Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Magnetic resonance spectroscopy (MRS) capability is available in most clinical magnetic resonance (MR) scanners. The additional information from MRS allows the assessment of cellular metabolism noninvasively. For the brain in particular, it has been proved that MRS of protons or hydrogen (1H MRS) provides additional clinically relevant information for several disease processes such as brain tumors, metabolic disorders, and systemic diseases, and it is the most accessible method for studying and monitoring patients with neurometabolic disorders ( Table 25.1 ).
| Tumors | New tumor |
| Evaluate progression | |
| Tumor vs. encephalitis/other lesions | |
| Hypoxic ischemic injury | Patients at risk for cerebral malperfusion (congenital heart defects—status, postoperative) |
| Status, postoperative cardiac arrest, apneic episodes | |
| Trauma, nonaccidental trauma | |
| Birth asphyxia | |
| Other | New seizures, worsening seizures |
| Unknown neurologic condition, altered mental state, developmental delay, global hypotonia | |
| Metabolic disorders, phenylketonuria | |
| Leukodystrophies—monitoring | |
| Liver problems/failure—preliver transplant |
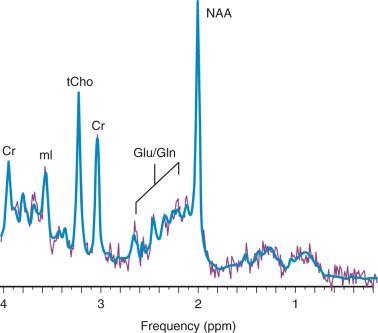
In MR imaging (MRI), the signal from hydrogen nuclei in water molecules is used to create images or anatomic maps. In contrast, 1H MRS analyzes the signal of protons attached to other molecules. The output of MRS is a collection of peaks at different radiofrequencies representing proton nuclei in different chemical environments, that is, the spectrum (typical MR spectra of normal occipital gray matter is shown in Fig. 25.1 ). MRS can measure a variety of metabolites ( Table 25.2 ). The x axis, or chemical shift axis, is a measure of the frequency shift of a proton relative to a universally fixed reference substance (tetramethylsilane at 0 parts per million [ppm]). In spectra in vivo, the protons of water (usually not shown) resonate at 4.7 ppm. The ppm scale has been selected instead of Hertz (Hz = sec −1 ) because it is independent of the magnetic field strength. The y axis is a measure of the signal intensity, which is proportional to the concentration of the metabolite or chemical.
| Long Name | Role, Regulation, Location | Altered in Magnetic Resonance Spectrum When: | |
|---|---|---|---|
| NAA | N-acetylaspartate | Synthesized in neurons, diffuses along axons, broken down in oligodendrocytes, high in neurons and axons | Nonspecific but quantitative marker for neuronal/axonal damage |
| Cr | Creatine (creatine + phosphocreatine) | Synthesized in liver; functions as “battery” to replenish adenosine triphosphate levels | Abnormal in diseases of creatine synthesis, absent in cells that lack creatine kinase |
| tCho | Choline-containing metabolites | Involved in membrane synthesis and breakdown, osmolyte (GPC) | High in proliferative tumors, gliosis, leukodystrophies, low in hypoosmotic conditions, after radiation therapy |
| mI | Myo-inositol | High in astrocytes, osmolyte, sugar-like molecule, involved with phosphatidyl inositol (membrane lipid) metabolism | High in glial-based tumors (but low in glioblastoma multiforme), adrenoleukodystrophy, depleted in hepatic encephalopathy, low in other encephalitis (infections), liver disease |
| Glu | Glutamate | Excitatory neurotransmitter, high in neurons | High in seizures? Low in hepatic encephalopathy |
| Gln | Glutamine | Glutamate detoxification, osmolyte, high in glial cells, ammonia detoxification | High in hepatic encephalopathy, encephalitis (infections), ischemic-hypoxic injury |
| Lac | Lactate | End product of glycolysis, accumulates in cystic necrotic tissue, anaerobic metabolism indicator | Often high in tumors, (secondary) hypoxic-ischemic injury, mitochondrial disorders |
| Lip | Lipids | Membrane degeneration marker, necrosis marker | Aggressive tumors, infections, diseases associated with membrane breakdown |
| Glc | Glucose | Principle substrate for energy metabolism | Diabetes, disrupted energy metabolism |
| Tau | Taurine | Membrane stabilization | Elevated in medulloblastoma and possibly other primitive tumors |
The most prominent peak of the 1H spectrum is the resonance at 2.0 ppm from three equivalent protons of the acetyl group of the N-acetylaspartate (NAA) molecule (see Fig. 25.1 ). The role of NAA and its regulation in vivo are not well understood. In the normal brain, NAA is synthesized in neurons, diffuses along axons, and is broken down in oligodendrocytes. NAA is present in high concentrations only in normal neurons and axons, and from an MRS perspective, it is a marker of viability for mature neurons and axons. The concentrations of NAA increase after birth, becoming the dominant peak around 6 months. Proton spectra of any disease associated with neuronal or axonal loss will exhibit a reduction of NAA. Brain NAA increases rapidly as the brain matures, peaks at approximately 10 to 15 years, and then decreases slightly over time as the number of neurons and axons declines even in the normal brain.
