Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Clinical classification of acute and chronic prostatitis
Acute bacterial prostatitis
Chronic bacterial prostatitis
Chronic abacterial prostatitis
Prostate is swollen and tender on palpation
Often refractory to antibiotic therapy because the prostate is a “safe haven” for bacteria
Patients may present with recurrent urinary tract infections
Cultured organisms are the same as those seen in urinary tract infections (i.e., Escherichia coli , other gram-negative rods, and Enterococcus and Staphylococcus species)
Same as acute prostatitis, but symptoms are of longer duration
Most common form of clinical prostatitis
Presents similar to acute and chronic bacterial prostatitis
By definition, no organisms are cultured (idiopathic); however, infection by Chlamydia, Ureaplasma , or Mycoplasma species has been suggested
Nonspecific (idiopathic) granulomatous type
Patients are between 20 and 70 years of age (mean age, 60 years)
Patients present with obstructive symptoms, dysuria, fever, and chills; may have a history of urinary tract infection
Prostate on palpitation can be firm and indurated (may clinically mimic carcinoma)
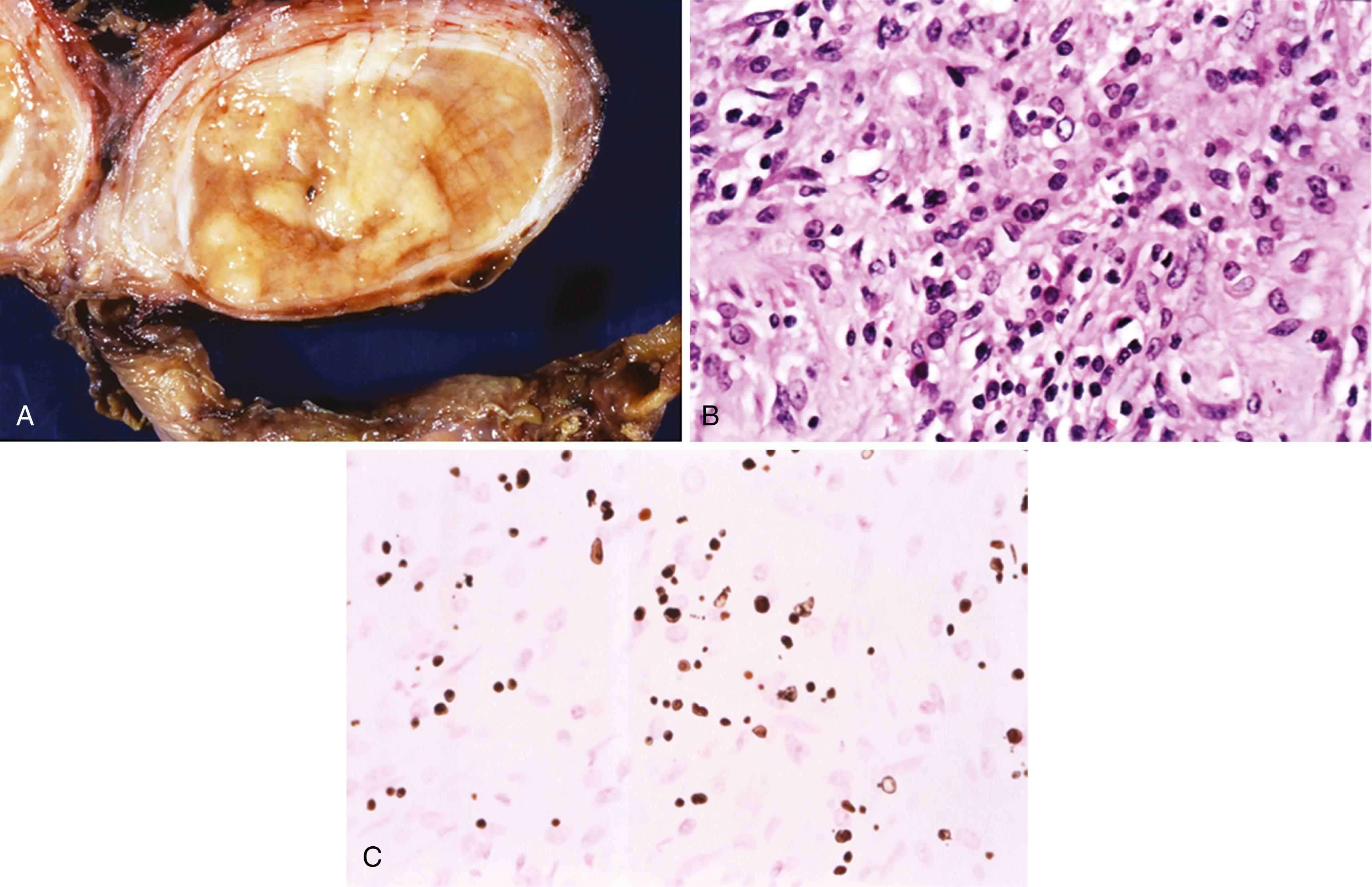
After bacillus Calmette-Guérin (BCG) therapy: history of intracystic BCG therapy for transitional cell carcinoma (TCC) may be remote ( Figure 11.1B )
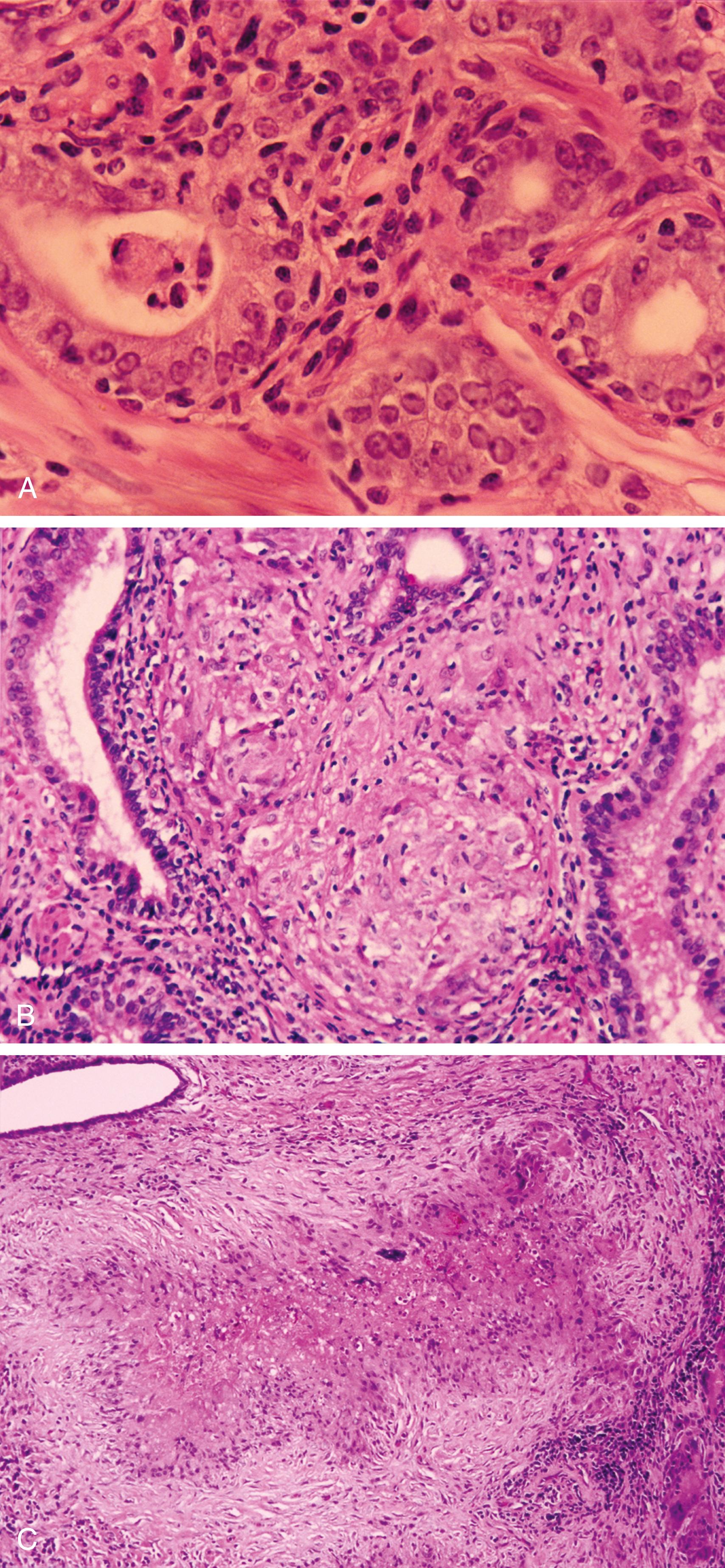
Post-transurethral or postbiopsy granulomatous type: history of procedure up to 5 years ago
Infectious granulomatous type: history of infection by any one of the following
Bacteria (tuberculosis, syphilis, or brucella)
Fungi (cryptococcosis, blastomycosis, or coccidioidomycosis)
Viruses (herpes)
Parasites (schistosomiasis, echinococcosis)
Primarily affects men older than 50 years
Symptoms include fever, frequency, dysuria, and hematuria
Urine culture is often positive for E. coli
Acute and chronic bacterial and chronic abacterial prostatitis
Prostatic enlargement; may be soft and swollen
Granulomatous prostatitis
Enlarged with firm, nodular parenchyma
Areas of infarction and necrosis with infectious granulomas are often seen
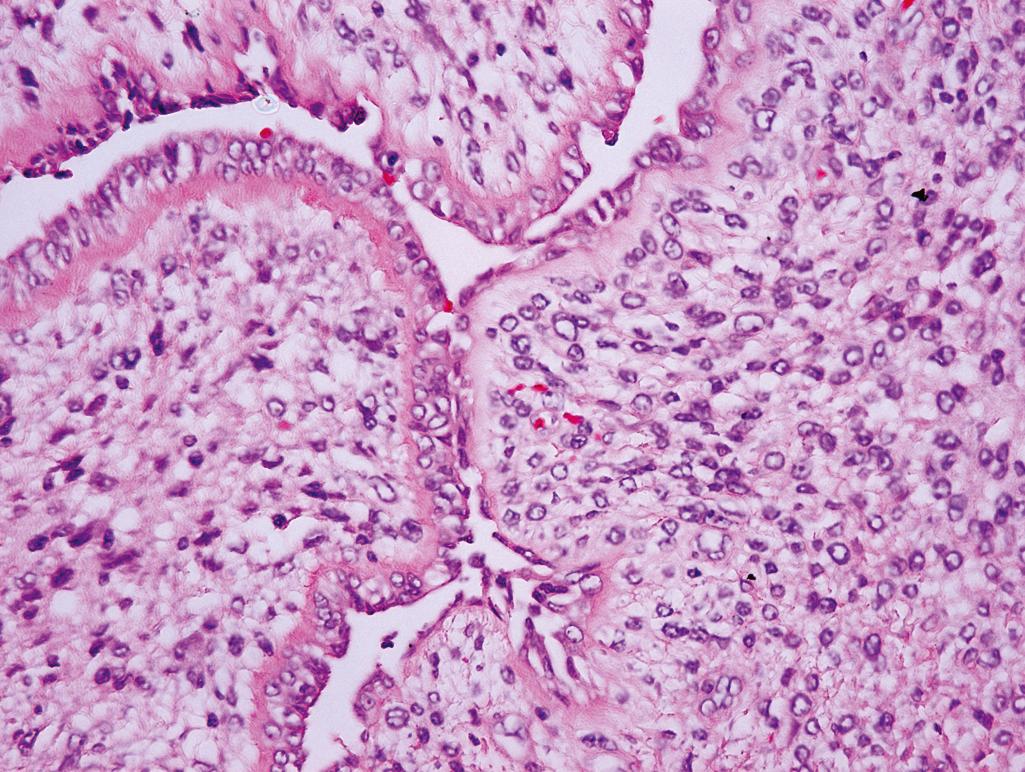
Acute and chronic bacterial prostatitis
Prominent neutrophilic infiltrate with abscess formation
Neutrophils and necrotic debris may fill prostatic ducts and acini
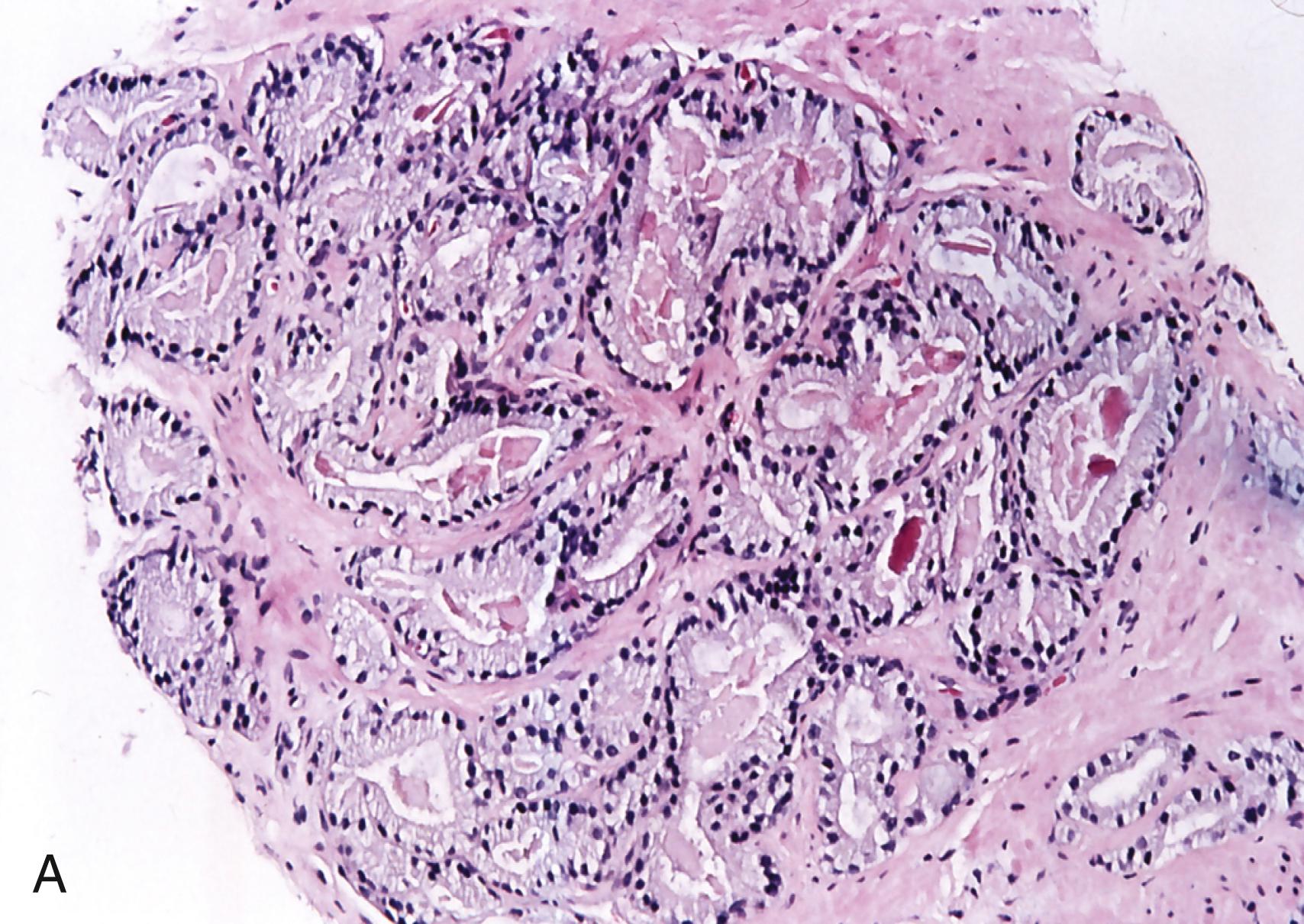
Reactive glandular epithelium showing mild cytologic atypia; nuclei with prominent nucleoli may be seen ( Figure 11.1A )
Glands may appear atrophic and have a pseudocribriform architecture owing to little glands budding off within lumen
Stroma is edematous and hyperemic
Chronic abacterial prostatitis
Presence of neutrophils and lymphocytes in prostatic ducts and epithelium
Reactive glandular epithelium showing mild cytologic atypia; nuclei with prominent nucleoli may be seen
May be associated with glandular atrophy
Granulomatous prostatitis
Nonspecific (idiopathic) granulomatous type
Admixture of histiocytes, plasma cells, eosinophils, neutrophils, lymphocytes, and giant cells
Cells arranged in sheets around ruptured ducts and acini
Post-BCG therapy
Mostly histiocytes and giant cells associated with ducts or acini ( Figure 11.1B )
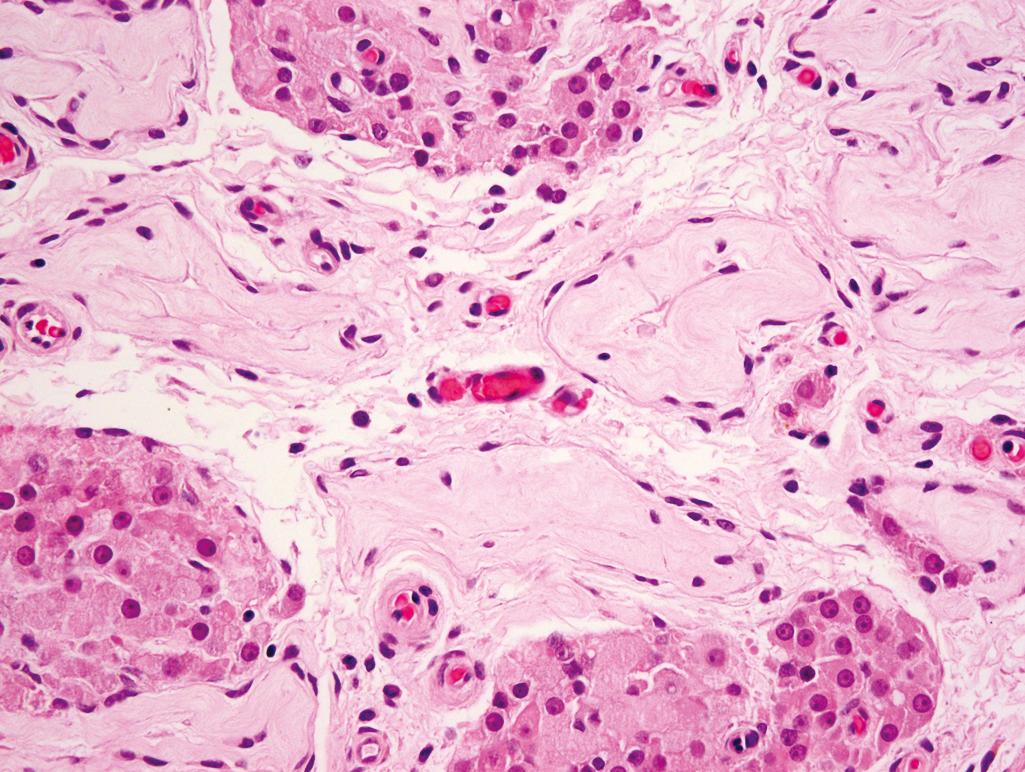
Posttransurethral or postbiopsy granulomatous type
Central zone of fibrinoid necrosis surrounded by palisading histiocytes and some multinucleated giant cells ( Figure 11.1C )
Minimal chronic inflammatory infiltrate
Eosinophilic infiltrate typically presents after recent prostate surgery (1 month after resection)
Infectious granulomatous type
Granulomatous inflammation, with or without necrosis
Eosinophils often present with parasitic infection
Malakoplakia
Hansemann cells: histiocytes with clear or eosinophilic cytoplasm arranged in sheets with surrounding mixed chronic inflammatory infiltrate
Michaelis-Gutmann bodies: round, target-shaped structures found intracellularly and extracellularly
Granulomatous prostatitis
Nonspecific granulomatous type
Stains positively for histiocytic markers, negative for epithelial markers
Infectious granulomatous type
May identify causative organism with special stains (Gomori methenamine silver, periodic acid-Schiff [PAS], acid-fast bacillus stains)
Malakoplakia
Von Kossa calcium, iron, and PAS highlight Michaelis-Gutmann bodies
Noncontributory
Rare in the prostate gland
Proliferation of neoplastic lymphoid cells that typically infiltrate the prostatic stroma in diffuse sheets while sparing the ducts and acini
Infiltration into surrounding periprostatic tissues is common
Monoclonal lymphoid population seen with flow cytometry and immunohistochemistry
Most common tumor subtype is diffuse large cell lymphoma, B-cell type
Infiltrating tumor composed of a diffuse and focally glandular proliferation
Malignant cells have pleomorphic nuclei and prominent nucleoli
Neoplastic glands lack basal cell layer (negative high-molecular-weight cytokeratin [HMWCK] staining)
Inflammatory cell infiltrate is unusual in adenocarcinoma
Patients typically have evidence of systemic disease; rare to have isolated prostate involvement
Characterized by noncaseating granulomas composed of epithelioid histiocytes and giant cells
Special stains for organisms are negative
Preferable to diagnose inflamed prostate specimens as having acute or chronic inflammation than as acute or chronic prostatitis (i.e., the latter are clinical diagnoses)
Biopsy is not required because most prostatitis cases are effectively treated with antibiotics
Patients with chronic prostatitis often have frequent recurrences, and histology correlates poorly with clinical findings (e.g., stromal and periglandular mononuclear cell infiltrates are normal in older men)
All forms of prostatic disease may cause mild elevation of prostate-specific antigen (PSA)
Patients may be asymptomatic or present with urinary retention and hematuria
Typically occurs in a background of nodular hyperplasia
In general, the greater the degree of nodular hyperplasia, the greater the likelihood of infarction
Central pale-yellow zone surrounded by hyperemic tissue
Acute infarction
Central coagulative necrosis with surrounding hemorrhage ( Figure 11.2 )
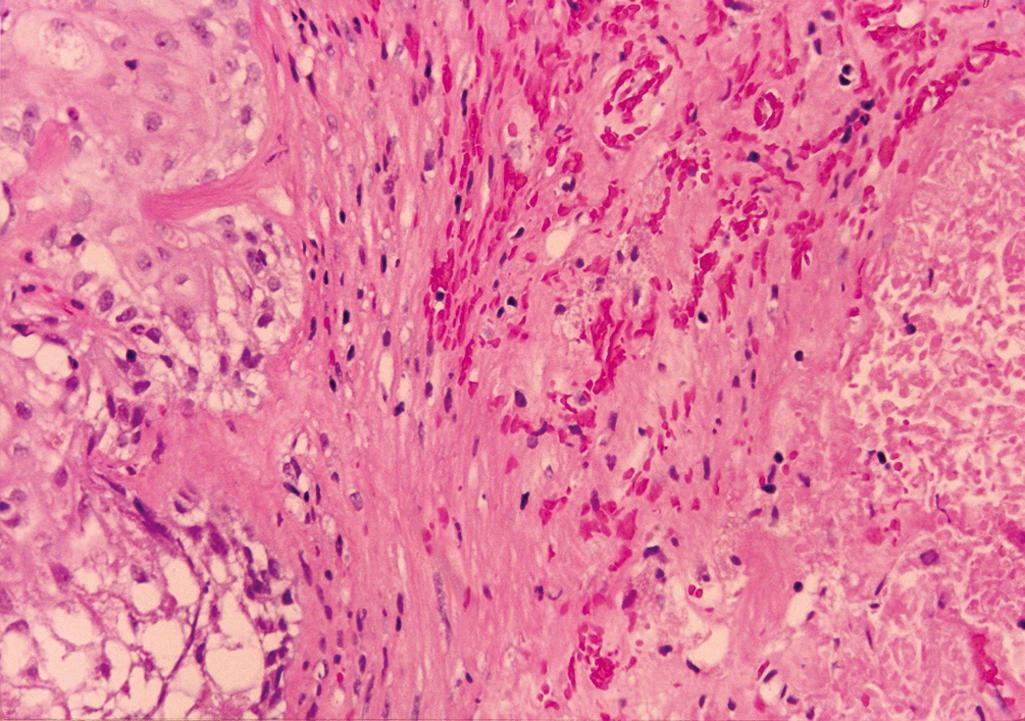
Adjacent glands show reactive and metaplastic changes; typically squamous metaplasia
Reactive glandular epithelium is characterized by cells with enlarged nuclei, prominent nucleoli, and mitotic figures
Remote infarction
Central fibrous scar with hemosiderin admixed with small glands often showing squamous metaplasia
Noncontributory
Noncontributory
Rare in the prostate gland
