Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The eyelids are affected by skin cancer and are often overlooked when considering suspicious skin lesions.
Eyelid skin cancer treatment must remove the offending lesion while preserving as much normal tissue as possible.
Like all skin cancers, basal cell carcinoma is the most common type to affect the eyelids.
The majority of eyelid skin cancers are the result of cumulative ultraviolet (UV) exposure; thus patients must be educated on the importance of protecting the eyelids and eyes.
Eyelid skin cancers are usually treated with Mohs micrographic surgery or excision under frozen section control, followed by eyelid reconstruction, to ensure the highest rates of eradication and proper eyelid function.
Untreated eyelid skin cancers may extend posteriorly to the orbit, thus endangering the globe as complete eradication involves orbital exenteration.
Medial canthal eyelid skin cancers are more prone to deep invasion given their proximity to nerves and vessels along the embryologic fusion lines.
Carcinoma of the skin is the most common malignancy in the United States, accounting for half of all cancers. Each year in the United States, nearly 5 million people are treated for skin cancer. Forty percent to 50% of Americans who live to age 65 years will be diagnosed with at least one skin cancer during their lifetime. , Approximately 5%–9% of all cutaneous cancers arise in the eyelids. The most common periocular malignancies are basal cell carcinoma (BCC), squamous cell carcinoma (SCC), sebaceous cell carcinoma, and malignant melanoma. Kaposi sarcoma is not as common as in previous periods when human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) was not treated as optimally as in the present day. Systemic diseases such as basal cell nevus syndrome, xeroderma pigmentosum (XP), and Muir-Torre syndrome are associated with periocular cutaneous tumors. The goals in the management of malignant eyelid lesions are to establish early accurate diagnosis, to achieve a permanent cure by total eradication of the tumor, and to preserve or restore eyelid function and cosmesis.
BCC, the most common skin cancer, accounts for 90% of all eyelid malignancies. , The relative frequency of BCC to SCC of the eyelid has been estimated to be as high as 40:1. The tumor primarily involves the lower eyelid (50%–66%) and the medial canthus (25%–30%).
Actinic damage is an important predisposing factor in the genesis of BCC and other epithelial skin tumors. The ultraviolet (UV) rays most responsible for cutaneous carcinogenesis are 290–320 nm (UVB) in wavelength. Individuals with a fair complexion are particularly susceptible to the effects of UV radiation. The majority of patients with BCC are between 50 and 80 years of age; 5%–15% are between the ages of 20 and 40 years. Overall, increased UV exposure during childhood and adolescence seems to be a critical risk factor for developing tumors in adulthood. In addition, positive family history incurs an odds ratio of 2:2 for development of disease. ,
Among young adults with BCC, two distinct groups can be identified. Most young patients tend to be of light complexion and eye coloring without a family history of BCC. A second group possesses characteristics of the basal cell nevus syndrome. The appearance of even a single isolated BCC in a young adolescent should suggest the possibility that the lesion may be a forme fruste of basal cell nevus syndrome. BCCs occurring in younger persons and those with immunodeficiency syndromes , are usually more aggressive. Patients who have been treated for BCC show a higher risk for developing a subsequent lesion.
Martin et al. noted that approximately 10% of patients with a history of radiation therapy to the head developed cutaneous neoplasm within the treatment field. The median interval between exposure and development of carcinoma was 21 years. Histologically, two-thirds of these radiation-induced skin cancers were BCC and one-third were SCC.
BCC is a tumor with varied clinical and histologic manifestations and develops from the basal layer and epidermal appendages; the malignant character depends on the destructive growth of the primary tumor, rather than on metastasis. The most common clinical presentations include the nodular, noduloulcerative, pigmented, cystic, morpheaform or sclerosing, plaque, and superficial varieties.
