Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The surgical approach in the management of patients with lung cancer continues to evolve and improve. Conventional surgical approaches (including standard posterolateral thoracotomy, muscle-sparing thoracotomy, trans-sternal thoracotomy, and median sternotomy) remain viable options for some patients with resectable lung cancer. However, minimally invasive procedures have increasingly gained acceptance as a standard surgical modality for early-stage lung cancer, with increasing application to more advanced disease, as a means of minimizing operative morbidity without sacrificing oncologic efficacy.
Minimally invasive procedures, using operative telescopes and video technology, are referred to synonymously as thoracoscopic procedures or video-assisted thoracic surgery (VATS). For clarity, the terms VATS and thoracoscopic refer to totally thoracoscopic approaches, where rib spreading is avoided and visualization depends on video monitors. A hybrid procedure, which employs rib spreading and direct visualization in addition to thoracoscopy, may be referred to as video-assisted thoracotomy . Thoracoscopy has been widely used diagnostically in the management of patients with lung cancer; thoracoscopic wedge resection to confirm malignancy prior to thoracotomy for anatomic resection is commonly performed. In addition, thoracoscopic therapeutic procedures, including pleurodesis for malignant pleural effusion and pericardial window for pericardial effusions, are also frequently performed. The application of thoracoscopic anatomic resections is no longer new and is increasingly used internationally. In the most recent analysis of the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database, thoracoscopic lobectomy constituted 45% of all lobectomies performed.
Thoracoscopic lobectomy is defined as the anatomic resection of an entire lobe of the lung, using a videoscope and an access incision (<8 cm), without the use of a mechanical retractor and without rib spreading. The anatomic resection includes individual dissection and stapling of the involved pulmonary vein, pulmonary artery, and bronchus and appropriate management of the mediastinal lymph nodes, as would be performed with thoracotomy. In selected patients, thoracoscopic anatomic segmentectomy may be performed, adhering to the same oncologic principles that guide resection at thoracotomy. Theoretical advantages to minimally invasive resection include reduced surgical trauma and inflammation, decreased postoperative pain, shorter chest tube duration, shorter length of stay, preserved pulmonary function, and numerous short-term and long-term outcomes.
The history of minimally invasive thoracic surgery began in 1910 when Jacobeus used a cystoscope to lyse adhesions to collapse the lung to treat tuberculosis. This technique was widely applied in the early part of the century but was largely abandoned after streptomycin was introduced in 1945. However, with the emergence of laparoscopic cholecystectomy, minimally invasive approaches were applied more widely. The first descriptions of the use of VATS for anatomic lobectomy were published in 1993 by Walker and colleagues. The first large single institution series, published in 2006, supported the safety and feasibility demonstrated in the Cancer and Leukemia Group B multi-institutional, prospective study.
In general, the indications for thoracoscopic lobectomy are similar to those for lobectomy using the open approach. Thus, the procedure is applied to patients with known or suspected lung cancer (clinical stage I-II) if the disease appears amenable to complete resection by lobectomy. Preoperative staging and patient selection for thoracoscopic lobectomy should be conducted as for conventional thoracotomy. With increasing focus on operative planning and experience with the VATS techniques, the indications for thoracoscopic lobectomy are evolving. Whereas initially a history of prior surgery, the presence of an endobronchial lesion, or even the administration of induction chemotherapy were once regarded as contraindications, the experience that has since been gained, together with improvements in instrumentation and thoracoscopic imaging, have now changed this situation in most hospitals with experience in VATS. As such, recent studies have shown that lobectomy by VATS in cases of lung cancer with prior chemotherapy can be carried out safely and effectively, without an increase in the rate of complications. And although endobronchial lesions were previously considered a contraindication to VATS resections, bronchoplastic procedures and pneumonectomy are now commonly performed minimally invasively.