Infiltrative architecture composed of irregular nests or cords of malignant cells with squamous differentiation; areas of keratinization often seen
Will typically have prominent desmoplastic changes in the surrounding stroma
Low-power magnification reveals uniform proliferation of small glands with irregular contours and irregular stromal spacing
Neoplastic glands are lined by a single layer of epithelium (basal cell layer is absent)
Higher-power magnification demonstrates cuboidal or columnar cells with abundant cytoplasm, enlarged nuclei, and prominent nucleoli
Perineural infiltration is often present
Commonly seen at autopsy in men with marked hypotension who had a urethral catheter in place
Common in males after 60 years of age
Patients may have symptoms of urinary obstruction (inability to initiate or terminate urinary flow) or may be asymptomatic
Multilobulated surface
Variably sized nodules typically located around the prostatic urethra
Peripheral zone appears compressed and atrophic
Small foci of infarction may be seen
Well-circumscribed, nonencapsulated nodules
Composed of hyperplastic epithelial and stromal components ( Figure 11.3A )
Epithelial component
Large, irregularly shaped glands
Glands with a double cell layer and some with pseudostratification of secretory cells
Columnar cells with pale-staining granular cytoplasm
Papillae with fibrovascular cores
Chronic inflammatory infiltrate that surrounds glands
Stromal component
Composed of fibroblasts and smooth muscle cells
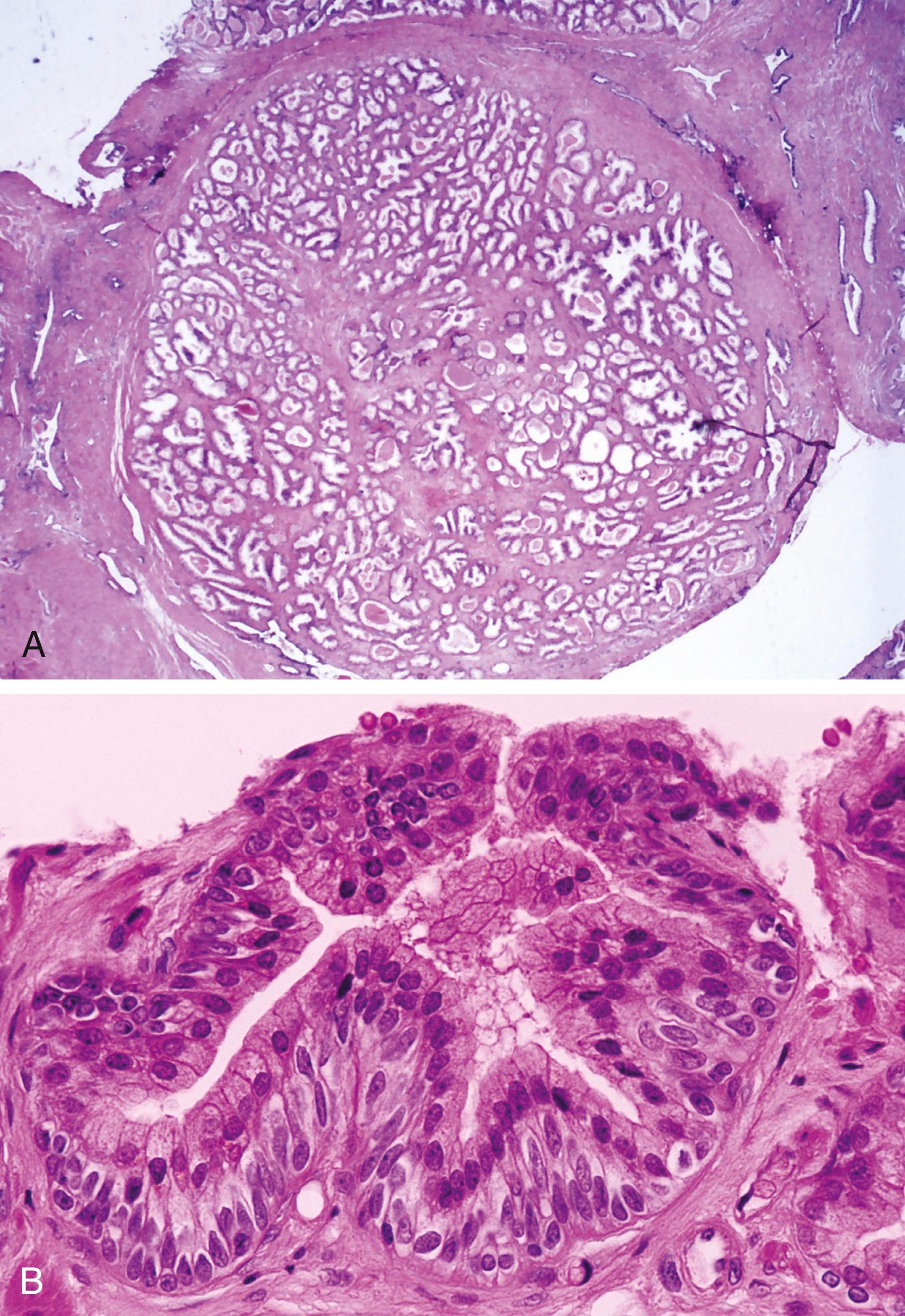
Basal cell hyperplasia (BCH)
Typically an incidental finding
Forms well-defined, small solid nests of basal cells or glandular structures; may have an infiltrative architecture
Hyperplastic glands with proliferation of uniform basaloid cells that may occlude the glandular lumens ( Figure 11.3B )
Glands showing peripheral nuclear palisading
Hypercellular, fibroblastic stroma
Often seen together with typical benign (nodular) prostatic hyperplasia
BCH with nucleolomegaly (“atypical BCH”)
Architecture is similar to that of BCH
Differentiating feature is basaloid cells with prominent nucleoli
Almost always associated with benign (nodular) hyperplasia
Characterized by acini distended by a proliferation of cells with clear cytoplasm forming uniform round spaces
Cells are cuboidal to columnar and have small hyperchromatic nuclei, indistinct nucleoli, and clear cytoplasm
Basal cell layer is intact
Lobular or focally infiltrative glandular proliferation
Glands may be round or compressed and have an angulated, slitlike appearance
Glands have a double cell layer (may be difficult to appreciate) and a thickened basement membrane
Cells contain medium-to-large nuclei with fine chromatin and typically indistinct nucleoli
Stromal component contains plump spindle cells arranged randomly or in fascicles
Architecturally similar to Gleason grade 1 or 2 adenocarcinoma
Circumscribed proliferation of variably sized acini that may show focal infiltration at the periphery
Tightly packed small glands intermixed with larger glands
Basal cell layer may be discontinuous and indistinct but is usually focally present in at least some glands
Glandular cells typically have pale to clear cytoplasm, small nuclei, and inconspicuous nucleoli; prominent nucleoli may occasionally be seen; however, macronucleoli (>3 μm) should not be present
Corpora amylacea is often present (much less common in adenocarcinoma)
HMWCK: highlights basal cell layer in benign (nodular) prostatic hyperplasia, BCH, clear cell cribriform hyperplasia, and sclerosing adenosis
(MSA) and S-100 protein positive in some basal and spindle cells in sclerosing adenosis (indicates myoepithelial differentiation)
Noncontributory
Glands are large and branched with intraluminal papillary projections
Nuclei are elongated, pseudostratified, and perpendicular to the basement membrane
Low-power microscopy reveals uniform proliferation of small glands with irregular contours and irregular stromal spacing
Neoplastic glands are lined by a single layer of epithelium (basal cell layer is absent)
Higher-power microscopy demonstrates cuboidal or columnar cells with abundant cytoplasm, enlarged nuclei, and prominent nucleoli
Cells in clear cell cribriform hyperplasia have distinct clear cytoplasm, small nuclei with indistinct nucleoli, and a prominent basal cell layer
Treatment for persistent, symptomatic hyperplasia is often transurethral resection; occasionally treated with suprapubic prostatectomy
May be treated with various drugs, including
Finasteride (androgen-converting enzyme inhibitor)
α 1 -Adrenergic blockers
Medically treated benign (nodular) prostatic hyperplasia often shows stromal and glandular involution with luminal cell dropout and BCH
Describes a condition in which transitional epithelium is within the prostatic ducts and acini
Often seen in infants and neonates
Generally no clinical symptoms
May be associated with infarction, estrogen therapy, androgen ablation, or radiation therapy
Common in neonates
Generally no clinical symptoms
Nonspecific
Localized to peripheral prostatic ducts and acini
Urothelium admixed with alternating areas of cuboidal and columnar epithelium
Cells are spindle to ovoid to polygonal and have ovoid nuclei overlapping in a streaming manner; nuclei are uniform and have prominent nuclear grooves
May completely fill gland lumen forming a solid nest
Differs from normal urothelium by lack of umbrella cells and presence of eosinophilic secretory lining cells
Squamous differentiation (polygonal cells with eosinophilic cytoplasm) with variable keratin formation and intercellular bridging
May be associated with infarcts and nodular prostatic hyperplasia; if associated with prostatic infarction, mild nuclear atypia may be seen
Haphazardly scattered or small groups of tall, mucin-filled goblet cells
Cells have small, dark, basally oriented nuclei and abundant mucin-filled cytoplasm
Can be found in association with normal and hyperplastic prostate glands, as well as in areas of urothelial metaplasia, BCH, or atrophy
HMWCK positive in urothelial and transitional cells and squamous metaplasia
Alcian blue and mucicarmine positive for intracytoplasmic acid mucin in mucinous metaplasia
PAS positive for neutral mucin in mucinous metaplasia (diastase resistant)
Noncontributory
Carcinoma in situ (intraductal TCC) is typically present adjacent to the invasive component
Infiltrative component consists of single or small groups of cells showing hyperchromatic, pleomorphic nuclei with chromatin clumping, multiple nucleoli, and angulated nuclear borders
Mitotic figures and tumor necrosis are common
Desmoplasia is typically associated with the invasive stromal component