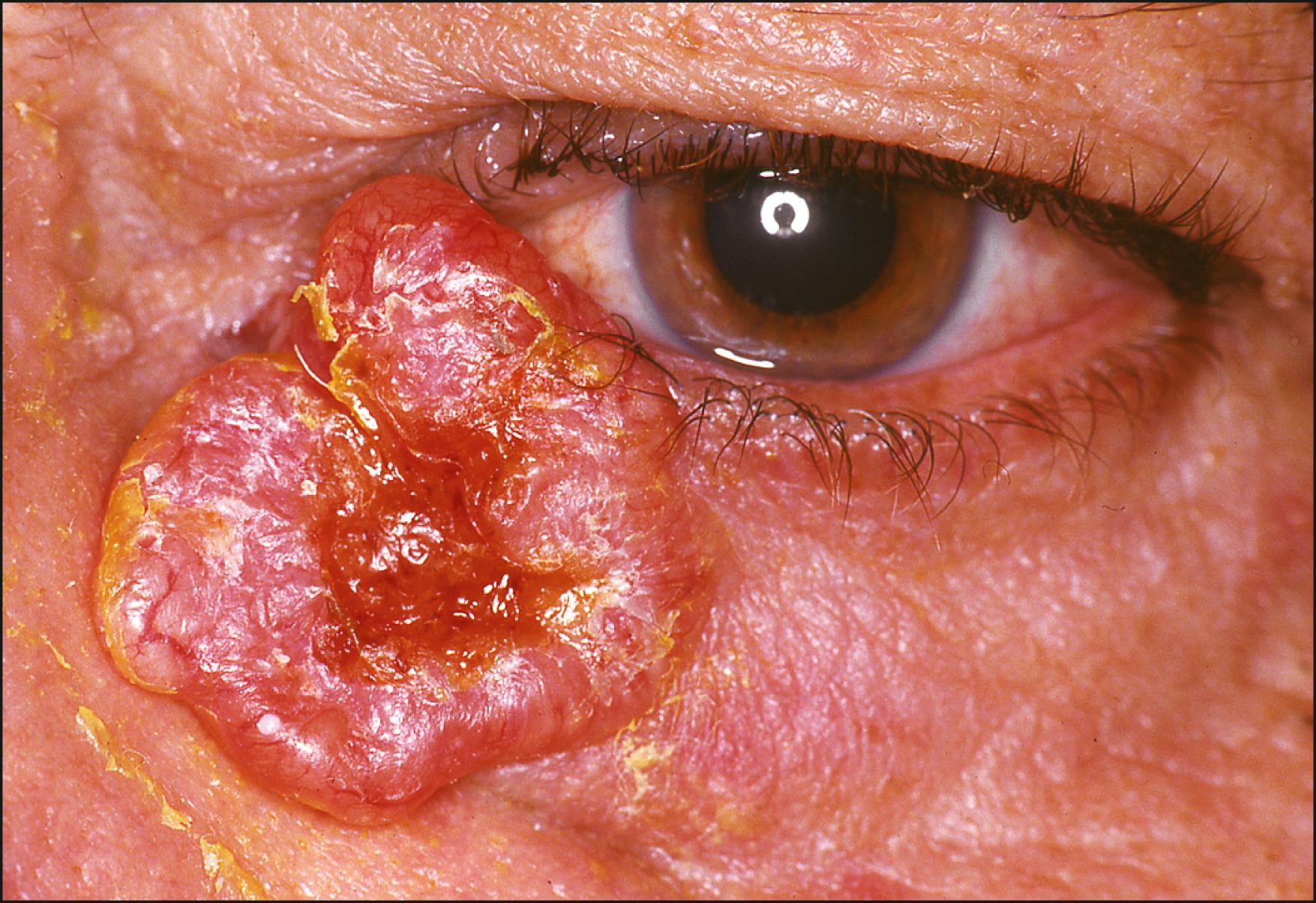
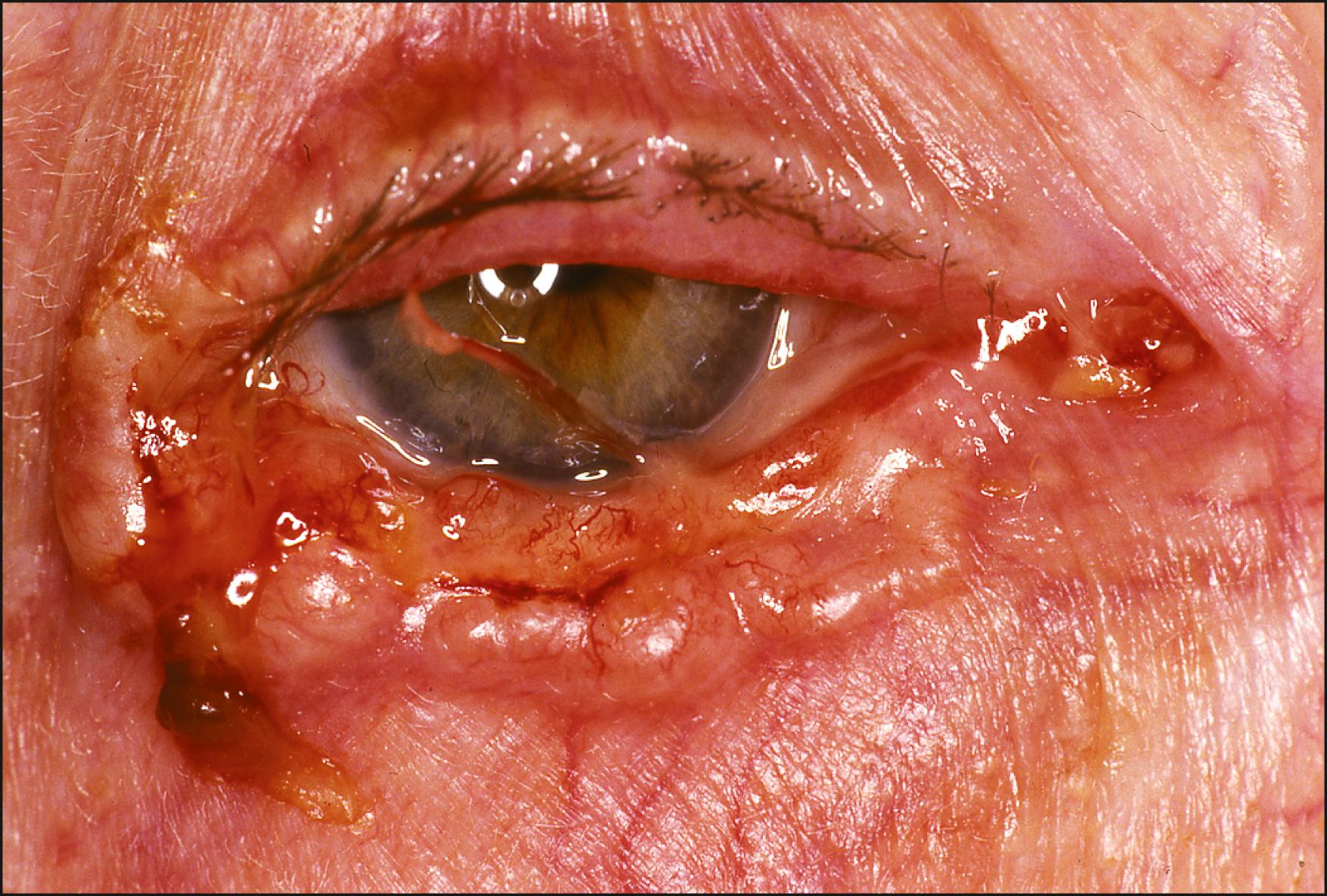
The typical nodular type appears as a firm, pearly, dome-shaped nodule, often displaying multiple telangiectatic vessels ( Fig. 28.1 ). A hyperkeratotic crust, typical of benign and malignant squamous lesions, is generally not seen with BCCs. Some lesions may bleed without pain, whereas others heal spontaneously to form scar tissue as they enlarge. With increasing radial growth, the interior of the tumor may outgrow its blood supply, leading to central ulceration. Eventually, this may appear as a slowly enlarging ulcer, forming the common noduloulcerative variant with characteristic rolled and indurated borders (rodent ulcer) ( Fig. 28.2 ).
Histologically, BCC is composed of solid lobules of small, regularly shaped cells with basophilic and scanty cytoplasm. The most distinctive histopathologic feature of this tumor is the peripheral palisading or “picket-fence” formation of peripheral nuclei at the edge of the mass.
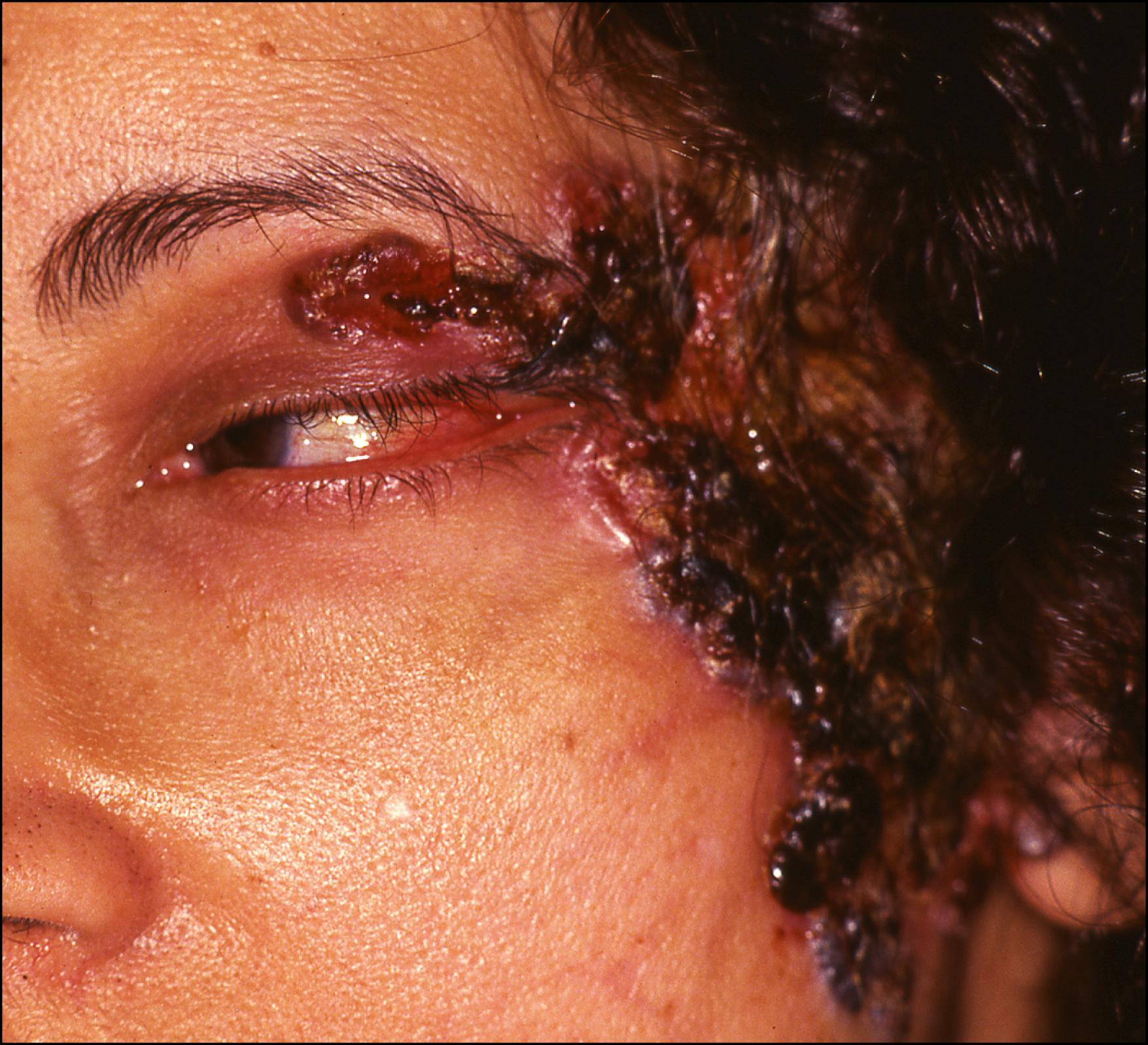
A variant of the noduloulcerative type, characterized by intermingled crusting, ulceration, erythema, and scarring, is referred to as field-fire BCC. It spreads peripherally and may become large ( Fig. 28.3 ). Clinically, it is extremely difficult to determine the margins of this variant. These lesions require extensive surgical extirpation and reconstruction.
