Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
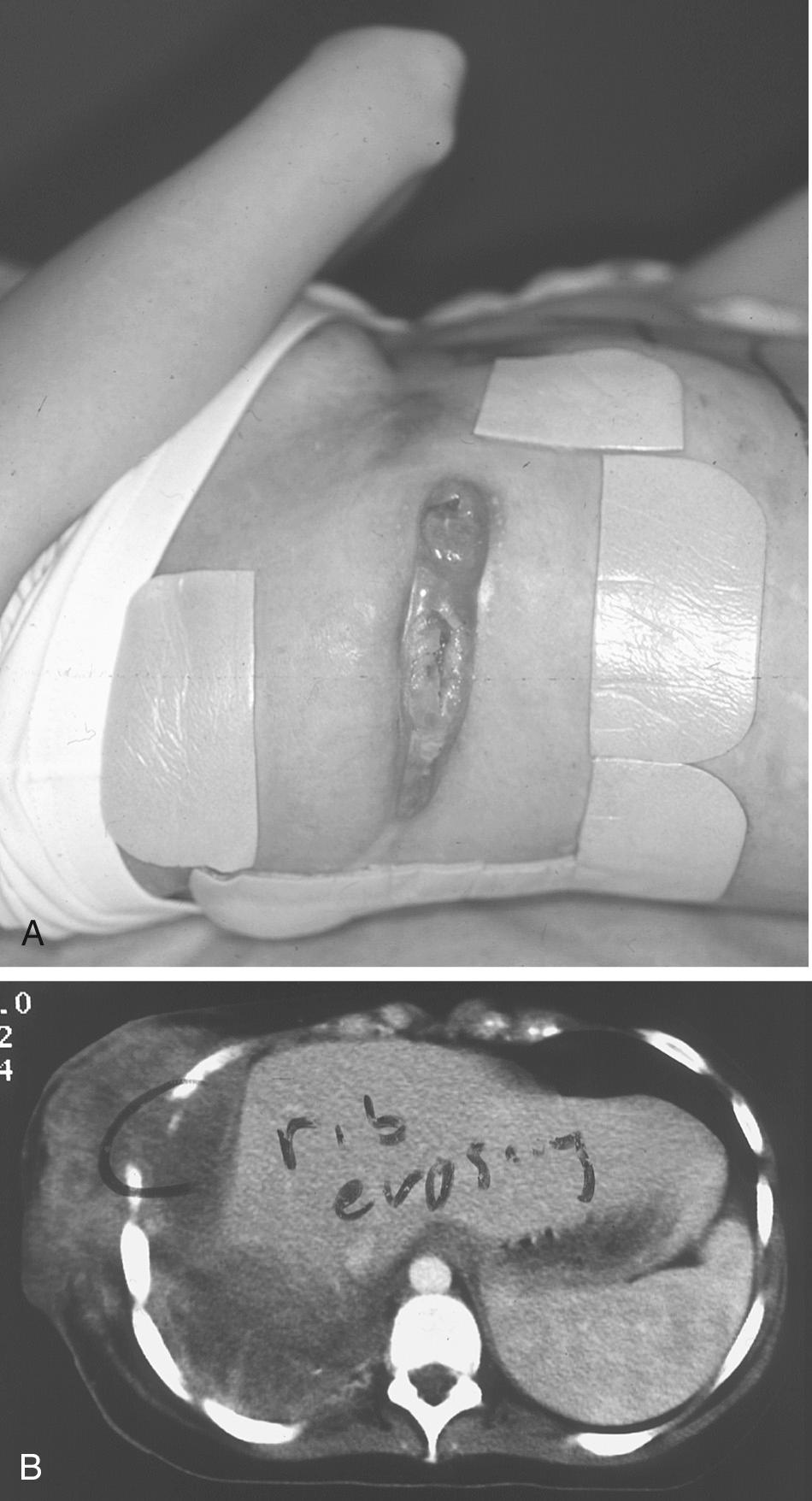
Lung cancer continues to be the leading cause of cancer-related deaths in both men and women. Over 75% of non–small cell lung cancer (NSCLC) is advanced in stage at presentation, with extensive locoregional disease or distant metastasis. Most resectable lung cancers are confined to the pulmonary parenchyma, but 5% to 8% extend beyond the lungs and invade the pleura, soft tissues, or osseous structures of the chest wall. Chest wall invasion for surgical staging (T3) is defined as any tumor involvement into or beyond the parietal pleura. Pathologists can further describe these tumors by their depth of chest wall invasion, but this is infrequently reported in this subset of T3 patients.
