Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Survival at birth depends upon the lung attaining an adequate size and degree of structural maturity during fetal life. This chapter deals with mechanisms underlying normal and impaired lung growth and lung maturation before birth.
The airways of the fetal lung contain a liquid that is actively secreted by the epithelium; this ‘lung liquid’ causes the lung to develop in an expanded state, which is necessary for normal lung growth and maturation.
The degree of lung expansion in a fetus is determined by the lung’s physical environment, including intrathoracic space, fetal breathing movements (FBMs) and amniotic fluid volume. Mechanical stress in lung tissue stimulates gene networks, leading to tissue growth and differentiation. The long-term absence of the physiological stretch stimulus leads to lung hypoplasia.
Clearance of lung liquid begins with the onset of labour caused by (i) imposed fetal postural changes that cause loss of lung liquid via the nose and mouth and (ii) active reabsorption across the lung epithelium. Luminal liquid remaining after birth is cleared because of transpulmonary pressure gradients generated by inspiration.
Pulmonary blood flow (PBF) is generally low during fetal life but can increase with FBMs. At birth, pulmonary vascular resistance decreases markedly, thereby permitting increased blood flow through the lungs, which is necessary for adequate gas exchange.
Lung aeration at birth underpins the cardiovascular transition at birth, including the marked increase in PBF. With the loss of umbilical venous return at birth, the increase in pulmonary venous return takes over the critical role of supplying preload for the left ventricle.
Maturation of the lung in preparation for birth involves extracellular matrix remodelling, alveolar epithelial cell differentiation and surfactant production. These changes are driven by mechanical stress in lung tissue and corticosteroid signalling.
In fetal life, the lungs play no role in gas exchange, but at birth, they must immediately take over from the placenta the critical role of gas exchange. This transition is normally uneventful, which is remarkable given that before birth, the lungs are liquid filled with a low blood flow. For the lung to function as an organ of gas exchange at birth, it must cease the secretion of fetal lung liquid, and the airways must be cleared of luminal liquid; the lungs must produce adequate amounts of surfactant; and pulmonary vascular resistance (PVR) must be reduced, allowing them to receive the entire output of the right ventricle. During normal fetal development, the lung becomes progressively prepared for these dramatic changes in physiology at birth. However, if lung growth or maturation in utero is impaired or if an infant is born before term, the newborn infant may develop respiratory distress syndrome (RDS). This chapter focuses on the processes controlling prenatal lung growth and maturation and highlights the physiological changes that underpin the transition to newborn life. Some of the more common respiratory complications in neonates and their fetal origins are discussed together with strategies for their treatment.
Pulmonary morphologists recognise five or six major stages in human lung development ( Table 11.1 ).
| Stage | Gestational Age | Major Events |
|---|---|---|
| Embryonic | 4–7 wk | Appearance of ventral bud in foregut. Epithelial tube branches and grows into surrounding mesenchyme. Vascular connections formed. |
| Pseudoglandular | 5–17 wk | Development of bronchial tree, paralleled by formation of vascular tree. Lung periphery contains parenchymal precursors. |
| Canalicular | 16–26 wk | Addition of further generations of airways and vascular tree. Differentiation of type I and type II epithelial cells. Formation of thin air–blood barrier. Start of surfactant production. |
| Saccular stage | 25–40 wk (term) | Formation of additional airway generations. Dilation of prospective gas-exchanging airspaces. Maturation of surfactant system. |
| Alveolar stage | 36 wk–18 mo | Start of alveolar formation by outgrowth of secondary septa. |
| Microvascular maturation | Birth–3 yr | Change from double- to single-capillary network. Reduction in interstitial tissue mass; fusion of capillaries; preferential growth of single-layered capillary network areas. |
The lung first appears as an outgrowth of the primitive foregut (i.e., endodermal tissue) at 22 to 26 days postconception. This bud divides to form the left and right bronchi, which then undergo dichotomous branching to form the major units of the bronchial tree. During early embryonic development, epithelial cells that are endodermal in origin form the developing ‘airways’ and grow into the surrounding tissue, which is derived from splanchnic mesoderm. This mesodermal tissue gives rise to the mesenchymal cells that ultimately form the nonepithelial structures of the lung, including blood and lymph vessels, airway cartilage and smooth muscle, fibrous tissue and other components of the lung parenchyma.
During the pseudoglandular stage, the lung resembles a typical exocrine gland. The major bronchi and associated functional units of the lung (i.e., acini) progressively form, accompanied by branches of the pulmonary arterial tree. As a result, each major ‘airway’ is accompanied by a branch of the pulmonary artery. The formation of each acinus (respiratory unit) results from repeated branching of the distal extremities of blind-ending tubes or ‘airways’ composed of epithelial cells ( Fig. 11.1 ). This process of branching (branching morphogenesis) is induced by airway epithelial cells interacting with adjacent mesenchymal cells, which are supplied by a loose network of capillaries (see Fig. 11.1 ). Airway epithelial cells gradually differentiate (in a centrifugal direction) into specific cell types: ciliated cells (by 11–13 weeks), goblet cells and mucous glands.