Pigmented BCCs are rare, accounting for less than 10% of BCCs. They are similar to the nodular type with respect to age, sex, location, duration, and rate of recurrence. Pigmented BCC is seen more often in dark-complexioned individuals, with a spectrum of tumor colors ranging from light brown to black. Melanin pigmentation may occur in all types of BCCs with the possible exception of the morpheaform type. These lesions are sometimes misdiagnosed as pigmented nevi or malignant melanomas. The pigmentation is caused by benign melanocytes containing melanin granules that proliferate along with the tumor basal cells.
Cystic BCC may develop with mucin accumulation or degenerative necrosis within solid lobules of proliferating basaloid cells. No particular biologic import is associated with the cystic variety, except that they may be difficult to differentiate from an innocuous, benign epithelial inclusion cyst or apocrine hidrocystoma.
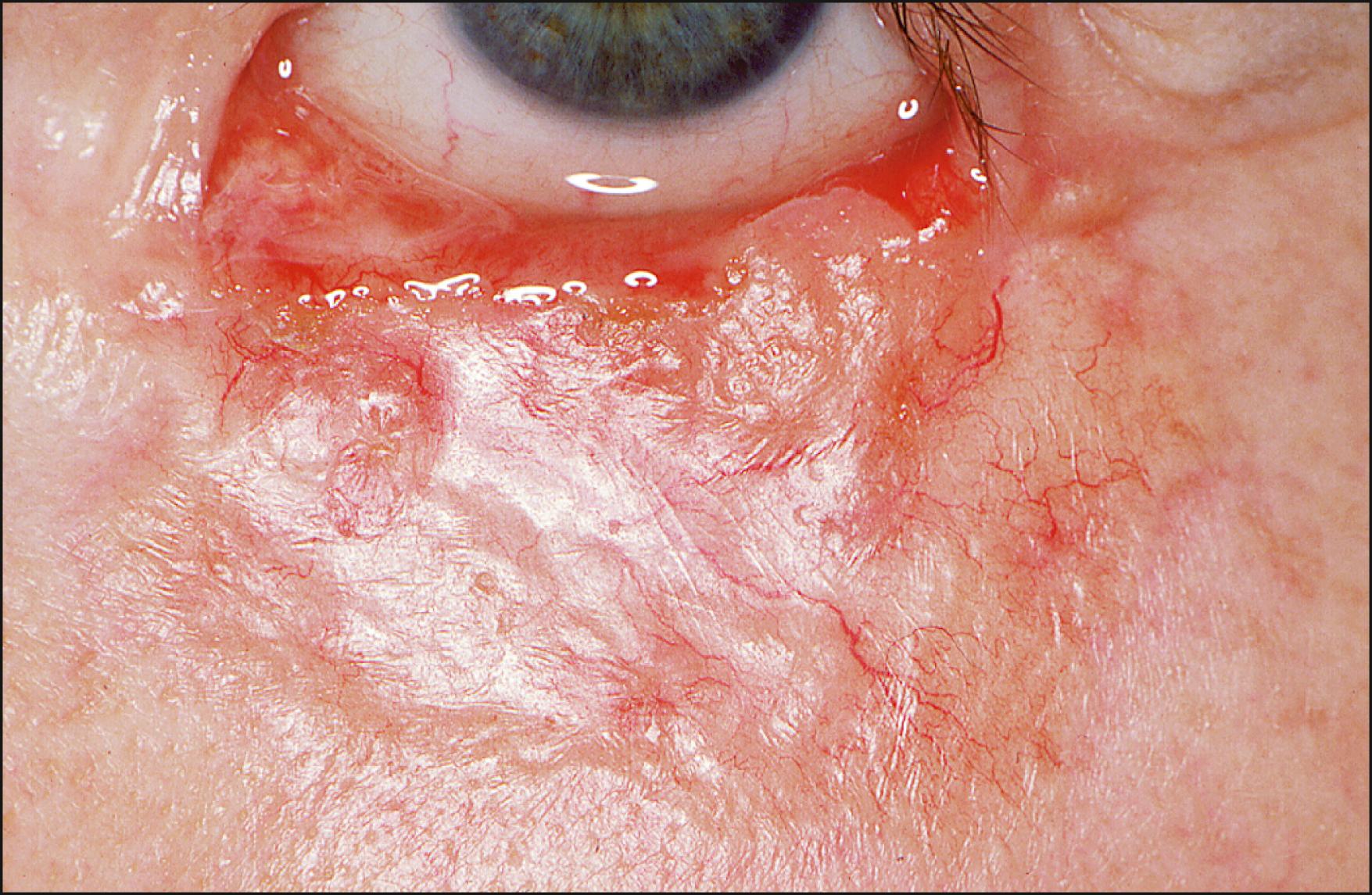
Morpheaform or sclerosing type appears as a flat or slightly depressed, waxy, poorly demarcated, indurated plaque whose pale color has been likened to old ivory ( Fig. 28.4 ). The overlying epidermis remains unaltered for a long time. Ulceration, rolled edges, and crusting are conspicuously absent, but telangiectasias are prominent. If situated along the eyelid margin, the tumor can simulate a