Historically, chest wall invasion by a tumor of any histology was considered unresectable. Early surgical experience demonstrated that the surgical violation of the parietal pleura resulted in a sucking chest wound with immediate pulmonary collapse, often leading to the rapid demise of the patient. Dr. M. Michellau presented to the Institute of France in Paris in March of 1818 with a fungating mass protruding from his left chest wall. Dr. Richerand proposed a resection of the involved rib and pleura, an unprecedented operation at that time. On March 31, 1818, Dr. Richerand resected the left sixth and seventh ribs of Dr. Michellau with an unexpected occurrence of acute respiratory distress on entrance into the chest cavity. The patient was saved by covering the aperture with a linen cloth plastered with cerate. Despite a rocky postoperative course, Dr. Michellau survived and returned home 27 days postoperatively. The pathology of the lesion is not known, but a primary rib malignancy is suspected.
In the summer of 1883, a brilliant young surgeon, H. M. Block, in what was then called Danzig, East Prussia (now Gdansk, Poland), carried out the first planned pulmonary resection. Dr. Block had performed successful open chest surgery on experimental animals and was eager to apply his experience to humans. He chose a young female relative with a diagnosis of bilateral pulmonary tuberculosis and performed a thoracotomy to resect her diseased lung. Although the details of the operation are not known, we do know that the operation had a tragic end. A few days later, the short, brilliant career of Dr. Block ended with a self-inflicted gunshot wound to the head.
Murphy described his experiments and clinical experiences with open pneumothorax during his address to the American Medical Association in 1898. Parham, in 1898, was the first in the United States to report resection of a bony chest wall tumor involving three ribs. A controlled pneumothorax with soft tissue coverage was created. This patient survived, although many others who followed did not.
The difficulties of operating with an opened pleural space in a spontaneously breathing patient were all too apparent to the surgeons of those times. Working without adequate control of the airway and ventilator support was difficult, and the patient quickly deteriorated once the chest was open. Many ideas were investigated to overcome these deficiencies of the anesthetic techniques.
Major surgical and anesthesia advances were introduced in 1904 at the German Surgical Congress in Berlin. Two techniques, designed to surmount the open chest problem, were proposed. Ferdinand Sauerbruch, from the surgical clinic of von Mickulykz at the University of Breslau, introduced his method of unterdruck (low-pressure) ventilation. Lung expansion was maintained after thoracotomy by keeping an experimental animal's entire body inside a negative-pressure chamber (at −15 cm H 2 O) while the head remained outside the chamber with the anesthesiologist. Brauer described the benefits of uberdruck (high-pressure) anesthesia, in which the lung was kept expanded by placing the patient's head in a glass positive-pressure chamber.
Surprisingly, the unterdruck method initially was the preferred technique. Sauerbruch and von Mickulykz built a negative-pressure operating room large enough to accommodate an entire surgical team, in which successful thoracic operations were carried out. These rooms continued to be built as late as 1918 in Munich by Sauerbruch, making this approach the favored method in Germany throughout the 1930s. Sauerbruch's ideas and methods so dominated his associates and contemporaries that little progress in other anesthetic techniques issued from Germany during this era.
Major progress, however, had already begun and would continue in France, England, and the United States during roughly the same period. The use of positive-pressure ventilation of the lungs was slowly being developed. Reliable delivery of positive pressure to the lungs was possible only by direct intubation of the trachea, and at that time, a tracheotomy was the only technique for tracheal intubation. Most surgeons were unwilling to perform a tracheotomy simply to deliver an anesthetic under positive pressure. The first systematic use of intubation through the mouth using bellows to inflate the lung was by the Frenchman DePaul, who intubated and resuscitated neonates in the mid-1800s. Other French surgeons, Tuffier, Quenu, and Doyen, and Milton in Egypt, also used positive pressure during thoracotomies in the last few years of the 19th century.