Rare in prostate gland
Infiltrative growth pattern composed of malignant cells with squamous features (keratin formation and intercellular bridging)
Must exclude secondary involvement from extraprostatic sites (e.g., urinary bladder)
Adenosquamous carcinoma composed of typical squamous cell carcinoma admixed with adenocarcinoma (patients usually have a history of radiation or hormonal therapy)
At least 25% of tumor consists of extracellular mucin lakes
Neoplastic cells and glands float within lakes of extracellular mucin
Cribriform pattern is most common, with mucin within the gland lumina and dissecting between the stroma
Neoplastic cells have variable degree of cytologic atypia
None of the metaplastic cell types are associated with subsequent development of prostatic adenocarcinoma
High-grade prostatic intraepithelial neoplasia (PIN) is considered to be a premalignant condition based on morphologic, epidemiologic, and genetic features
In autopsy series, high-grade PIN precedes carcinoma by 10 years and is common in the fourth decade of life
Currently, high-grade PIN is associated with adenocarcinoma on rebiopsy in 25% of patients, significantly less than the 50% association reported in patients biopsied in the late 1980s (see “Pearls”)
Presence of high-grade PIN mandates rebiopsy; however, it is unclear that chasing PIN has any benefit (i.e., it simply results in the detection of clinically insignificant prostate cancers)
Clinical significance of low-grade PIN is unclear; should not be diagnosed
Nonspecific
Low-grade PIN
Morphologic features not rigorously defined and subjective; should not be diagnosed
High-grade PIN
Four patterns include tufted, cribriform, micropapillary, or flat
Cells have enlarged nuclei with prominent nucleoli ( Figure 11.4 )
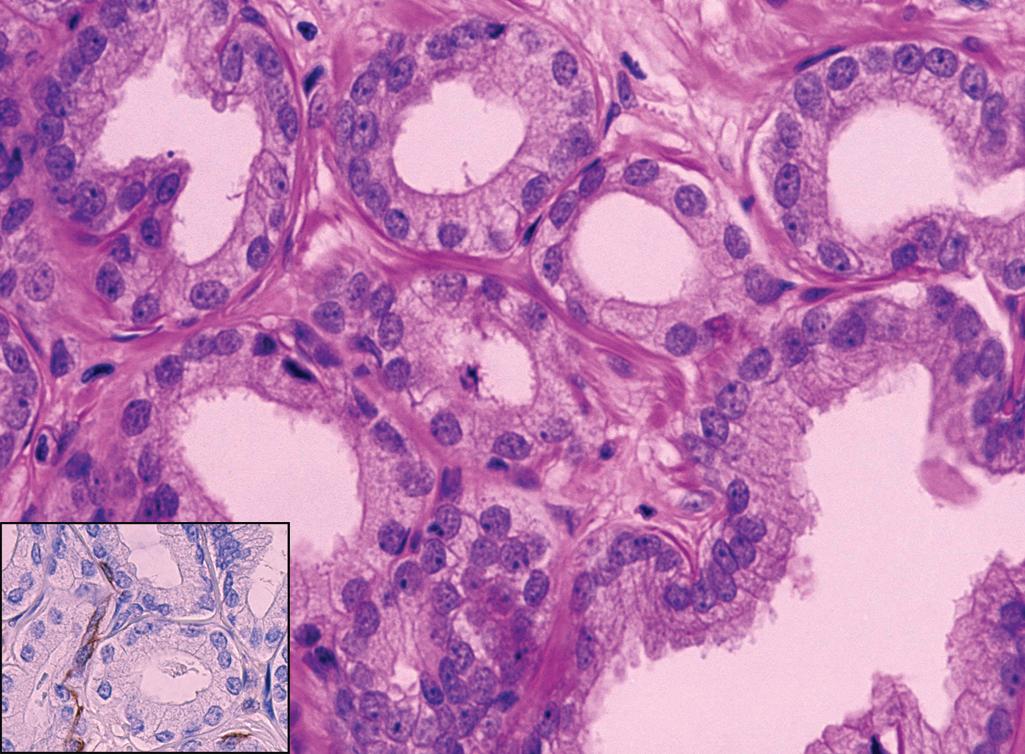
Basal cells are present but may be attenuated
HMWCK and p63: basal cell layer is immunopositive but may be thin and attenuated
α-Methylacyl coenzyme A racemase (AMACR): high-grade PIN may be positive but more apical and granular and less intense than carcinoma
Morphometric studies: high-grade PIN and adenocarcinoma have similar cytologic features (i.e., nuclear area, nuclear perimeter size, nuclear shape, amount and distribution of chromatin, and nucleolar changes)
Low-power magnification reveals a uniform compact proliferation of small glands with irregular contours and irregular stromal spacing
Neoplastic glands are lined by a single layer of epithelium (basal cell layer is absent)
Higher magnification demonstrates cuboidal or columnar cells with abundant cytoplasm, enlarged nuclei, and prominent nucleoli
Perineural infiltration is often present
Negative staining for HMWCK (no basal cell layer)
The decreasing association between high-grade PIN and carcinoma is due to the following:
Increased number of cores performed per biopsy procedure with better targeting of peripheral zone
Changing patient population (younger, PSA screened) with lower prevalence or lower volume of adenocarcinoma; Bayesian reasoning dictates that the positive predictive value of any test result (high-grade PIN on biopsy) is a function of the prevalence of disease (carcinoma) in the population being tested
Diagnosis of high-grade PIN should be made conservatively (cells must show both nucleomegaly and nucleolomegaly)
Most common cause of cancer in men; second most common cause of cancer death after lung cancer
One in five American men will be diagnosed with prostate cancer
Occurs predominantly in men older than 50 years
More prevalent in black men and rare in Asians
Familial predisposition exists
Because of the typical location of prostatic carcinoma (posterior aspects of the peripheral zone), urinary symptoms occur late; asymptomatic tumors are often detected by digital rectal examination or after routine examination that detects elevated PSA
Advanced disease may cause obstructive symptoms (difficulty initiating or terminating urination, frequency, or dysuria)
Metastases to bone may cause osteoblastic or osteolytic lesions; however, in men, the demonstration of osteoblastic bone metastases is virtually diagnostic of metastatic prostate carcinoma
Back pain is a common finding in patients with metastatic disease
Screening methods
Digital rectal examination: cancer focus may be nonpalpable or indurated
PSA levels greater than 4 ng/mL (some advocate 2 ng/mL) prompt biopsy
Random bilateral biopsies now standard of care in select patients with nonpalpable disease
Transrectal ultrasound with biopsy
Elevated PSA is not sensitive or specific for prostatic cancer; other benign conditions, including inflammatory processes or nodular hyperplasia, may cause slight PSA elevation
PSA levels do not distinguish between significant and insignificant cancers; identification of such biomarkers (e.g., EPCA-2, PCAs, TMPRSS2-ERG) is an active area of research
Elevated PSA in patients after treatment for prostatic carcinoma is a useful indicator of recurrent or progressive disease
Associated with a previous or current high-grade prostatic adenocarcinoma
May have a history of radiation therapy for prior adenocarcinoma of prostate
Serum PSA may be normal or only slightly elevated
Carcinosarcoma and sarcomatoid carcinoma are often used interchangeably; however, by convention
Carcinosarcoma should be reserved for tumors that have distinct carcinomatous and sarcomatous elements by histology and immunohistochemistry
Sarcomatoid carcinoma should be used for tumors that show a transition between the two elements
Often multifocal
Preference for the posterior aspects of the peripheral zone (about 75% of tumors); this location renders tumor more likely to be palpable on digital rectal examination
Small tumors typically show no gross abnormalities
Neoplastic tissue is firm, gritty, and less spongy than the surrounding non-neoplastic prostate parenchyma; may show focal yellow discoloration
Cut surface may be glistening or mucinous
Constitutes more than 95% of prostate cancer
Low-power magnification
Majority show haphazard proliferation of fairly uniform acini infiltrating between benign elements
Occasionally the acini are intermediate-sized (“microcystic”) or dilated and filled with complex, branching epithelial cells (“pseudohyperplastic”)
High-grade tumors tend to grow in cords, nests, or sheets;
See Gleason grading system ( Figure 11.6 )
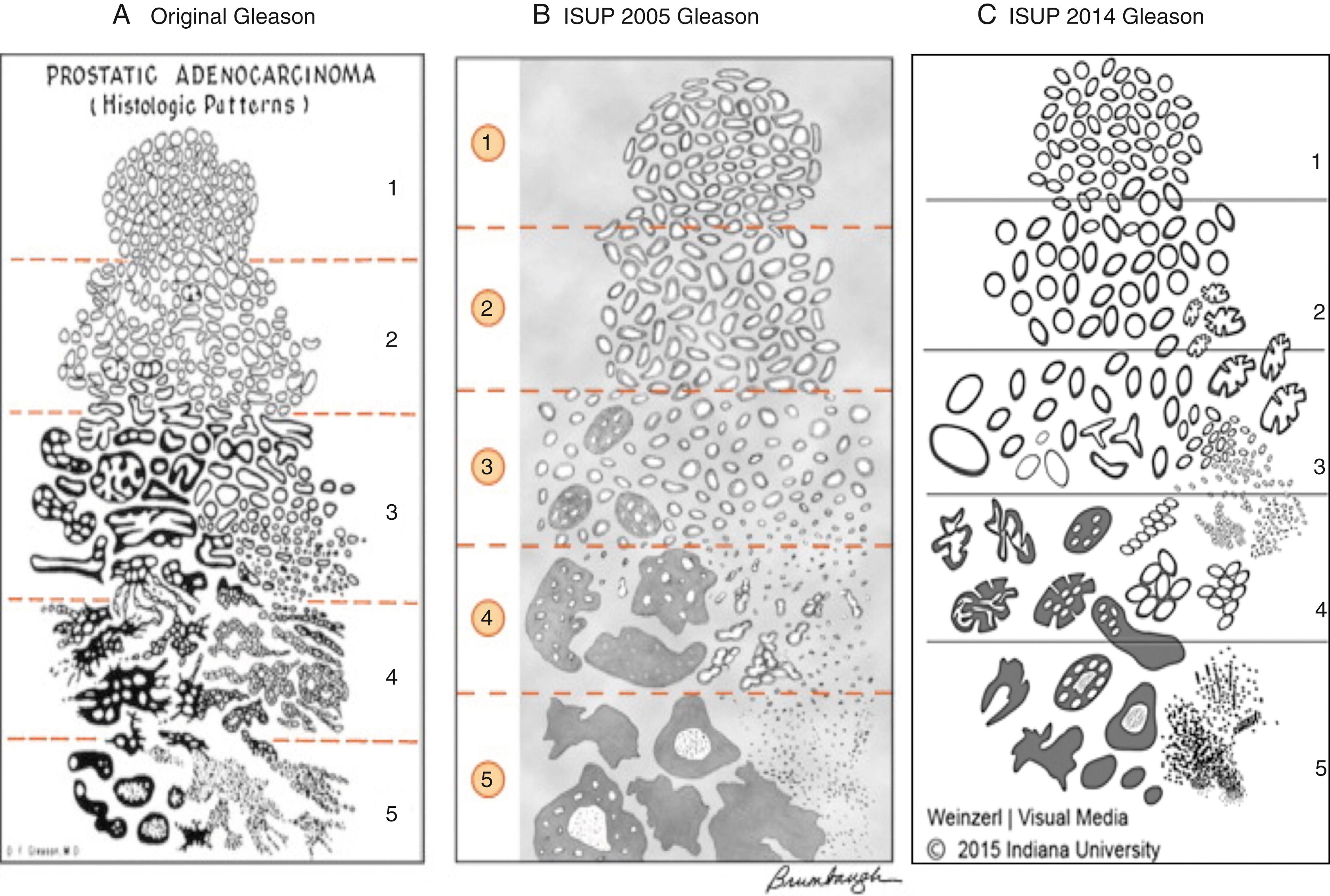
High-power magnification
Acini are lined by a single layer of epithelial cells; basal cell layer is absent
Epithelial cells are cuboidal or columnar and have abundant amphophilic cytoplasm and enlarged, variably pleomorphic nuclei with one or more prominent macronucleoli