localized, chronic blepharitis. Histologically, the morpheaform type is characterized by discrete, elongated islands of basaloid cells encased in a dense, fibrous stroma beneath an intact epidermis. These finger-like cords of tumor can project deep into the dermis and connective tissue, beyond the area of suspected clinical involvement. This subtype often displays a tendency toward invasion of the deeper orbital structures and a propensity for recurrence. The subclinical extension of tumor cells into deep tissue planes and the surgeon’s inability to determine the tumor margins render its complete removal difficult. The recurrence rate of an incompletely excised morpheaform tumor is 10 times greater than that of the nodular type. To ensure complete excision, this type is best managed by the Mohs micrographic technique.
Superficial BCC is a less common variety usually found on the trunk. These flat, superficial lesions appear as erythematous, scaly patches or plaques with well-defined pearly borders. Like the nodular subtype, they are less aggressive and enlarge slowly without tendency to invade or ulcerate. They are often mistaken for psoriatic plaques or Bowen disease.
Regardless of histologic type, BCCs are usually only locally invasive. They are typically indolent; however, if neglected, uncontrolled tumor growth can infiltrate nearby structures including the orbit, paranasal sinuses, and cranial cavity. Risk factors for subclinical spread include tumor diameter greater than 2 cm, location on central face or ears, long-standing presence prior to initial treatment, incomplete excision, aggressive subtype, and perineural or perivascular involvement. Tumor deaths resulting from direct intracranial extension of BCCs may occur. The reported incidence of BCC metastasis is estimated to be less than 0.1%. If metastatic disease develops, it is usually found in the regional lymph nodes. ,
Ophthalmologists are afforded an excellent method of magnified examination of eyelid lesions through the use of slit lamp biomicroscopy. Distortion or destruction of meibomian orifices of the eyelid margin or focal loss of lashes should alert the clinician to the possibility of underlying malignancy. Flaky crust on the surface of a lesion suggests SCC, whereas a more translucent, white, or flesh-colored lesion stretching the skin is likely to represent a BCC. If a lesion attains a diameter of 1 cm in less than 3 months, a diagnosis of SCC or keratoacanthoma is likely. A careful search for an elevated translucent telangiectatic border is important in differentiating BCC from all other lesions. Despite magnified examination of suspicious lesions, clinical appearance alone may be insufficient to establish a diagnosis and biopsy is required for definitive histologic verification.