In the late 1800s, two physicians from New York, Joseph O'Dwyer and George Fell, described intubation techniques and positive-pressure ventilation. Dr. O'Dwyer developed a practical method of endotracheal intubation for the treatment of diphtheria, which was applied in thousands of cases and resulted in a gratifying decrease in the mortality rate of that dreaded disease. Dr. Fell also used a crude device to maintain ventilation in patients suffering from drug overdoses. In New Orleans, Parham and Matas used the combined Fell-O'Dwyer apparatus in 1886 to administer positive-pressure surgical anesthesia.
The use of positive-pressure ventilation identified the need for cuffed endotracheal tubes for the reliable delivery of anesthesia to the lungs. Eisenmenger first described a cuffed endotracheal tube in 1893. Placement of such tubes was facilitated by Kirstein, who introduced direct laryngoscopy in 1895 for safe, reliable placement of endotracheal tubes in the trachea. In 1907, Chevalier Jackson improved the laryngoscope and produced the instrument that is still in use today and bears his name. A practical endotracheal tube design for general use was introduced by Guedel in 1928, and its use became widespread starting in the 1930s. In 1938, the first operative use of ventilators was made with the Freckner Spiropulsator, developed in Sweden. In 1942, Griffith and Johnson, in Montreal, Canada, introduced curare to facilitate intraoperative controlled ventilation.
With these international advances in intubation techniques and airway management, positive-pressure anesthesia slowly became a clinical reality during the last few years of the 19th century and the first few decades of the 20th century. As a direct result, surgeons were now willing to transgress the parietal pleura to address the complex pathologies of the thoracic cavity with increasing frequency. From 1904 to 1929, surgeons began to specialize and perform a series of pulmonary resections, and by 1929, thoracic surgery had become an established specialty. Most early lung resections and thoracoplasties were performed because of infections. Surgical morbidity and mortality rates were horrifying at first, and only the bravest patients and the most resilient surgeons chose to continue to work in the field. Sepsis was the predominant cause of death and was correlated mainly with an open pleural cavity.
In 1947, Coleman reported long-term survival after en bloc excision of the chest wall with pulmonary resection. Concurrently, significant strides in chest wall reconstructive techniques were occurring through the use of fascia lata grafts, autogenous rib grafts, large cutaneous flaps, and latissimus dorsi muscle flaps, as described by Campbell in 1950 and Grillo et al in 1966. Over the past 40 years, we have witnessed further refinements in surgical procedures, the use of prophylactic antibiotics, improved anesthesia delivery and monitoring, and the implementation and use of critical care units for postoperative ventilation. Today these advances permit the safe and effective resection of locally advanced lung cancer with extensive chest wall involvement on a routine basis.
Pancoast tumors comprise a distinct surgical entity and are discussed separately.
Patients with lung cancer are usually 50 to 70 years old; lung cancer is rarely seen in patients younger than 30 years, although with the ongoing epidemic of children and young teenage smokers, advanced lung cancer can be seen even in these younger age groups ( Fig. 20-1 ). Lung cancer with chest wall invasion most typically presents in patients in their seventh decade, with a median age of 64 to 66 years (range, 38-93 years). Overall, lung cancer incidence and mortality continue to be disproportionately higher in men than in women, although the gap is narrowing. Lung cancer with chest wall invasion has an overwhelming predominance in men; women represent only 10% to 30% of patients in several recent studies. Current or previous smoking history is elicited in approximately two thirds of patients, with an average 50-pack-year history per patient.
The lung parenchyma has no sensory nerve fibers, which accounts for the often late clinical presentation of most bronchogenic tumors. Most patients have presenting symptoms related to compression, invasion, or obstruction of the lung parenchyma or airways, or invasion of the chest wall or mediastinal structures. Metastasis to distant organs, including neurologic symptoms and bone pain, unfortunately are common.