Mitotic figures are a helpful feature of malignancy but are uncommon, especially in low-grade tumors
Features pathognomonic for carcinoma include glomeruloid structures, mucinous fibroplasias (collagenous micronodules), circumferential perineural invasion, and extraprostatic extension
Blue-tinged mucinous material, amorphous eosinophilic material, and crystalloid within lumina of neoplastic glands (less common in benign glands) may be seen
Corpora amylacea is rare (much more common in benign conditions)
Uncommon variant
At least 25% of tumor consists of extracellular mucin lakes
Neoplastic cells and glands float within lakes of extracellular mucin
Cribriform pattern is most common, with mucin within the gland lumina and dissecting between stromal muscle fibers
Neoplastic cells have variable degrees of cytologic atypia
Typically associated with an acinar-type adenocarcinoma
Considered Gleason grade 4 and is associated with aggressive biologic behavior
At least 25% of tumor consists of cells with a cytoplasmic vacuole that displaces the nucleus to the side
Cells diffusely infiltrate the stroma and invade perineural and vascular spaces as well as the prostatic capsule
Other patterns of prostatic adenocarcinoma in the same tumor are typically seen
Histologically characterized by cells with abundant foamy cytoplasm and bland nuclei
May be underdiagnosed on needle core because of lack of nucleomegaly and nucleolomegaly
Usually associated with higher-Gleason-score acinar-type adenocarcinoma; therefore, prognostic significance per se unclear
Two patterns on low-power microscopy
Crowded glands lined by pseudostratified epithelium (truly pseudohyperplastic)
Large acini
High-power microscopy shows nucleomegaly and nucleolomegaly
May be underdiagnosed on needle core biopsy because the pseudostratified epithelium looks like hyperplasia or high-grade PIN on low-power microscopy and the large acinar pattern deviates from the more typical small acinar pattern
Biphasic tumor: admixture of carcinoma and sarcoma components
Sarcoma component consists of spindle cells with pleomorphic nuclei and high mitotic rate
Common sarcoma patterns
High-grade sarcoma (not otherwise specified), fibrosarcoma, leiomyosarcoma, osteosarcoma, rhabdomyosarcoma, chondrosarcoma
Should not be confused with the very rare carcinoma that shows giant, pleomorphic cells (“pleomorphic giant cell adenocarcinoma”)
Carcinoma component is usually high grade
Glandular proliferation with an infiltrative growth pattern
Neoplastic cells have large nuclei with prominent nucleoli
Glands lack a basal cell layer
Generally associated with an adjacent acinar adenocarcinoma
Androgen deprivation therapy
Smaller acini or single cells with loss of nucleolomegaly and cytoplasmic clearing (special stains may be required); residual carcinoma showing treatment effect should not be Gleason graded
Adjacent benign tissue shows stromal hyperplasia and gland involution with BCH and squamous metaplasia
Radiation therapy
Smaller acini or single cells with cytoplasmic vacuolization (special stains may be required); residual carcinoma showing treatment effect should not be Gleason graded
Adjacent benign tissue shows glandular atrophy, nucleomegaly, nucleolomegaly, and BCH
AMACR
Sensitive and specific marker for conventional (acinar) prostate cancer; positive in 82% to 100% of cases
Less sensitive in low-grade and hormone-treated conventional prostate cancer and prostate cancer variants, such as foamy gland, pseudohyperplastic, atrophic-type, and ductal-type prostate cancers
Positive but less intense, noncircumferential or only focal in high-grade PIN, atypical adenomatous hyperplasia, atrophy, nephrogenic adenoma, and benign glands adjacent to cancer
AMACR is less useful in evaluating metastases because many tumors in other organs are immunopositive
HMWCK and p63
Stains basal cell cytoplasm (HMWCK) and nuclei (p63); therefore, negative in adenocarcinoma because basal cells are absent
PSA and prostatic acid phosphatase (PAP) positive for tumor cells of mucinous, signet ring cell, and ductal-type variants of adenocarcinoma; also positive in epithelial component of sarcomatoid carcinoma
Carcinoembryonic antigen (CEA) positive in some ductal-type variants of carcinoma
Vimentin: spindle cell component of sarcomatoid carcinoma positive
Desmin, smooth muscle actin (SMA), and S-100 protein: variable positivity in spindle cell component of sarcomatoid carcinoma
Genetic studies of clinical relevance: Familial studies have demonstrated an 8q24 ( MYC oncogene) genetic variant that may be associated with prostate cancer risk; men with germline BRCA2 mutations have a 20-fold increased risk of prostate cancer; rearrangements of ETS family genes (most commonly ERG or ETV1 ) next to the androgen-regulated TMPRSS2 promoter has been identified as an early molecular event in the development of prostate cancer, illustrating the role of androgen stimulation in the pathogenesis of prostate cancer; testing for homologous recombination deficiency, particularly somatic or germline mutations of the homologous recombination repair genes ( BRCA1 , BRCA2 , and ATM ) predict response to poly(ADP-ribose) polymerase inhibitors in hormone refractory prostate cancer.
Lobular or focally infiltrative glandular proliferation composed of glands with a double cell layer (may be difficult to appreciate) and a thickened basement membrane
Cells contain medium-sized to large nuclei with fine chromatin and indistinct nucleoli
Stromal component contains plump spindle cells arranged randomly or in fascicles
Cellular spindle cell component is positive for actin and S-100 protein (indicates myoepithelial differentiation)
Architecturally similar to Gleason grade 1 or 2 adenocarcinoma
Circumscribed proliferation of variably sized acini that may show focal infiltration at the periphery
Tightly packed small glands intermixed with larger glands
Basal cell layer may be discontinuous and indistinct but is usually focally present in at least some glands (positive staining for HMWCK)
Glandular cells typically have pale to clear cytoplasm, small nuclei, and inconspicuous nucleoli; distinct nucleoli may occasionally be seen; however, macronucleoli (>3 μm) should not be present
Corpora amylacea is often present (much less common in adenocarcinoma)
Often seen adjacent to unequivocal adenocarcinoma
Usually large acini lined by hyperplastic, pseudostratified epithelial cells with elongated, large nuclei with prominent nucleoli
The “flat type” of PIN may show small acini with a single cell layer
Basal cell markers (HMWCK or p63) often show attenuated basal cells
AMACR often shows granular, apical cytoplasmic positivity in contrast to more diffuse staining seen in carcinoma
