adenoma (MPA). As a group, these account for approximately 3% to 5% of all salivary gland malignancies, and carcinoma ex pleomorphic adenoma is by far the most common of these.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here