Tumor size may preclude the option of thoracoscopic lobectomy in some patients, as some large specimens (tumors greater than 6 to 8 cm in diameter) may not be amenable to removal without rib spreading, possibly negating the benefit of minimal access surgery. However, no absolute size criteria have been applied. Although it is controversial, some have argued that the thoracoscopic approach may allow recruitment and resection of some patients considered medically inoperable, who could not undergo conventional thoracotomy. A study by Cattaneo and colleagues demonstrated improved tolerance of thoracoscopic lobectomy as compared with thoracotomy lobectomy in patients older than 70 years of age. Several studies have further demonstrated that VATS lobectomy is beneficial in reducing pulmonary complications in patients with poor preoperative pulmonary function. The minimal physiologic requirements for resection have not been agreed on; however, the selection of patients for thoracoscopic lobectomy must take into account that conversion to thoracotomy may be necessary.
Absolute contraindications to thoracoscopic lobectomy include the inability to achieve complete resection with lobectomy, T3 (chest wall) or T4 (local invasion) tumors, active N2 or N3 disease, and inability to achieve single-lung ventilation. Relative contraindications include tumors visualized in the lobar orifice at bronchoscopy (although successful thoracoscopic sleeve resection has been reported), the presence of complex, calcified benign hilar lymphadenopathy that would complicate vascular dissection, and prior thoracic irradiation. Prior thoracic surgery; T3 tumors that involve the pericardium, mediastinal pleura, or diaphragm; incomplete or absent fissures; and benign noncalcified mediastinal adenopathy should not be considered contraindications. Increasing experience has allowed successful thoracoscopic lobectomy after induction therapy, including for patients with stage IIIA (N2) disease. Finally, chest wall involvement would obviate thoracoscopic resection for most patients, but successful hybrid thoracoscopic lobectomy with en bloc chest wall resection has been demonstrated to be safe and feasible.
The efficacy of mediastinal lymph node dissection (MLND) has been questioned. Several studies have examined the extent of MLND by VATS versus open lobectomy. In one study by Kondo and coworkers, thoracotomy was performed for reassessment of lymph nodes following MLND using VATS and yielded few additional lymph nodes (mean = 1.3 lymph nodes, median 0 lymph nodes). Similarly, Sugi et al found no difference between the number of lymph nodes dissected among patients who underwent VATS (mean = 8.4 ± 1.0) versus patients who had open lobectomy (mean = 8.2 ± 1.5). More recently, a retrospective review by Watanabe and colleagues of 770 patients with cN0-pN2 non–small cell lung cancer who underwent VATS ( n = 450) or open lobectomy ( n = 320) examined the total number of lymph nodes, number of lymph node stations, and number of mediastinal nodes and mediastinal stations by VATS versus open lobectomy, and found no difference in any of these categories. Data from the recent American College of Surgeons Oncology Group Z0030 trial ( N = 752, VATS = 66, open = 686) has also confirmed the efficacy of MLND during VATS procedures by demonstrating a similar number of lymph nodes removed and lymph node stations assessed as compared with thoracotomy.
Other studies comparing the efficacy of a lymph node dissection of a VATS lobectomy with standard thoracotomy have demonstrated similar results. Nevertheless, it remains that some surgeons doubt the efficacy of VATS MLND. To date, few studies have disputed the efficacy of MLND by VATS, with one study by Denlinger and colleagues ( n = 79 VATS, n = 464 open) showing fewer lymph nodes sampled by VATS compared with thoracotomy (7.4 ± 0.6 versus 8.9 ± 0.2, P = 0.03) and fewer N2 nodes (2.5 ± 3.0 versus 3.7 ± 3.0, P = 0.004). In a recent study by D'Amico et al analyzing data from the National Comprehensive Cancer Network database with a more balanced number of VATS versus open procedures ( N = 388, n = 199 VATS, n = 189 open), VATS and thoracotomy were found to result in a similar number of mediastinal lymph nodes resected (median = 4 for both groups) and N2 nodes resected (median = 3 for both groups). The percentage of patients with at least three mediastinal lymph node stations assessed, as recommended by the current guidelines, was also similar in the VATS and open group (66% VATS versus 58% open, P = 0.12).