Biopsy of any suspicious eyelid lesion should be performed to establish a definitive diagnosis. If a lesion is small, excisional biopsy with at least 2–3 mm of margin of normal tissue should be considered. Biopsy can be accomplished by simple elliptical excision and closure of the defect. If the size of the anticipated excisional defect is such that closure will cause eyelid malposition, an incisional biopsy should be performed first. Similarly, if it is anticipated that a graft or flap will be required to repair the defect, the surgical margins must be examined microscopically.
In simple surgical excisions, the specimen should be examined histologically to confirm the margins are clear. Excisional biopsy of even small lesions is controversial because inadequate resection is common. Doxanas et al. reported a 27% incomplete excision rate of BCCs managed by excisional biopsy; only 25% of the incompletely excised tumors recurred. This low rate of recurrence is thought to result from local stimulation of the immune system secondary to surgical trauma. Because of the frequency of incomplete excisions, some advocate incisional biopsy to establish the diagnosis first, followed by excision under frozen section control to ensure all surgical margins are free of residual tumor cells.
Several different treatment modalities have been used to manage periocular BCCs. Surgical excision, cryosurgery, radiotherapy, and Mohs micrographic surgery have been reported to achieve 5-year cure rates of 90%. Currently, the best available options are surgical excision with standard frozen section control or Mohs micrographic surgery. Reports suggest 5-year cure rates using these two forms of treatment in excess of 95%. Hamada and colleagues found that a 2-mm margin with the nodular subtype and 4 mm for all other subtypes are adequate in preventing recurrences. , Conventional frozen section control is widely used, but in many instances only a small fraction of the periphery of the excised tumor is actually examined microscopically.
The fresh tissue technique of micrographic surgery, an adaptation of Mohs original fixed tissue technique, was developed to maximize examination of margins and conservation of tissue. This resection is best accomplished by dermatologists trained in Mohs technique. A close working relationship between the Mohs and reconstructive surgeons results in eradication of periorbital tumor with optimal functional results. The Mohs fresh tissue technique involves removal of the gross mass of the tumor plus a small peripheral margin of normal tissue. A thin layer of tissue, approximately 2 mm thick, is further excised from the entire base and edges of the wound. The initial specimen is divided into 4–7-μm-thick portions on glass slides; the edges are marked with different colored dyes to maintain orientation. Frozen sections are obtained from the undersurface and skin edge of each specimen. Locations of residual tumor are marked on a map and only those areas are reexcised. Surgical resection is continued until there is a microscopically proven tumor-free plane. The defect is then reconstructed by the oculoplastic surgeon. This interdisciplinary collaborative team approach allows two unbiased specialists to offer their shared expertise in achieving the optimum surgical outcome for the patient.
Excision of BCC using frozen section control can also yield excellent results. Cure rates of 99% have been reported. , Frozen section control of tumor margins is performed by noting the clinical boundaries of the tumor edges and excising an additional 3-mm cuff of normal-appearing tissue. A pathologist examines each margin; if no tumor cells are noted, immediate repair is undertaken. If tumor remains in the excised portion, further surgery is performed until the margins are tumor free.
A basic principle of cancer surgery is to obtain tumor-free margins. As techniques that ensure the adequacy of tumor-free margins become more popular and available, radiation therapy is used less frequently to treat primary BCCs. For individuals with inoperable disease, multiple medical problems, elderly patients unable to tolerate surgical resection, or patients in whom surgery will result in extensive disfigurement with potential loss of useful ocular function, radiation therapy is an acceptable palliative alternative.
Reported cure rates do not approach those of histologically controlled surgical excision. The 5-year tumor control rate of 92%–95% for periocular BCCs treated by radiation therapy is slightly less than those associated with histologically monitored surgical excisions. , When compared with surgical excision with frozen margin control, radiation therapy is associated with higher rates of persistent tumor and recurrences. ,
This modality also lacks histologic control of the tumor margins beyond the treatment field. Postradiation complications are potentially vision threatening, and daily treatments for a period of weeks may be necessary. Radiation-induced atrophy and vascular damage to skin may compromise its potential use as flaps for future reconstruction. Side effects and complications after radiotherapy include skin atrophy, ectropion, entropion, nasolacrimal duct stenosis, keratitis, conjunctival keratinization, cataract, loss of eyelashes, and globe perforation.
In patients with extensive, widespread skin cancer that cannot be managed by standard methods of treatment, chemotherapy can be considered as an alternative. Chemotherapy may result in remission of the tumor or induce shrinkage of the lesion, allowing for other forms of treatment or less radical surgery.
In recent years, vismodegib and sonidegib have been used to treat locally advanced and metastatic BCC. These medications are US Food and Drug Administration (FDA)-approved oral, small-molecule, Hedgehog pathway inhibitors. Their overall response rates range from 30% to 58% for locally advanced and metastatic BCC. The side effects include muscle spasms, alopecia, dysgeusia (taste disturbance), weight loss, fatigue, diarrhea, and increased serum creatine kinase. These medications show promise in patients whose disease is considered inoperable or those for whom radical excision and reconstruction are medically contraindicated.