Atrophic glands may have a focally infiltrative architecture and thus may mimic atrophic-type adenocarcinoma
Atrophic glands have open lumens and are lined by cells with an increased nuclear-to-cytoplasmic ratio and inconspicuous nucleoli
Atrophic-type adenocarcinoma is associated with an adjacent acinar adenocarcinoma
Cells in clear cell cribriform hyperplasia have distinct clear cytoplasm, small nuclei with indistinct nucleoli, and a prominent basal cell layer
Typically, TCC involves the urethra or prostatic ducts in patients with a history of carcinoma in situ of the urinary bladder who have been treated conservatively
Carcinoma in situ (intraductal TCC) is typically present adjacent to the invasive component
Lacks glandular differentiation
Mitotic figures and tumor necrosis are common
Stains negative for PSA and PAP
BCH, Cowper’s glands, mesonephric remnant hyperplasia, mucous gland metaplasia, nephrogenic adenoma, paraganglion tissue, radiation changes, seminal vesicles/ejaculatory duct, squamous metaplasia, urothelial metaplasia, verumontanum mucosal hyperplasia, and xanthoma cells
Prostatic carcinoma is typically multifocal, and gross examination usually underestimates the extent of disease
Tumors of the transitional zone are less aggressive than tumors of the peripheral zone
Metastases typically involve the pelvic or para-aortic lymph nodes and axial skeleton (most commonly lumbar vertebrae)
Only in recent years has a survival advantage been demonstrated with the onset of widespread PSA screening; however, this same advantage has also been observed in Europe, where PSA screening is less common
AMACR staining quality may be affected by technologist expertise, run-to-run variability, and the use of monoclonal (P504S) versus polyclonal antibodies, with the latter showing more background staining
Endocrine therapy is used to deprive tumor cells of testosterone and is typically used in patients with widespread metastatic disease (orchiectomy or estrogen administration decreases or eliminates testicular production of testosterone)
Cryotherapy and radiation implants are being used more frequently as alternatives to radical prostatectomy
In general, the presence of lymph node metastases precludes radical prostatectomy
Small prostatectomies should be completely submitted for histopathologic examination, but larger prostatectomies may be partially submitted as long as all of the posterior aspect, apex, and base are submitted (see Iremashvili et al., 2013); the 2018 AJCC TNM guidelines require documentation of the following:
Pathologic stage of primary tumor (“T”):
pT2: (Organ confined) Document volume (%), and laterality of tumor (one half or both sides)
pT3: (Extraprostatic extension) Includes complete invasion through prostatic pseudocapsule, invasion into bladder neck, and seminal vesicle invasion
pT4: (Invasion of surrounding structures) Includes invasion into rectum, levator muscles, or pelvic wall
Pathologic stage of regional lymph nodes (“N”):
N1: Spread to regional lymph nodes, defined as nodes in the true pelvis below the bifurcation of the common iliac arteries
Pathologic stage of distant metastasis (“M”):
M1a: Spread to lymph nodes beyond regional lymph nodes
M2b: Spread to bone
M1c: Spread to other sites (regardless of bone involvement)
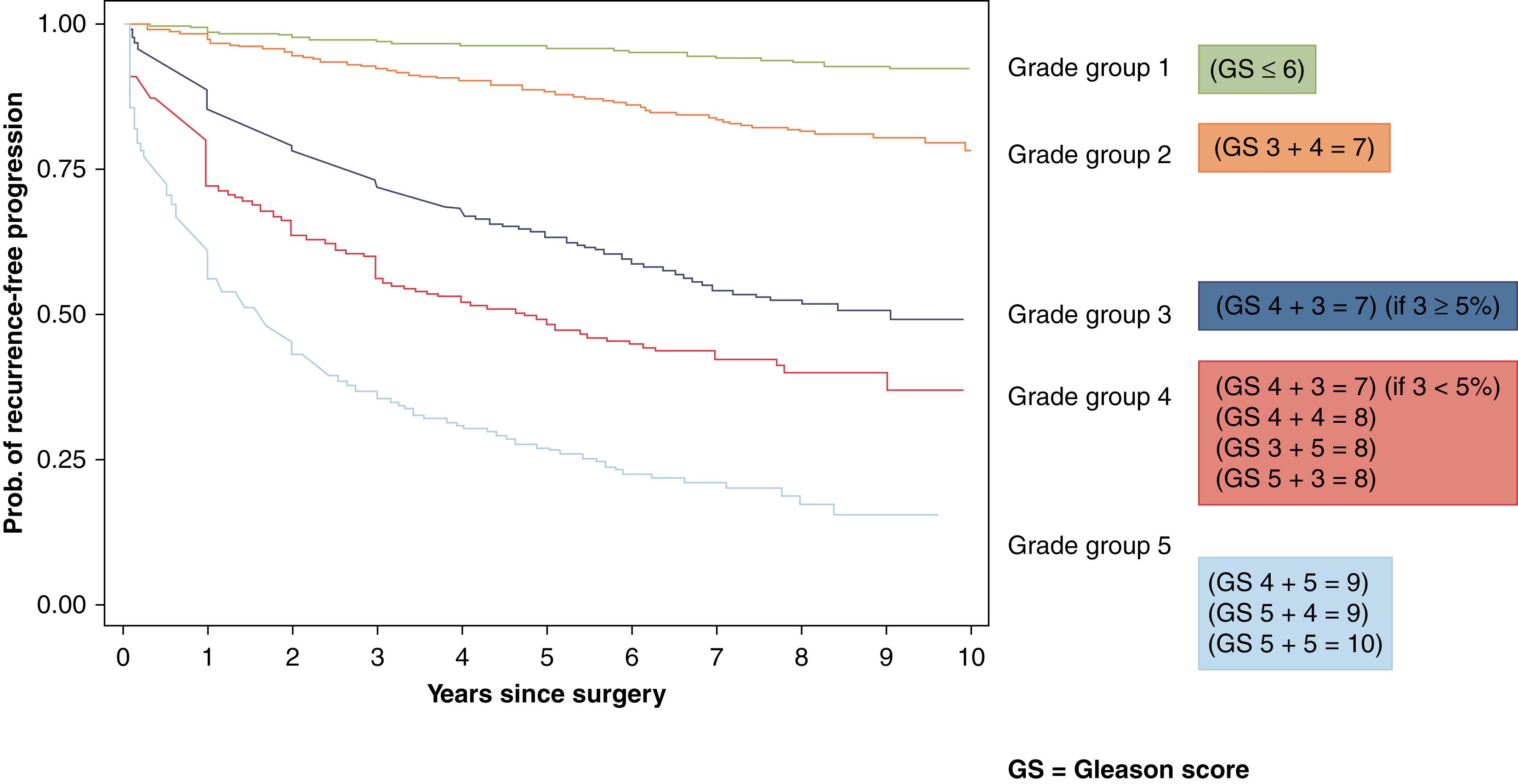
Overall clinical stage takes into account clinical stage, pathologic stage, grade group assigned to the prostate cancer and PSA level ( Figure 11.7 )
Patients may present with obstructive symptoms and hematuria
Cystoscopy may show papillary proliferation distending prostatic urethra
Prostatic ducts and prostatic urethra may be distended by tumor
Invasive adenocarcinoma showing growth along prostatic ducts
Papillary and cribriform architecture composed of pseudostratified (endometrioid-like) columnar cells with elongated nuclei
Gleason pattern 4. Presence of comedonecrosis should be classified as Gleason pattern 5
Basal cell markers negative
None
PIN
Glandular proliferation less complex, glands less dilated, and basal cells may be attenuated but still present
Acinar adenocarcinoma with cribriform architecture
Cuboidal (noncolumnar) cells with rounded nuclei creating rounded (nonelongated) cribriform spaces
Usually associated with conventional, acinar adenocarcinoma; pure ductal adenocarcinoma very rare
Nothing specific
Nothing specific
Noninvasive carcinoma distending acini and/or ducts
Architecturally may be solid, cribriform or micropapillary. Comedonecrosis may be present
Noncontributory
Noncontributory
Glandular proliferation architecturally much less complex, ducts and acini much less dilated, and much less cytologic atypicality
The term “intraductal” is a misnomer as the tumor may be intra-acinar
Does not impact upon the Gleason score
Serum PSA and PAP levels are typically not elevated
Benign and malignant basal cell lesions are relatively uncommon (reported in less than 6% to 9% of cases); benign basal cell proliferations make up most of them
Nonspecific
Basal cell tumors consist of a spectrum of disease ranging from BCH to atypical BCH to basal cell adenoma to basal cell carcinoma (BCC); historically, a basal cell tumor with a cribriform architecture has been referred to as an adenoid basal cell tumor or adenoid cystic carcinoma (ACC); BCC and ACC are now considered parts of a morphologic continuum
Infiltrative clusters of basaloid cells
Often have prominent desmoplastic stromal response
Must demonstrate one or more of the following features: necrosis, perineural invasion, or infiltration outside prostatic capsule
Histologic features similar to those of ACC of the salivary glands
Cells form poorly circumscribed, infiltrative nodules surrounded by a loose or myxoid stroma
Nests show peripheral nuclear palisading around adenoid cystlike spaces that contain mucinous, eosinophilic, or hyaline material
Focal squamous differentiation with keratin production may be seen
Basaloid cells are uniform and have round hyperchromatic nuclei
Perineural invasion is rare
Low malignant potential; no reports of metastasis (believed by some authors to be part of BCH and adenoma)
PSA and PAP typically positive
HMWK: focal weak positivity in basaloid cells
Noncontributory
Often see other findings of androgen blockade including stromal and glandular involution and squamous metaplasia
Lacks cytologic atypicality, infiltrative growth and desmoplasia seen in adenoid cystic/BCC
Lobular or focally infiltrative proliferation composed of glands with a double cell layer (may be difficult to appreciate) and a thickened basement membrane