After bronchoscopy and mediastinoscopy (when indicated), single-lung anesthesia is established using a dual-lumen endotracheal tube or bronchial blocker. The patient is positioned in a full lateral decubitus position with slight flexion of the table at the level of the hip, which provides splaying of the ribs to improve thoracoscopic access and exposure. Care must be taken to secure and pad the patient so that the risk of neurologic injury is minimized. Once the patient is positioned, the anesthesiologist should reconfirm the desired position of the endotracheal tube. Before sterile preparation and draping, the chest is marked for the placement of thoracoscopic incisions.
Port placement is a matter of surgeon preference. Most surgeons use three or four incisions, although lobectomy can usually be accomplished using only two incisions. Using this strategy, the first incision, either a 5-mm or 10-mm port access used predominantly for the thoracoscope, is placed in the seventh or eighth intercostal space in the mid-axillary line. The location of this incision is chosen so that it does not compete with the anterior incision, yet it still provides anterior and superior visualization of the hilum. A port is used for placement of the thoracoscope, but ports are not used for the other incisions. Before making the second incision, evidence that the patient is unresectable, such as parietal pleural involvement, should be sought.
The second incision, an anterior access incision (usually 4.5, but up to 6.0 cm) for dissection and specimen retrieval, is placed in the fifth or sixth intercostal space, just inferior to the breast. The location of this incision, where the intercostal spaces are the widest, is chosen to provide access for hilar dissection and is usually not dependent on whether the planned procedure is an upper or lower lobectomy. A wound protector may be used in the access incision to improve soft tissue retraction, to facilitate suctioning, and to protect the incision during specimen removal. Additional incisions may be used, either in the axilla or posteriorly, to improve visualization or to provide retraction.
Instrumentation for thoracoscopic lobectomy is critical to successful completion of the procedure. The thoracoscope should be a 30-degree angled scope to optimize the ability to achieve panoramic visualization during dissection and to minimize competition with the operative instruments. Alternatively, a 45-degree angled scope or a flexible scope may be used, although this may be more difficult for an assistant to navigate. A spectrum of surgical instruments may be used for dissection, including conventional instruments and dedicated thoracoscopic or laparoscopic instruments. It is especially beneficial to use curved instruments for retraction during dissection, as they minimize the tendency for instruments to compete or collide with each other. Thoracoscopic (linear) mechanical staplers, such as the Endo GIA (Covidien, Norwalk, CT), are employed for control of the vessels (2.0- or 2.5-mm staples), bronchus (3.5- or 4.8-mm staples), and fissure.
After placing the access incision, the surgeon performs thoracoscopic exploration, which includes confirming the location of the tumor, excluding the presence of pleural metastases, and dividing the pulmonary ligament for lower lobectomy. If a malignant diagnosis has not been achieved preoperatively, thoracoscopic wedge resection is performed using an automatic stapling device, and the specimen is removed, either in a protective bag or with a wound protector. After frozen section confirms a malignant diagnosis, thoracoscopic lobectomy may be completed. MLND may be performed at this point or may be deferred until the lobectomy is completed.
The approach to the staging of mediastinal lymph nodes is controversial. Many advocate systematic sampling of mediastinal lymph nodes because of concerns about the adequacy and safety of formal dissection. Others accomplish MLND by complete resection of the mediastinal nodes thoracoscopically, including levels 2, 4, 7, 8, and 9 on the right and levels 5, 6, 7, 8, and 9 on the left. A minimum of three mediastinal stations should be assessed.
Hilar dissection is carried out through the access incision, to achieve visualization and mobilization of the hilar structures. When upper lobectomy is being performed, division of the posterior pleural reflection permits elongation of the hilum and facilitates further resection. For any anatomic thoracoscopic lobectomy, hilar dissection is begun with mobilization of the pulmonary vein, although alternative strategies may be employed depending on the size and location of the tumor. For upper lobectomy, the lung is reflected posteriorly and inferiorly to facilitate dissection. For lower lobectomy, the lung is retracted superiorly. Moving the thoracoscope to the anterior incision may improve visualization of the superior hilum and may facilitate placement of the linear stapler for upper lobectomy if it is introduced through the mid-axillary (camera) port.