Patients whose lung cancer has invaded the chest wall have similar presenting symptoms, including chest pain (40%-60%), cough (14%), recurrent lower respiratory tract infection (10%-25%), weight loss (10%-18%), hemoptysis (12%), and dyspnea (11%). As many as 25% of patients can be asymptomatic ( Table 20-1 ).
| Presenting Symptom | Percentage |
|---|---|
| Chest wall pain | 40%-60% |
| Recurrent lower respiratory tract infection | 10%-25% |
| Weight loss | 10%-18% |
| Hemoptysis | 12% |
| Dyspnea | 11% |
| Cough | 11% |
| Asymptomatic | 25% |
The right lung has a slight predominance in location of lung cancers, both in general and for those with chest wall invasion. Okada and colleagues described a marked predilection for the upper lobes in his series of lung cancers that invade the chest wall, although not all series confirm this finding. Lung cancers in general have a slight predilection for upper lobes rather than lower lobes, which theoretically may be related to the relative increase in ventilation (and associated carcinogens) to the upper portions of the lungs.
Squamous cell carcinoma is the classic smoking-related tumor, and for many years it was the most common histology. In recent years, however, adenocarcinoma has overtaken squamous cell carcinoma as the most common lung cancer worldwide. Several series of patients with lung cancer that invades the chest wall have demonstrated that the histologic diagnosis of squamous cell carcinoma remains the most common, followed closely by adenocarcinoma; large cell carcinoma and adenosquamous carcinoma comprise fewer of these tumors. Average tumor diameter in these patients, by computed tomography (CT) measurements, was 6.5 cm; tumors ranged from 2 to 18 cm in maximum diameter.
Chest roentgenography is useful for the identification of parenchymal lesions, although it has poor specificity and sensitivity for detecting chest wall involvement. Rib destruction is a reliable indicator of chest wall invasion, although it is identified on routine chest x-rays in only a fraction of cases. Tissue diagnosis of malignancy in these T3 lesions usually is obtained by transthoracic needle aspiration by our interventional radiologists.
Peripheral lung lesions with the suggestion of chest wall involvement may require additional radiographic testing to confirm invasion. These radiographic techniques include CT scans, nuclear medicine (scintigraphic) bone scans, magnetic resonance imaging (MRI), and positron emission tomography (PET) scans. Although gross tumor involvement of the chest wall is easily diagnosed with these radiographic modalities, confirmation of isolated parietal or mediastinal pleural invasion is more difficult and often unreliable.
The use of CT scans has greatly increased the precision of tumor localization, allowed accurate evaluation of contiguous organ involvement, improved assessment of lymph nodes, and improved the identification of pulmonary metastasis. A CT scan is excellent for assessing rib destruction and intercostal muscle tumor extension but is relatively inaccurate for invasion limited to the parietal or mediastinal pleura. Shirakawa and associates identified patients who had parietal pleural invasion by using inspiratory/expiratory CT scans. They demonstrated that a respiratory phase shift of greater than half of a vertebral body height in middle and lower lobe tumors reliably predicted the absence of parietal pleural invasion. The accuracy and negative predictive value were 90% and 86%, respectively, in tumors located in the lower and middle lobes. For upper lobe tumors, however, the respiratory phase shift did not correlate with operative findings regardless of whether invasion was present. This discrepancy is a result of the minimal normal respiratory phase shift of these lung fields when the patients were studied in the supine position.
MRI has the advantages of multiplanar imaging and high differential signal intensity, which are invaluable for determining vascular invasion and spinal involvement. Conventional MRI, unfortunately, is just as limited as CT scans for evaluation of parietal and mediastinal pleural invasion. Kodalli and associates used breath-hold inspiration and expiration MRI to assess parietal pleural invasion. Pleural invasion was excluded when tumor displacement exceeded 5 mm in reference to chest wall structures or relevant mediastinal structures (e.g., the aortic arch).