Tumor recurrence may be related to the histologic characteristics of the BCC, anatomic site, and failure of cure by the type of previous primary treatment, specifically nonsurgical modalities. BCCs situated in the medial canthal region are more likely to infiltrate deeply than those arising from the central eyelid margin. , The predilection for deep early penetration of a medial canthal tumor is thought to be related to its proximity to the embryologic fusion planes. These planes offer little resistance to penetration of cutaneous carcinomas and have been implicated in their depth of invasion, horizontal spread, and recurrence. Clinically, the high-risk areas for deep tumor invasion in the midface are depicted as the “H-zone.” The tissue planes of the inner canthus are formed by embryologic fusion of the frontonasal process with the lateral and maxillary processes. Anatomically, this produces clefts in the subcutaneous fascial planes, allowing tumor cells to follow the “path of least resistance” to the periosteum of the lacrimal and ethmoid bones, thus gaining access to the posterior orbit. Tumors located in this high-risk zone can invade deeply and ultimately require exenteration.
The increased mortality and morbidity associated with medial canthal lesions may also be attributed to: (1) access to the nose via the nasolacrimal duct, providing a route of tumor extension; (2) an array of arteries, veins, and nerves in this region facilitating tumor extension in multiple directions; (3) an unremarkable external appearance with orbital infiltration along the medial orbital wall, reducing early clinical suspicion; and (4) the surgeon’s unwillingness to aggressively resect tumors in this region for fear of damaging a myriad of fine anatomic structures.
SCC, a malignant neoplasm of the epidermal keratinizing cells, constitutes approximately 9% of all periocular cutaneous tumors and is the second most common eyelid malignancy. SCC of the eyelids is a potentially lethal tumor that can invade the orbit by direct or perineural extension, spread to regional lymph nodes, and metastasize to distal sites. Regional lymph node metastases have been reported in up to 24% of patients. SCC is also more likely to spread perineurally than BCC, accounting for possible “skip” lesions. Actinic keratoses (AKs) and Bowen disease are considered incipient skin cancers with the potential to progress to invasive SCC.
UVB rays (290–320 nm) and possibly UVA (320–400 nm) are thought to have carcinogenic effects on skin. UV radiation is believed to induce skin cancer by direct DNA damage or alterations in cellular immunity resulting from injury to Langerhans cells within the epidermis. , Most cutaneous SCCs arise from preexisting lesions such as AKs, radiation dermatoses, burn scars, and inflammatory lesions. Photochemotherapy such as psoralen therapy with UVA light has also been implicated in tumor formation. Thermal injuries to the skin have been reported to produce thermal keratoses and SCC. Other factors implicated in the development of SCC include chemical exposure (arsenic, polycyclic aromatic hydrocarbons, smoking), immunosuppression, prior radiation, and genetic diseases (albinism, XP).