Cells contain medium-sized to large nuclei with fine chromatin and indistinct nucleoli
Cellular spindle cell stroma with evidence of myoepithelial differentiation demonstrated by positive staining for S-100 protein and MSA
Circumscribed proliferation of variably sized acini that may show focal infiltration at the periphery
Tightly packed small glands intermixed with larger glands
Some glands may show a basal cell layer (positive for HMWCK)
Glandular cells typically have pale to clear cytoplasm, small nuclei, and inconspicuous nucleoli; prominent nucleoli may occasionally be seen; however, macronucleoli (>3 μm) should not be present
Typical cytologic features of malignancy, including cuboidal or columnar cells with abundant amphophilic cytoplasm, enlarged nuclei, and one or more prominent macronucleoli
Absence of basal cell layer (negative for HMWCK)
Typically treated with transurethral resection; controversy still exists regarding treatment for BCC
Basal cell lesions in the prostate form a spectrum of disease behavior that is typically benign
Malignant behavior in adenoid basal cell tumor has not been demonstrated
Rarely occurs de novo; patients usually have a history of treated prostate cancer
Most show no evidence of hormonal secretion; however, paraneoplastic syndromes (Cushing syndrome, hypercalcemia, syndrome of inappropriate antidiuretic hormone [SIADH] secretion and Eaton-Lambert syndrome) can occur
May have minor elevations of serum PSA
Metastasizes through hematogenous (liver, brain) rather than lymphatic channels
Nonspecific
Neuroendocrine differentiation seen in the following circumstances:
Conventional prostate adenocarcinoma frequently contains isolated cells positive for neuroendocrine markers only (not apparent on H&E [hematoxylin & eosin] slides)
Conventional prostate adenocarcinoma with a neuroendocrine component apparent on H&E slides
Conventional prostate adenocarcinoma with intermingled cells showing Paneth cell-like neuroendocrine differentiation
Carcinoid-like tumors (“well-differentiated neuroendocrine tumor”)
Small cell neuroendocrine carcinoma
Large cell neuroendocrine carcinoma
Post-therapy neuroendocrine differentiation (which some have designated “treatment-related neuroendocrine prostate cancer” or “Aggressive variant of prostate cancer”)
Neuron-specific enolase (NSE), chromogranin, and cytokeratin typically positive
PSA and PAP: often negative or only focally positive
AMACR: 50% positive
Secretory products may be present within neoplastic cells
Adrenocorticotropic hormone (ACTH), serotonin, calcitonin, human chorionic gonadotropin (HCG), thyroid-stimulating hormone (TSH), and bombesin
Electron microscopy: neuroendocrine cells contain round, regular membrane-bound neurosecretory granules, measuring 100 to 400 nm
Clinical history is important
Lacks associated acinar adenocarcinoma that is usually seen in primary neuroendocrine carcinoma of the prostate gland
Primary prostatic lymphoma is rare
Neoplastic lymphoid population infiltrating around ducts and acini (typically spares prostatic glands)
Infiltration into surrounding periprostatic tissue is common
Positive for leukocyte common antigen (LCA)
Negative for cytokeratin, NSE, chromogranin, and other neuroendocrine markers
Many acinar-type prostatic adenocarcinomas show immunohistochemical evidence of neuroendocrine differentiation, the significance of which is unknown
Neuroendocrine carcinoma of the prostate may respond to small cell carcinoma–directed chemotherapy but is clinically aggressive
Rare primary prostate gland tumor (represents 1% to 3% of primary prostatic gland malignancies)
Common symptoms include hematuria or urinary obstruction
PSA not elevated
Three modes of prostatic involvement
Primary tumor of prostatic urethra, ducts, or acini
Secondary mucosal involvement from a prior or currently active bladder cancer
Direct invasion from bladder cancer infiltrating through the bladder wall
Nodular proliferation in prostatic urethra
Nonspecific nodular architecture with involvement of prostatic ducts and acini
Carcinoma in situ (intraductal TCC) is typically present adjacent to the invasive component; may involve the urethra, the prostatic ducts and acini, and occasionally the ejaculatory ducts and seminal vesicles
Infiltrative component consists of small groups or single cells with hyperchromatic, pleomorphic nuclei with chromatin clumping, multiple nucleoli, and angulated nuclear borders
Mitotic figures and tumor necrosis are common
Elicits a desmoplastic stromal reaction
Pagetoid spread and squamous metaplasia may be seen
HMWCK, CK7, and CK20 variably positive
PSA and PAP negative
Noncontributory
Gleason grade 5 adenocarcinoma with comedonecrosis may be difficult to distinguish from TCC
Focal gland formation can typically be found after careful evaluation of multiple sections
Not associated with TCC in situ
Positive for PSA, PAP, or AMACR
Typically urethral or prostatic duct TCC is seen in patients with a history or urothelial carcinoma elsewhere
Prostatic stromal involvement by TCC is, by definition, stage T4 disease and carries a poor prognosis
Rare in the prostate gland
Typically found in older age group (mean age of about 70 years)
Two clinical scenarios
Primary, de novo squamous cell carcinoma
Associated with treated (radiation or hormone ablation) adenocarcinoma
Serum PSA and PAP are usually normal
Metastatic bone lesions osteolytic, in contrast to adenocarcinoma, which causes osteoblastic bone lesions
Most commonly associated with squamous cell carcinoma of the urinary bladder
May be associated with Schistosoma haematobium infection
Nonspecific
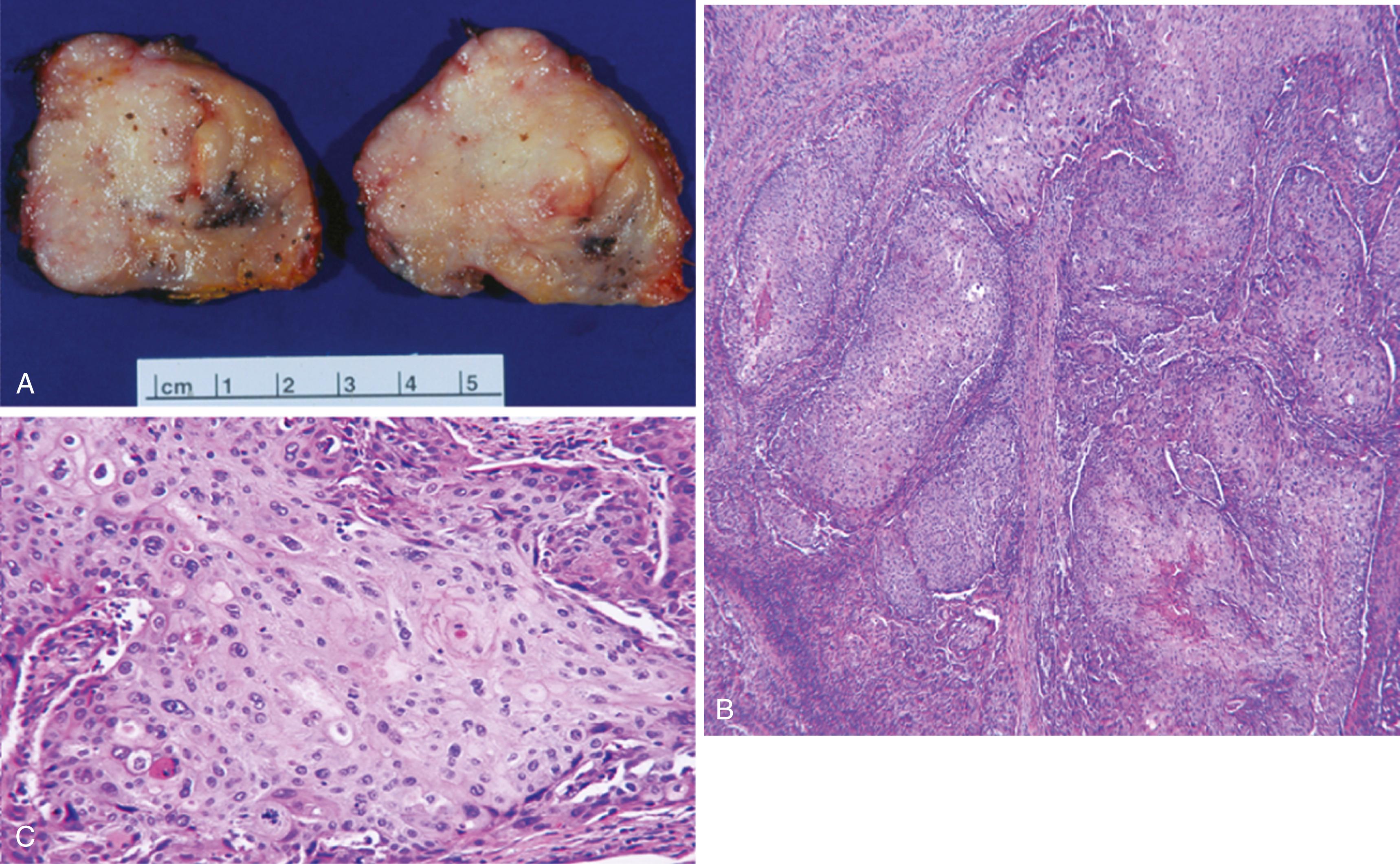
Similar histologic features to squamous cell carcinoma of other sites
Malignant squamous cells arranged in cords and nests with an infiltrative architecture
Two forms of carcinoma
Pure squamous carcinoma
Rare
Infiltrative growth pattern composed of malignant cells with squamous features (keratin formation and intercellular bridging) ( Figure 11.8B and C )
No gland formation
No patient history of radiation or hormonal therapy
Must exclude secondary involvement from extraprostatic sites (e.g., bladder)
Adenosquamous carcinoma
Admixture of adenocarcinoma and squamous cell carcinoma
Typically associated with a history of radiation or hormonal therapy
PSA and PAP are positive in glandular component of adenosquamous carcinoma; pure squamous cell carcinoma is typically negative
Noncontributory
Commonly associated with prostatic infarction
Lacks significant cytologic atypia and tumor necrosis
Much more common to have a squamous cell carcinoma in the prostate gland as metastatic disease or direct extension from adjacent organs (i.e., urinary bladder) than as a prostatic primary