This study identified 100% sensitivity and specificity for pleural invasion for tumors located in the middle lobe and basilar segments of the lower lobes. Studies of upper lobe tumors and those located in the apical segments of the lower lobes demonstrated a positive predictive value of only 40% but a negative predictive value of 100%. The superiority of MRI to CT scans lies in its ability to assess lung and diaphragm movement in a coronal plane. Insufficient respiratory motion is evident by less than 1-cm movement of the diaphragm on coronal images, and necessary scans could be repeated by asking the patient to take a deeper breath. Although more recent reports have also commented on high sensitivity, specificity, and accuracy of respiratory dynamic cine MRI in evaluating lesions that do not have clearly demonstrated invasion on standard CT or MRI, this modality is not widely available outside of specialized centers.
Ultrasonography (US) has been compared with CT for detection of chest wall involvement. In a series from 2008, 90 patients with suspicion for chest wall involvement were evaluated preoperatively with CT and US. Their ultrasound criteria for determining chest wall invasion were any two of the following: (1) tumor ingrowth seen into the chest wall, (2) interruption of the pleural reflection, (3) invasion of the ribs, (4) impairment of movement with respiration. Based on these criteria, US was deemed more sensitive than CT (89% vs. 42%) with similar specificity (95% vs. 100%). Perhaps the real-time imaging of ultrasound during respiration leads to more accurate imaging data.
More invasive methods have been used for detection of parietal pleural invasion, including use of expiratory dynamic CT scans after the introduction of a diagnostic pneumothorax. Lack of invasion was diagnosed on the basis of appearance of an air space between the mass and adjacent structures. Sensitivity was 100% in both studies for chest wall invasion, although sensitivity dropped to 76% in cases of mediastinal invasion.
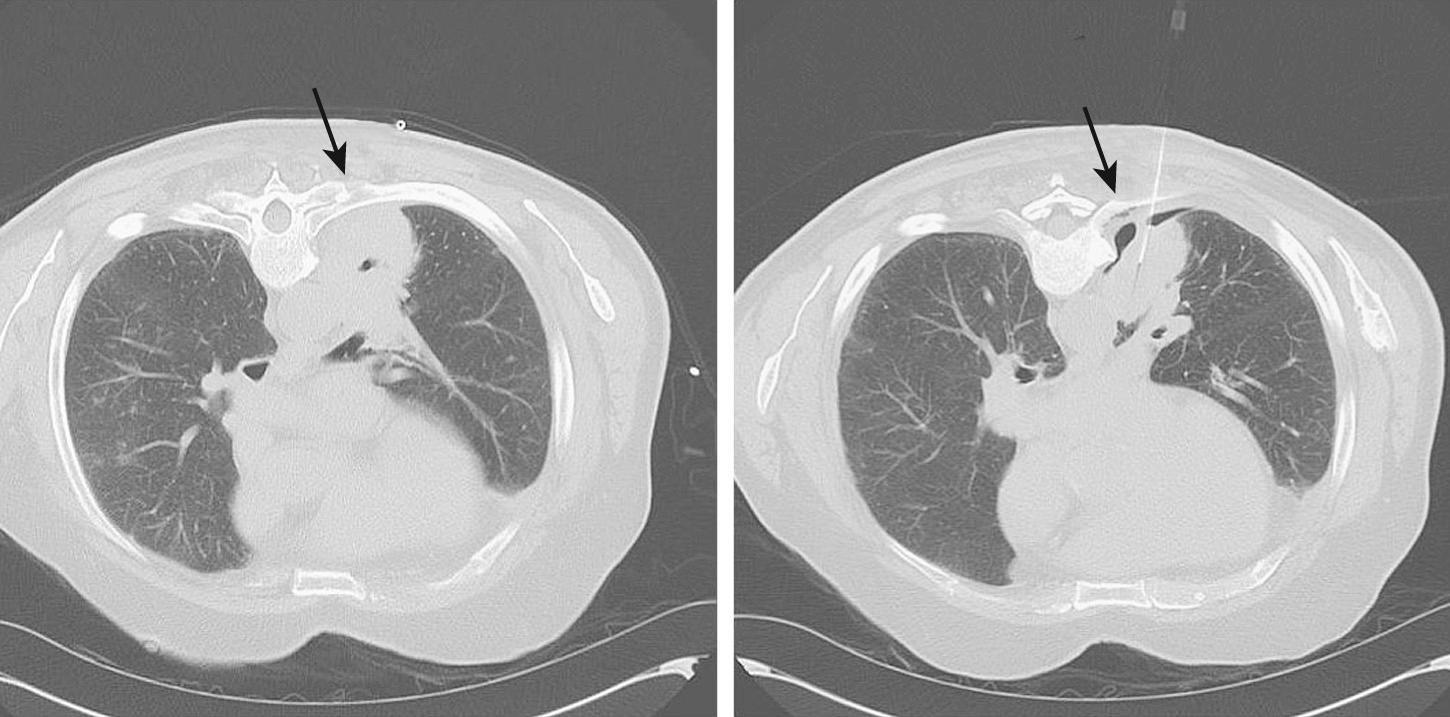
Specificity for tumor involvement was 80% in a study by Watanabe and coworkers. Benign pleural adhesions caused false-positive results. Complications were reported as mild and included chest pain, shortness of breath, and subcutaneous emphysema. Despite these results from a variety of imaging techniques, we do not feel that these tests are warranted because they potentially subject high-risk patients to a pneumothorax and ultimately are unlikely to alter the planned operative procedure. In some cases, review of the CT images obtained during transthoracic needle biopsy can reveal minimal pneumothoraces incidentally obtained as a result of biopsy. Often, these images will show clear separation from the chest wall ( Fig. 20-2 ).