AKs are proliferations of transformed, neoplastic keratinocytes confined to the epidermis and are induced by exposure to UV radiation. They are extremely prevalent and are seen in most middle-aged to elderly, fair-complexioned individuals with a history of significant sun exposure. AKs tend to increase in number with age and often develop on the face, forearm, scalp, and dorsal hands. They are typically round, flat, scaly, keratotic plaques with an erythematous base, measuring only a few millimeters in diameter ( Fig. 28.5 ). Occasionally, these lesions have a horny or wart-like appearance with the texture of fine sandpaper.
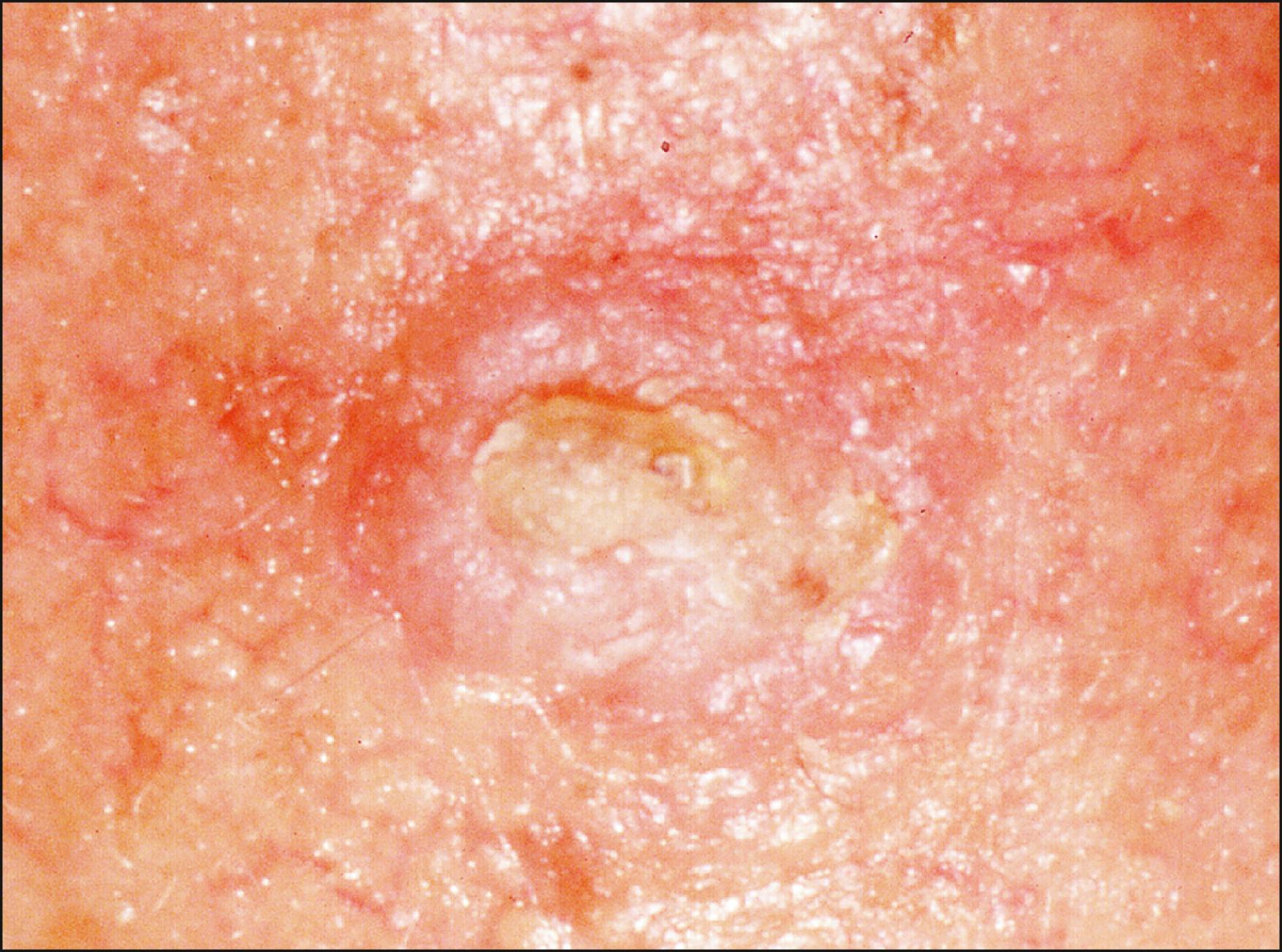
AKs are in a state of continual flux. Increased sunlight exposure may activate the lesions, especially in the summer months. Marks et al. reported that 25% of individual AKs observed over a 12-month period spontaneously resolved, although there was a generalized net increase in total number because of the development of new lesions. The risk of malignant transformation from a single AK lesion has been estimated to be less than 0.24% per year. Although the risk per lesion per year may be low, a patient with several AKs followed over a 10-year period may have an expected risk of malignant transformation of 16.9%. SCCs arising from AKs are thought to be less aggressive, with lower metastatic potential, than SCC arising de novo.
As mentioned earlier, the literature reflects controversy about the risk of progression. AKs are part of a continuum beginning with DNA damage and mutation, neoplastic transformation and proliferation, involvement of deeper structures, and finally metastasis and death. Most authorities believe that AKs represent SCCs in situ in their earliest stages, because these lesions demonstrate features of malignancy from their inception. These lesions share genetic tumor markers and identical p53 gene mutations with SCC involving the dermis. Treatment of periocular AKs depends on size and location of the lesions. Small lesions not involving the eyelid may be observed; however, within the periocular region, biopsy may be performed to establish a definitive diagnosis. With histologic confirmation of AK, the remainder of the lesion may be treated with excision or cryotherapy.
Bowen disease is synonymous with carcinoma in situ of the skin and has been implicated as a marker for internal malignancies. In contrast to most other squamous epithelial proliferative lesions that occur predominantly in sun-exposed areas, these lesions can appear in nonexposed regions of the body with a relatively high frequency. Bowen’s original description referred only to lesions of the integument. Therefore application of this designation to mucous membrane growths, such as those of the conjunctiva, should be avoided.
This condition produces a wide variety of clinical appearances. Typically, a patient presents with an isolated, slightly elevated, erythematous lesion with well-demarcated borders that fails to heal. The lesion has the appearance of a red, scaly patch or plaque, does not bleed or itch, and is devoid of hair. The average diameter is 1.3 cm, much larger than a typical AK lesion. Occasionally, Bowen disease may present as a scaly, crusty, pigmented keratotic plaque.
In Bowen disease, full-thickness epidermal cellular atypia and loss of polarity of the immature, neoplastic epithelial cell are the constant cytologic findings. The histopathologic hallmark is the lack of penetration of cancerous cells into the dermis. The basement membrane remains intact. Five percent of Bowen disease lesions may progress to invasive SCC; complete surgical excision is usually curative.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here