Behaves in an aggressive manner (mean survival of 14 months, regardless of therapy)
Unresponsive to androgen-deprivation therapy
Rare neoplasm
Wide age range
Patients present with symptoms associated with prostatic enlargement, which include urinary obstruction, hematuria, and dysuria
Multinodular solid, gray-white mass
Cut surface may be spongy or cystic
Variable size; may be larger than 25 cm in diameter
Biphasic tumor composed of epithelial and stromal components
Epithelial cells are cuboidal to columnar, arranged in double-layer lining glands, cysts, or slitlike spaces
Stellate to spindle stromal cells arranged in a loose, myxoid background
Glandular or cystic spaces compressed by cellular stroma into a leaflike configuration ( Figure 11.9 )
Likelihood of recurrence and malignant behavior is associated with a high stromal to epithelial ratio (stromal hypercellularity), cellular atypia, and high mitotic rate
Vimentin: stromal component is typically positive
PSA and PAP: epithelial cells may be positive
SMA negative
Noncontributory
Benign prostatic hyperplasia nodule with stromal overgrowth may be large
Lacks epithelial component and leaflike configuration
Solitary cystic tumor with a surrounding dense fibrous stroma
Numerous, variably sized cystic spaces lined by a benign-appearing prostatic epithelium
Lacks leaflike configuration
Rare reactive spindle cell proliferation that may occur after transurethral prostate resection (previously resected prostate tissue must show no evidence of a mesenchymal or spindle cell tumor)
Benign cytologic features and variable mitotic rate (uniform cells with no nuclear pleomorphism and no atypical mitotic figures)
Lacks epithelial component
Low-power view has variable cellularity and lacks leaflike configuration
High-power view has spindled cells insinuating themselves into bands of collagen
Monophasic hypercellular spindle cell neoplasm without epithelial component
Positive for SMA and desmin
Malignant spindle cell proliferation admixed with a malignant epithelial component
Spindle cell component may predominate; cytokeratin positivity, the distinguishing feature, may be weak and focal
Most are cured by surgical resection and follow a benign clinical course; however, biologic behavior is difficult to predict based on histologic features
Tumors with overtly malignant stromal component have given rise to distant metastases (most commonly lung and bone)
Diagnosis on needle biopsy may be difficult
Most common sarcoma of the prostate
Occurs primarily between birth and 6 years of age
Most common in the head and neck, followed by the genitourinary tract
About 20% of childhood cases occur in the genitourinary tract
Rare cases reported in older men
Presents with pelvic mass and urethral obstruction
Pelvic mass may cause bladder displacement and rectal compression
Large, gray-white mass typically measuring 5 to 10 cm
Appears grossly circumscribed but is typically infiltrative microscopically
Most common subtype
Mixture of sheets of primitive, undifferentiated, round to spindle cells admixed with haphazardly arranged rhabdomyoblasts in a myxoid stroma
Primitive cells are small and round with dark nuclei and minimal cytoplasm
Variable numbers of strap cells, with or without cross-striations
Variable mitotic activity
These patterns are rare
Botryoid pattern consists of polypoid fragments covered with urothelium that often extends into the urethra or bladder
Vimentin, MSA, desmin, and myoglobin positive
Stains negatively for cytokeratin, LCA, NSE, PSA, and PAP
Electron microscopy: rhabdomyoblasts have cytoplasmic myofilaments and Z bands
Flow cytometry: tumor cells are typically aneuploid
Must rule out metastasis from other primitive childhood small round blue cell tumors
Non-Hodgkin lymphoma
Typically found in older age groups
Neoplastic lymphoid cells infiltrating stroma in diffuse sheets or patches; ducts and acini are typically spared
Positive for LCA
Composed of a monoclonal lymphoid population
Presence of strap cells or rhabdomyoblasts is diagnostic
Treatment typically consists of surgery, chemotherapy, and radiotherapy
The prostate and urinary bladder are considered “unfavorable prognosis” sites
Most common in older men (mean age, 60 years)
Presents with urinary obstruction symptoms
Primary lymphoma involves the prostate gland without extraglandular involvement (i.e., liver, spleen, lymph nodes, peripheral blood)
Secondary involvement of the prostate gland by a systemic lymphoma is more common than primary prostate lymphoma
Systemic symptoms (fever, chills, night sweats, and weight loss) are infrequent and typically seen only in patients with disseminated disease
Diffuse enlargement of the prostate gland
Tan, homogeneous, rubbery parenchyma
Proliferation of neoplastic lymphoid cells that typically infiltrate the prostatic stroma in diffuse sheets while sparing the ducts and acini
Infiltration into surrounding periprostatic tissues is common
Most common subtype is diffuse large cell lymphoma, B-cell type; small cleaved cell lymphoma is also relatively common
Hodgkin disease is rare
LCA positive (non-Hodgkin lymphoma)
Refer to Chapter 14 for specific immunohistochemistry profiles
Flow cytometric immunophenotyping using fresh tissue is useful in documenting clonality and for subtyping lymphomas (refer to Chapter 14 )
Mixed inflammatory infiltrate with germinal center formation
Inflammation is typically within duct lumina and in the glandular epithelium
Nonclonal lymphocytic population
Admixture of histiocytes, plasma cells, eosinophils, neutrophils, lymphocytes, and giant cells
Inflammatory cells cause destruction of the prostatic ducts and acini
Characteristic prostatic adenocarcinoma associated with a neuroendocrine carcinoma, which may range from a low-grade neuroendocrine carcinoma (carcinoid) to a small cell undifferentiated carcinoma (oat cell carcinoma)
Areas of necrosis are typical in small cell carcinoma
Positive for cytokeratin, NSE, chromogranin, and other neuroendocrine markers
Negative for LCA
Typically found in younger age group
Mixture of sheets of primitive, undifferentiated, round to spindle cells admixed with haphazardly arranged rhabdomyoblasts in a myxoid stroma
Positive for MSA, desmin, and myoglobin
Negative for LCA
Surgery is used mainly for relief of urinary obstruction symptoms
Poor prognosis; death typically results within 2 years of diagnosis
Usually unilateral (75%)
Occurs in 3% to 4% of term infants and in up to 20% of premature infants
Undescended testicles typically descend by 3 months of age (<1% remain undescended at 1 year of age)
Associated with an inguinal hernia in 10% to 20% of cases