For most patients, preoperative assessment of chest wall involvement is not critical, because it does not often alter resectability or treatment strategy. However, for the marginal patient, who may be borderline for toleration of lung resection, identifying chest wall invasion, and thus the necessity for combined pulmonary and chest wall resection, may lead the clinician to pursue definitive nonoperative treatment with chemoradiotherapy.
Accurate staging of NSCLC is critical for effective clinical management. Standard staging includes a complete history and physical examination, laboratory tests (including calcium, alkaline phosphatase, and liver function tests), chest x-ray, CT scan of the chest and upper abdomen to include the adrenal glands, and full body PET scan. In addition, brain MRI is obtained when a patient has neurologic symptoms or when the lesion is locally advanced or nodal disease is suspected.
Surgical-pathologic staging is performed according to the International Staging System for Lung Cancer, using information about the primary tumor (T), nodal status (N), and distant metastasis (M) ( Table 20-2 ). “T” status denotes characteristics of the primary tumor, including size and local aggressiveness. Chest wall invasion denotes at least T3 status (for a full description of TNM staging, refer to the most current AJCC Lung Cancer Staging guidelines). “Chest wall involvement,” however, includes a wide pathologic spectrum, from invasion of the parietal pleura only to full-thickness chest wall replacement by tumor.
| Stage 0 | Carcinoma in situ | |||||
| Stage IA | T1a,b | N0 | M0 | |||
| Stage IB | T2a | N0 | M0 | |||
| Stage IIA | T1a,b, T2a | N1 | M0 | T2b N0 M0 | ||
| Stage IIB | T2b | N1 | M0 | T3 | N0 M0 | |
| Stage IIIA | T3 | N1 | M0 | T1-3 | N2 M0 | T4 N0-1 M0 |
| Stage IIIB | T4 | N2 | M0 | Any T N3 M0 | ||
| Stage IV | Any T | Any N | M1a,b |
Nodal status (N) comprises the second feature of the staging system. Clinical evidence of ipsilateral hilar node involvement is classified as N1, which is not considered a contraindication for surgical resection. Ipsilateral adenopathy of the mediastinum increases the nodal status to N2. The most effective treatment modality for these patients is controversial and is discussed later. Spread of tumor to the contralateral mediastinal, contralateral hilar, or any scalene or supraclavicular nodal basins denotes an N3 status; patients with N3 disease generally are not considered surgical candidates.
Patients without evidence of distant metastatic disease are considered to have M0 disease, and they may be candidates for surgical resection. However, in patients with distant metastatic disease (M1), systemic treatment in the form of chemotherapy is currently the treatment of choice, often in combination with radiation therapy for local control.