The next prominent peak at 3.2 ppm is commonly referred to as choline or trimethylamines . Choline is a complex peak comprising several metabolites that contain choline, and therefore the term total choline (tCho) is used in this chapter. Compounds that contain choline are involved in the synthesis and breakdown of phosphatidylcholine (lecithin). Phosphatidylcholine is the major phospholipid component of eukaryotic cells, accounting for approximately 60% of total cellular phospholipids.
The second tallest peak in occipital gray matter spectra is creatine (Cr) at 3.0 ppm. For normal brain tissue, the Cr peak comprises contributions from free Cr and phosphocreatine in approximately equal proportions. Phosphocreatine is in rapid chemical exchange with free Cr and is used to replenish adenosine triphosphate (ATP) levels, if required. Like NAA, Cr levels also are low in the newborn.
tCho, Cr, and NAA can be detected readily and quantified in long echo time (TE) MRS. Short TE acquisition methods are necessary for reliable quantitation of myo-inositol (mI), which is a sugar-like molecule that resonates at 3.6 ppm in the proton spectrum. It has been identified as a marker for astrocytes and is an osmolyte. mI also is involved in the metabolism of phosphatidyl inositol, a membrane phospholipid. Similar to choline, mI is altered in response to alteration of membrane metabolism or membrane damage. Both tCho and mI are high in the newborn brain but decrease rapidly to normal levels within the first 12 to 24 months after birth.
Lactate (1.33 ppm) indicates anaerobic metabolism. Lactate is the product of anaerobic glycolysis and increases when subsequent oxidation of lactate in the tricarboxylic acid cycle is impaired (for example, by lack of oxygen or mitochondrial disorders). With the exception of healthy-term neonates who may have trace amounts of lactate in the brain in the first days of life (lactate/choline <0.15), significantly elevated lactate concentrations is indicative of pathologic tissue. Lactate is typically elevated in brain infarcts, contusions, infections, tumor necrosis, metabolic disorders, and cysts. Lactate (1.33 ppm) can be differentiated from other lipids and macromolecules (0.9–1.3 ppm) using TE = 144 ms that causes the lactate peak to reverse ( Fig. 25.2 ).
![Figure 25.2, Comparison of magnetic resonance spectroscopy (MRS) of neonatal hypoxic-ishemic encephalopathy (acquired at multiple echo times [TEs]) with neonatal metabolic disease. Figure 25.2, Comparison of magnetic resonance spectroscopy (MRS) of neonatal hypoxic-ishemic encephalopathy (acquired at multiple echo times [TEs]) with neonatal metabolic disease.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MagneticResonanceSpectroscopyandPositronEmissionTomography/1_3s20B9780323497480000253.jpg)
The protons of the methyl groups (-CH 3 ) of lipid molecules resonate at 0.9 ppm, whereas protons of the methylene groups (-CH 2 -) resonate at 1.3 ppm in the 1 H spectrum. Both resonances are broad and also may comprise contributions from other macromolecules. In normal tissue, the concentration of free lipids is small, and very little signal should be present in this part of the spectrum. Lipid signals increase upon breakdown of cell membranes and release of fatty acids, therefore lipids are marker of severity of brain imaging. NAA and lactate can be detected using either short echo (35 ms) or long echo (144 or 288 ms) time. MI, glutamate/glutamine, and lipids can be detected only during use of the short echo technique (see Fig. 25.2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here