Most cryptorchid testes are found in the inguinal canal
Right testicle is more commonly involved
Patients with undescended and surgically descended cryptorchid testes have decreased fertility and increased risk for certain germ cell and non–germ cell tumors
Normal descent of testes is under hormonal control
Cryptorchid testes are smaller and softer than normal testes
Histologic changes in cryptorchid testis occur by age 2 years
Seminiferous tubules may be small or ring shaped and have areas of tubular sclerosis or atrophy ( Figure 11.10 )
Spermatogonia may be decreased in number and irregularly distributed or totally absent
Sertoli cells are increased in number; Leydig cell hyperplasia may be prominent
Interstitium is typically widened and edematous
Normally descended testis contralateral to the cryptorchid testis often shows many of the same histologic features
Noncontributory
Noncontributory
Causes of testicular maldescent include anatomic abnormalities of the gubernaculum, hormonal dysfunction, mechanical impairment, and gonadal dysgenesis
Testicles normally descend from their intra-abdominal location to the scrotum in two phases, both of which are under hormonal control; defects in the transabdominal phase are much less common than defects in the inguinal-scrotal phase
Patients with cryptorchidism have a 5 to 10 times higher risk for testicular malignancy than the general population; orchiopexy does not reduce the risk for cancer but does make detection easier
Most common consequence is infertility
Early orchiopexy (surgical placement of the testis in the scrotum) may have a positive effect on fertility; orchiopexy after 4 years of age does not increase fertility
Usually unilateral
May be difficult to distinguish from cysts of paratesticular adnexa by ultrasound
Albugineal cysts are usually uniloculated, centered in the visceral tunica albuginea, and contain clear fluid
Epidermoid cysts are usually uniloculated, abut the visceral tunica albuginea; and contain laminated, granular, friable material ( Figure 11.11 )
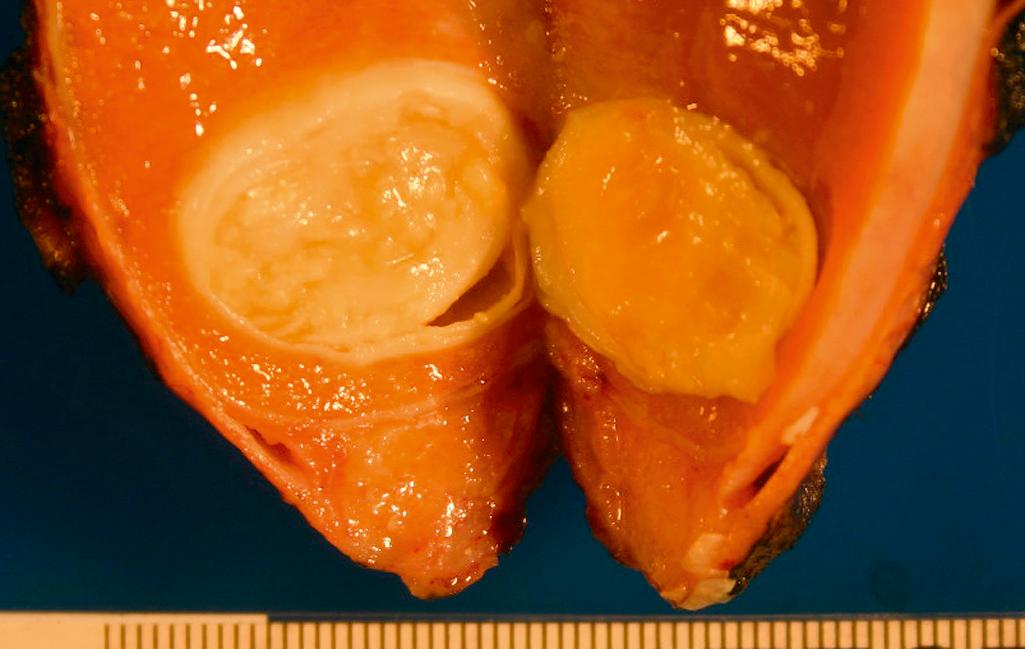
Rete testes cysts (cystic dysplasia of the rete testes) are usually multiloculated, retiform, centered in the testicular hilum, and contain clear fluid
Albugineal cysts are at least partially lined by low cuboidal serosal epithelium
Epidermoid cysts are lined by attenuated squamous epithelium, contain keratinaceous material, and, by definition, lack adnexal structures and germinal elements
Rete testes cysts (cystic dysplasia of the rete testes) are lined by attenuated, low cuboidal epithelium
Noncontributory
Noncontributory
Dermoid cysts are lined by keratinizing squamous epithelium but also have adnexal structures
Teratomas may be mostly cystic lined by keratinizing squamous epithelium but also have teratomatous elements
Complete submission of epidermoid cysts is required to rule out dermoid cysts and teratoma
Most are idiopathic; may be associated with inguinal hernia, scrotal trauma, orchitis, or testicular tumors
May be secondary to congenital lack of closure of the processus vaginalis, resulting in a communication with the peritoneal cavity
Characterized by accumulation of serous fluid between the parietal and visceral tunica vaginalis
Occasionally patients present with acute testicular enlargement secondary to hemorrhage; lack of transillumination may necessitate orchiectomy
Clear serous fluid-filled cavity compresses adjacent testis
Hemorrhage or infection may cause fluid to become opaque
Tunica may be thickened in long-standing lesions
Fluid-filled cavity lined by flattened or cuboidal mesothelial cells
Mesothelium may be hyperplastic or cytologically atypical
Noncontributory
Noncontributory
Usually located near rete testis or caput epididymis
Contains spermatozoa
Usually located anterior or lateral to testis
May arise within the tunica vaginalis, tunica albuginea, epididymis, or rarely the spermatic cord
May be multiloculated
Mumps is most common; coxsackievirus B is also relatively common
Although the mumps viral syndrome occurs primarily in adolescent children, mumps orchitis is seen in postpubertal individuals
Manifests with testicular pain
Usually appears shortly after or during the viral syndrome, which includes parotitis
Testicular involvement is seen in 15% to 30% of mumps infections
May be bilateral
Infrequent in childhood
E. coli is the most common causative agent
May be acute or chronic
Usually associated with infection elsewhere in the genitourinary tract
Usually a chronic process
Associated with a variety of organisms; often associated with systemic or extratesticular infection
May be idiopathic
Acute: testicle is swollen and edematous
Chronic: testicle is firm and often has a thickened tunica
Acute inflammation seen during acute infection
Long-term infection results in patchy interstitial fibrosis and atrophy of seminiferous tubules; often involves both testes
Often associated with bacterial epididymitis
Prominent neutrophilic infiltrate with abscess formation
Chronic bacterial orchitis may show granulomatous inflammation; lacks intratubular giant cells
Characterized by edema and diffuse lymphoplasmacytic inflammation
Defining features include obliterative endarteritis with perivascular lymphocytes and plasma cells
Gumma formation may be seen
Stains for microorganisms can be useful to identify bacteria and fungi
Noncontributory
Specific agents (e.g., mycobacteria, brucellosis, fungi) must be demonstrated by special stains, culture, or serology
Sarcoidosis
Isolated (i.e., nonsystemic) testicular involvement is extremely rare
Characterized by noncaseating granulomas composed of epithelioid histiocytes and giant cells
Idiopathic granulomatous orchitis
No organisms are identified
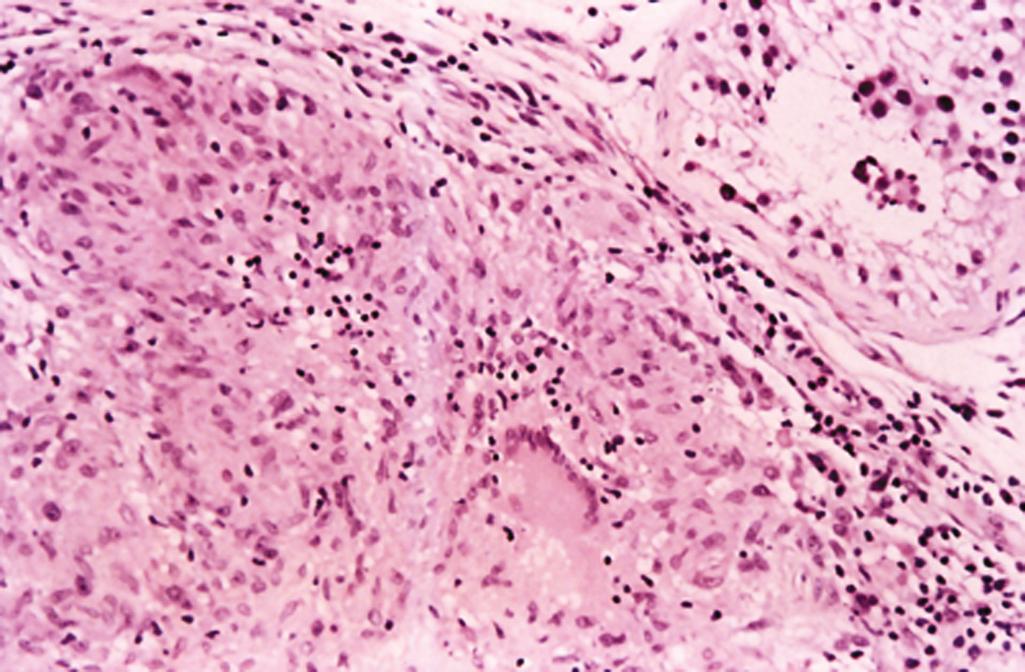
May be associated with a florid granulomatous reaction, but diagnostic foci of seminoma are at least focally present
Germ cell neoplasia in situ seen in residual seminiferous tubules
Placental alkaline phosphatase (PLAP), OCT3/4, and CD117 immunopositivity seen in seminoma cells
Diagnostic Michaelis-Gutmann bodies readily demonstrated by iron or calcium stain
Often associated with chronic E. coli infection
Healing infection typically shows prominent granulation tissue and fibrosis
Tuberculosis may involve the testes; more common in underdeveloped countries or immunocompromised patients
Typically presents with testicular enlargement with or without tenderness
Often associated with chronic bacterial infections, particularly E. coli
Rarely seen in children
Testicular enlargement with focal areas of firm, tan-yellow tissue ( Figure 11.13A )
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here