In 1997 an important change was made to the TNM staging system regarding tumors with chest wall involvement. Before 1997 a NSCLC that was defined as T3 N0 M0 was classified as stage IIIA. Survival data of patients with these tumors revealed that their clinical course with surgery alone was more favorable than the clinical course of other patients with stage IIIA disease (i.e., those with hilar or mediastinal lymph node involvement; T3N1, T3N2, T1N2, or T2N2). Subsequently, T3 N0 M0 tumors had been downstaged to stage IIB with this revision.
Because the survival rate of lung cancer patients with chest wall involvement and nodal disease is significantly worse than that of patients with chest involvement alone, the clinical challenge for surgeons is to identify nodal involvement by noninvasive imaging or minimally invasive biopsy techniques before subjecting patients to extensive chest wall resections.
The importance of correct pretreatment staging cannot be overemphasized. Hilar and mediastinal lymph nodes can be assessed before surgery by using CT scans, MRI, and PET scans. Integrated CT-PET scans measure metabolic activity of tissues and can detect disease in otherwise normal size–appearing nodal tissue; however, the accuracy for subcentimeter nodules is reduced.
Despite advances in nuclear imaging techniques, mediastinoscopy remains the most sensitive and specific test for evaluating the mediastinal nodes and should be considered before undertaking any major chest wall resection. Another option available for staging the mediastinum is endobronchial ultrasound (EBUS)–guided fine-needle aspiration. EBUS involves the use of a specially designed bronchoscope with an ultrasound transducer at the tip that allows real-time image guided biopsy of nodes adjacent to the airway and can access some nodes at levels 10, 11, and 12. Sensitivity and specificity of EBUS have been reported to be higher than those of CT or PET and comparable to those of mediastinoscopy; in addition, it is less invasive. Because of the anatomic location, the preoperative assessment of N1 disease is more problematic via mediastinoscopy. Patients with T3N1 disease still should be considered for en bloc resections, and these patients have a better survival rate than those with N2 disease.
Flexible bronchoscopy is performed for transbronchial biopsies, brushings, and washings. Although it is unlikely that peripheral lung tumors involving the chest wall extend into the airway, it is the authors' practice to perform bronchoscopy immediately before resection to identify any unsuspected endobronchial disease and to assess the airway anatomy.
Bone scintigraphy scans can be used to detect occult bony metastases and confirm bony lesions in symptomatic patients. The vertebral column is the most commonly affected region for bone metastases. MRI is accepted as the most accurate imaging modality in detecting bone metastases within the vertebral column, and focal imaging can be guided by bone scan abnormalities or symptoms. Suspicious uptake in bones other than the vertebral column also requires further investigation, although MRI scans become less useful. False positives are seen in the bony thorax when a history of rib fractures or trauma is noted. False negatives can be appreciated when bone scans are correlated with PET scans. Positive lesions on PET scans, which are not seen on bone scans, may represent soft tissue metastasis. Durski and associates recommend that all patients be staged with a PET scan, and bone scans should be performed only if symptomatically indicated when PET scans are negative. In their series, the use of bone scans in addition to PET scans did not change the clinical stage of any of their patients, although it allowed more precise localization of skeletal abnormalities.
PET scans using F-fluorodeoxyglucose (FDG) are routinely used in addition to CT scans for both initial diagnosis and staging of NSCLC. One study suggests that obtaining both a PET and a CT scan is more cost-effective than performing a CT scan alone for staging. The reported sensitivity and specificity of PET for thoracic lymph node involvement are 70% to 100% and 81% to 100%, respectively. CT has sensitivity and specificity of 25% to 81% and 56% to 94%, respectively. The ACOSOG trial Z0050 evaluated the usefulness of PET for staging NSCLC. Their results support staging NSCLC patients with PET to reduce the rate of nontherapeutic thoracotomy but recommend confirming PET-positive mediastinal nodes with mediastinoscopy. In addition, their recommendations include using PET to guide tissue biopsy in the cases of suspected single-site distant metastases. Integrated PET-CT was compared with PET alone in a prospective blinded trial in which the same radiologist read an integrated scan and, at a later date, was asked to read the PET images only. Integrated PET-CT was shown to be more accurate at predicting T and N status of patients and was better at determining stage I and stage II disease. Integrated scans have become standard for preoperative evaluation of all our NSCLC patients.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here