The risk of intraoperative hemorrhage is minimized with careful hilar dissection, which is facilitated with the visual clarity and magnification available with the video thoracoscope. Unexpected bleeding from a major branch of the pulmonary artery or pulmonary vein may occur, however. In most cases, the source of the bleeding is easily identifiable and tamponade is possible, allowing conversion to thoracotomy. To minimize the risk of vascular injury, surgeons have employed various techniques to isolate the pulmonary arterial and venous branches, including ligatures to retract the vessels, and catheters to guide the stapling devices. These techniques may be helpful in difficult cases but are not required for most patients.
All lobectomy specimens are removed using a protective specimen bag to prevent implantation of tumor cells in the incision. The lobectomy specimen and hilum are inspected to ascertain that anatomic lobectomy has been performed. After retrieval, the hemithorax is irrigated with warm saline and the bronchial stump is inspected. If an air leak is encountered, repeat stapling or endoscopic suturing may be performed.
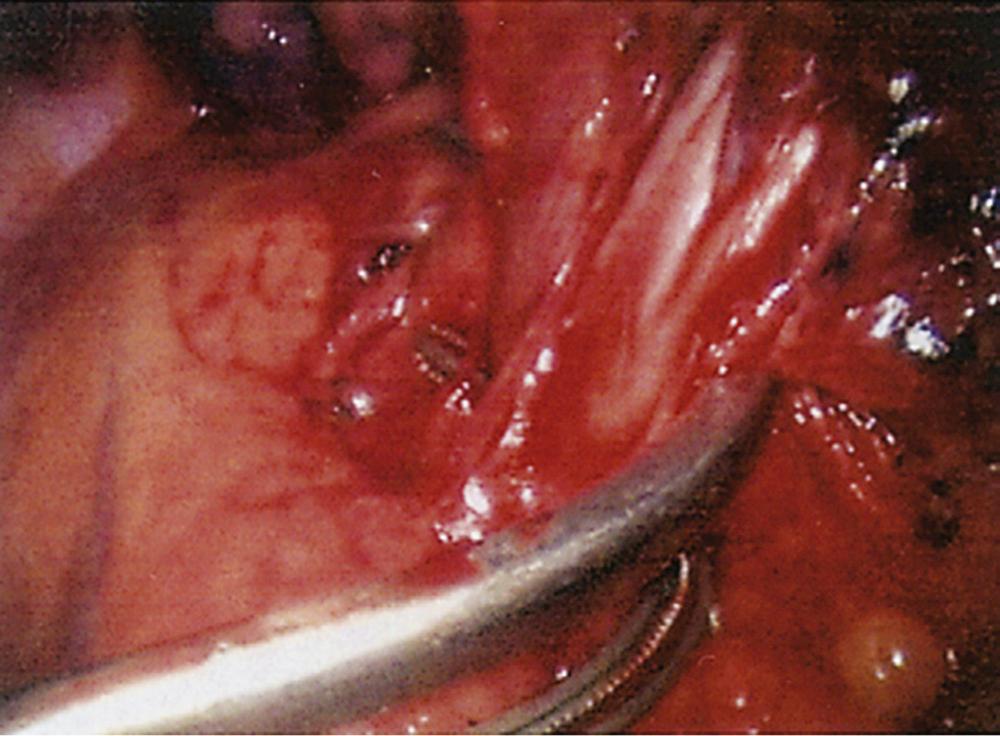
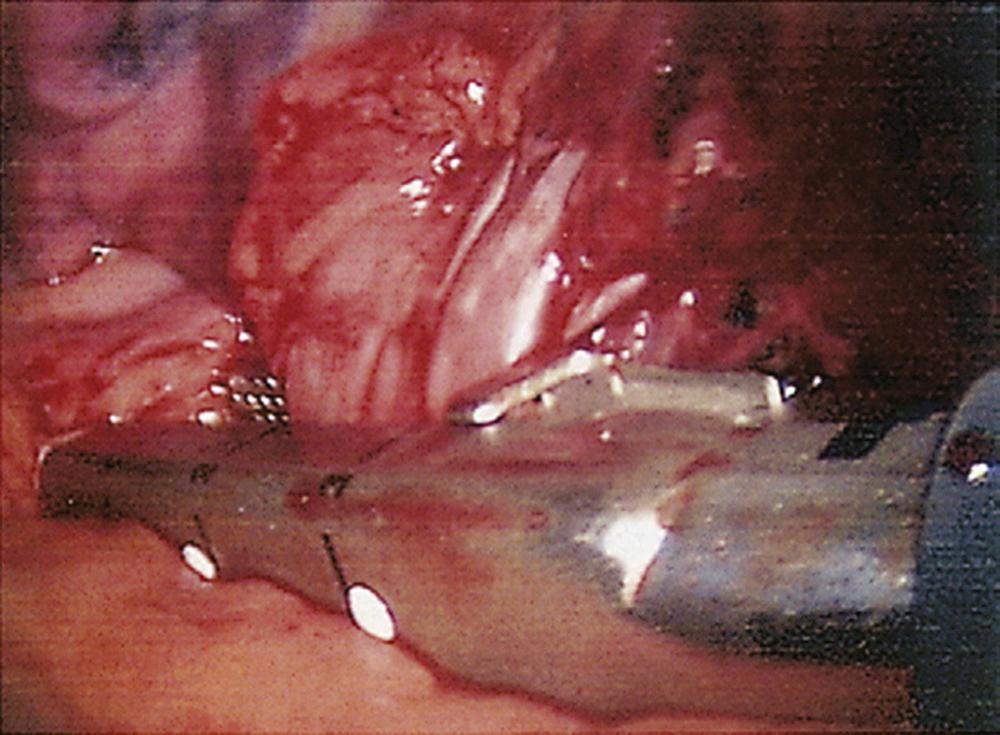
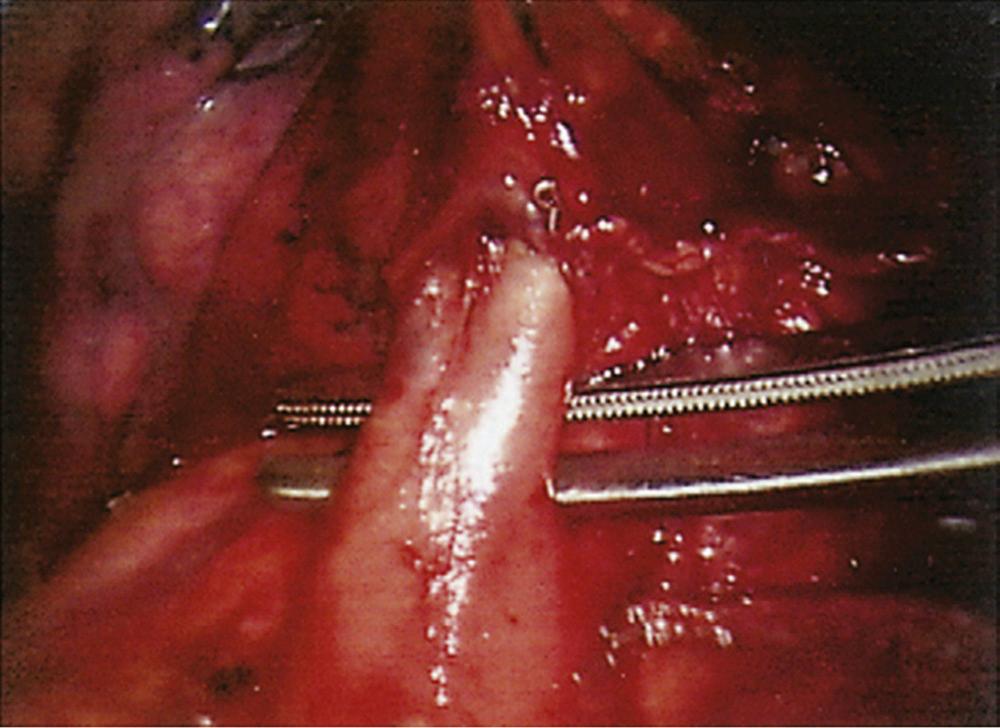
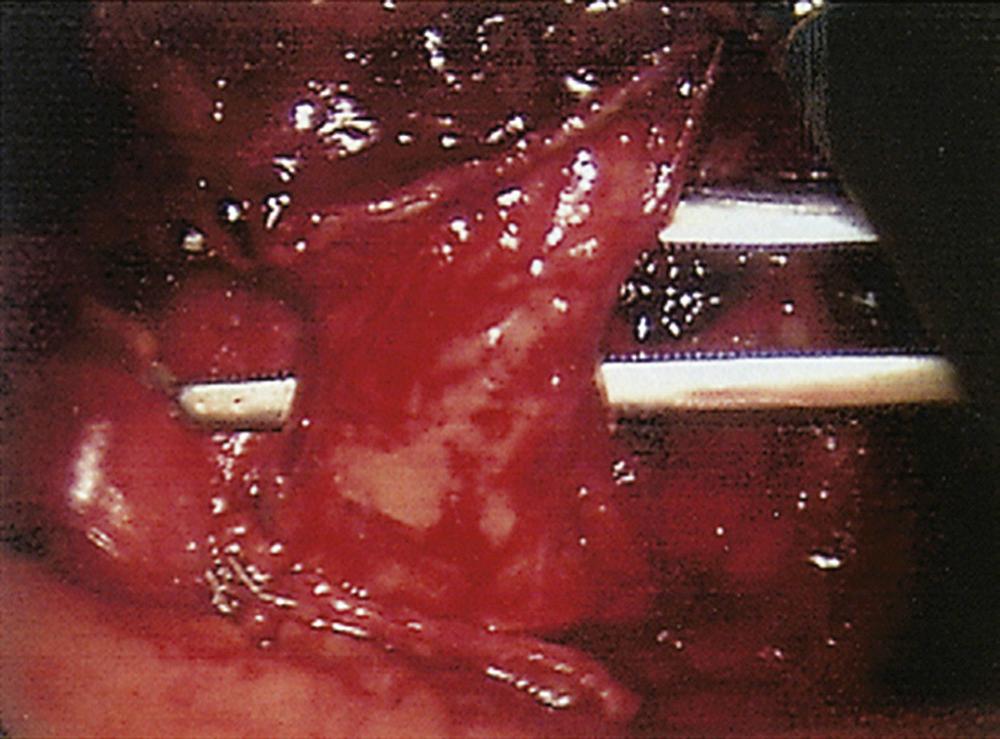
With the thoracoscope in the mid-axillary incision, the horizontal and oblique fissures are inspected and the presence of the tumor in the left upper lobe is confirmed. The lung is retracted posteriorly, and the superior pulmonary vein is identified and mobilized. The left superior pulmonary vein is then encircled using a curved clamp; dissection behind the superior pulmonary vein allows identification of the pulmonary artery ( Fig. 18-1 ). The stapling device is then applied ( Fig. 18-2 ), and the superior pulmonary vein is divided, exposing the pulmonary artery. The pulmonary artery is mobilized, focusing on the apical and anterior branches ( Fig. 18-3 ), which may then be stapled and divided. The left upper lobe bronchus is now visualized and may be stapled and divided ( Fig. 18-4 ). Subsequently, the branches of the posterior and lingular arteries are stapled ( Fig. 18-5 ). Finally, the fissures are completed and the specimen is retrieved.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here