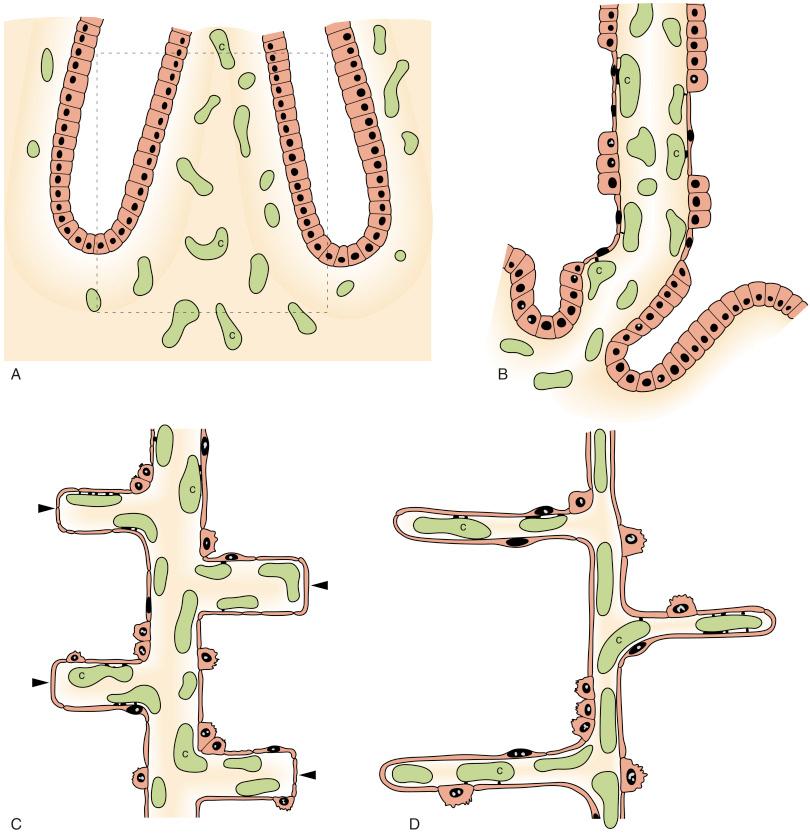
During the canalicular stage, the airways widen and lengthen, and mesenchymal tissue surrounding the distal airways becomes attenuated (see Fig. 11.1 ). This process (canalisation) results in a substantial increase in the ratio of lumen volume to tissue volume. During the canalicular stage, the functional units of the lung are formed, consisting of terminal bronchioles ending with expansions that subsequently form terminal sacs (primitive alveoli). A network of blood capillaries develops around the terminal air sacs, increasing the proximity of blood capillaries to the epithelial surface; this marks the beginning of the air–blood interface that is required for effective gas exchange (see Fig. 11.1 ). Thus the late canalicular stage is the earliest at which the lungs can support independent life.
The terminal sac, or saccular, stage of lung development sees a progressive enlargement of the distal ‘air spaces’. This enlargement results from further attenuation of perisaccular mesenchymal tissue and leads to a further increase in luminal volume relative to lung tissue volume. During the terminal sac stage, the development of secondary septa begins; these outgrowths from primary septa will eventually subdivide the terminal sac into multiple alveoli (see Fig. 11.1 ). The primitive primary septa, which separate adjacent saccules, are thicker than secondary septa and contain a double capillary network rather than the single capillary layer of the mature alveolus. Elastic fibres are formed by myofibroblasts within secondary septa and are deposited at their tips, thereby contributing to the inherent elastic (recoil) properties of the lung. The epithelial cells become differentiated into type I and type II epithelial cells. As a result of these structural changes, the separation between luminal ‘air’ and capillary blood becomes smaller, thereby enhancing the ability of the lung to exchange respiratory gases after birth.
During the alveolar stage, terminal sacs become subdivided by the outgrowth of secondary septa from the primary septa to form alveoli. Initially, these alveoli resemble shallow cups, but they deepen because of elongation of the secondary septa. The alveolar walls and the epithelial cells lining them become thinner, leading to the formation of definitive alveoli. The mean alveolar diameter increases greatly, from about 30 μm at 30 weeks to about 150 μm at 40 weeks. The final stage of alveolar maturation involves the restructuring of the capillary network, such that the more primitive double capillary network lining the terminal sacs and alveoli is transformed into a single layer of capillaries (see Fig. 11.1 ); this marks the existence of definitive alveoli.
By the time of term birth, the human lung contains 20 to 50 million alveoli. An adult human lung contains approximately 300 million alveoli, indicating that most are formed postnatally. The alveolar stage of lung development is thought to continue for at least 1 to 2 years after birth, although some alveoli may continue to be formed later in life. In species born at an earlier stage of development (e.g., rats and mice), the alveolar stage begins after birth; therefore, at birth, gas exchange occurs across terminal sacs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here