Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lung auscultation has long suffered from a complex and onomatopoeic terminology that goes back to the original stethoscope and its inventor. Recent application of computer technology has rekindled this art by facilitating acoustic analysis. Still, its major difficulty lies not in the identification of sounds (which is much easier than for cardiac sounds and murmurs), but in their interpretation. And despite recent attempts at standardization, terminology remains a vexing issue.
Lung and thoracic ultrasound, as well as related applications, are discussed at the conclusion of this chapter. Like auscultation, lung ultrasound was once viewed as having a “foreign” character destined for limited dissemination. However, this opinion has been dramatically reversed in recent years, and is now regarded as perhaps the most clinically useful applications of POCUS for the generalist.
Who invented lung auscultation?
Auscultation of the direct or immediate variety (that is, without the use of the stethoscope) has actually been around for a long time. References to breath sounds first appeared in the Ebers papyrus (c. 1500 BC), the Hindu Vedas (c. 1400–1200 BC), and the Hippocratic writings (4th century BC). In fact, Hippocrates himself taught and practiced auscultation, advising physicians to apply their ears to the patient’s thorax in order to detect various diagnostic sounds. Since then, chest auscultation was mentioned by Caelius Aeralianus, Leonardo Da Vinci, Ambroise Paré, William Harvey, Giovanni Battista Morgagni, Gerhard Van Swieten, William Hunter, and many others. The hypochondriacal Robert Hooke, an assistant to Robert Boyle and one of the first scientists to use the word cell (1664), even had a good insight in describing heart sounds. He wrote, “Who knows? It may be possible to discover the motions of internal parts … by the sound they make.” Yet, during the 18th and early 19th centuries, direct auscultation fell rapidly out of favor, being replaced by a newer diagnostic modality: chest percussion. It took a lot of serendipity (and plenty of shyness) to rekindle it as indirect auscultation, that is, one “mediated” by a newly invented cylindrical instrument, the stethoscope. The hero of this rediscovery was an introverted, diminutive, very asthmatic, very prudish, and very tuberculotic Breton physician, named René Théophile Hyacinthe Laënnec. In the fall of 1816 (a year after the battle of Waterloo), he was summoned to the bedside of a young woman with a chest illness. Because percussion was technically difficult (given the large size of the woman’s breasts) and since direct auscultation (i.e., placing the physician’s naked ear over the patient’s naked chest) was, in Laënnec’s own words, “inadmissible” (given the young lady’s age and gender), Laënnec came up with a totally different approach. He remembered that a few days before, while walking in the Tuileries garden in Paris, he had seen children scraping a stick of wood and listening to the other end. Imagining that something similar could be tried with patients’ chests, he fetched a cardboard, rolled it into a cylinder, applied it to the lady’s thorax, and to his amazement, was able to hear very distinct lung sounds. And all of this without even touching her! Being handy (he used to make flutes), Laënnec quickly manufactured a wooden contraption, shaped like a flute, which he started taking regularly on rounds. He dubbed it the cylinder (and this, in turn, gave his students a chance to dub him “the cylindromaniac”). Yet, in academic circles the tool came to be known as the stethoscope, from the Greek term for “inspector of the chest.” Whatever its name, the gadget allowed Laënnec to gather over 3 years an astounding wealth of clinical–pathological correlations, which he then published on August 15, 1819 – in a two-volume book titled De l’Auscultation Mediate . There he reported masterly descriptions of several chest diseases, many previously unheard (no pun intended), like bronchitis, bronchiectasis, pleurisy, lobar pneumonia, hydrothorax, emphysema, pneumothorax, pulmonary edema, pulmonary gangrene and infarction, mitral stenosis, esophagitis, peritonitis, cirrhosis (hence the eponym Laënnec’s cirrhosis), and, of course, tuberculosis. And since autopsy was the ultimate benchmark for all these conditions, several ended up acquiring a pathological name. The book also presented an entirely new terminology, rooted in daily life examples, and enriched by Laënnec’s fascination with Greek and Latin language. Among such neologisms were stethoscope, but also auscultation, rales, rhonchus, fremitus, crackled-pot sound, metallic tinkling, egophony, bronchophony, cavernous breathing, puerile breathing, veiled puff, and bruit. The first edition of De l’Auscultation sold for 13 francs (16 if purchased with a wooden stethoscope) and sold quite badly. But when the considerably rewritten second edition hit the press, stethoscopy had already become the standard of chest examination. By the time of Laënnec’s premature death from tuberculosis (in 1826, at age 45), many physicians were carrying stethoscopes, all personally made by Laënnec. In fact, the posthumous third and fourth editions of 1831 and 1837 sold very well, establishing the tool not only as a symbol of the art of medicine but also as the centerpiece of bedside assessment. To reach a diagnosis, physicians could now rely on “objective” findings (instead of subjective symptoms reported by patients). They could finally tell their patients what they had wanted to say for a long time, “Shut up and let me listen to your lungs!” A new era had begun: one in which the patient was going to become first a sound, then a laboratory number, and, finally, a flickering computer image.
How do modern stethoscopes differ from Laënnec’s original cylinder?
Not a lot. The binaural stethoscope (invented in 1954 by Camman of New York) clearly helped. Yet, even today’s expensive stethoscopes remain primarily a simple conduit for transmitting sound between the patient’s chest and the examiner’s ears. Even as conduits, they have flaws. For one, most of them selectively amplify specific frequencies, such as those below 112 Hz (a welcome feature for cardiologists, who deal with the low-pitched S 3 and S 4 , but a not-so-welcome feature for pulmonologists , who deal instead with higher-frequency sounds). That is also why pulmonary auscultation has benefited so much from sound analysis through computer technology.
In addition to issues of nomenclature, why is pulmonary auscultation difficult?
Because sounds are of too low a frequency to hear well, plus they often overlap. This renders chest auscultation akin to listening to a group of people whispering at the same time. This is especially difficult in cardiac auscultation since heart sounds and murmurs have lower frequency, plus the cardiac cycle is shorter (0.8 second versus 2–3 for the respiratory cycle). To tackle these challenges, physicians develop pattern recognition , a crucial skill in clinical medicine, but one requiring practice.
What are lung sounds ?
They are sounds generated by the lungs. Transmitted voice sounds are instead sounds generated by the larynx and subsequently transmitted through the lungs ( Fig. 12.1 ).
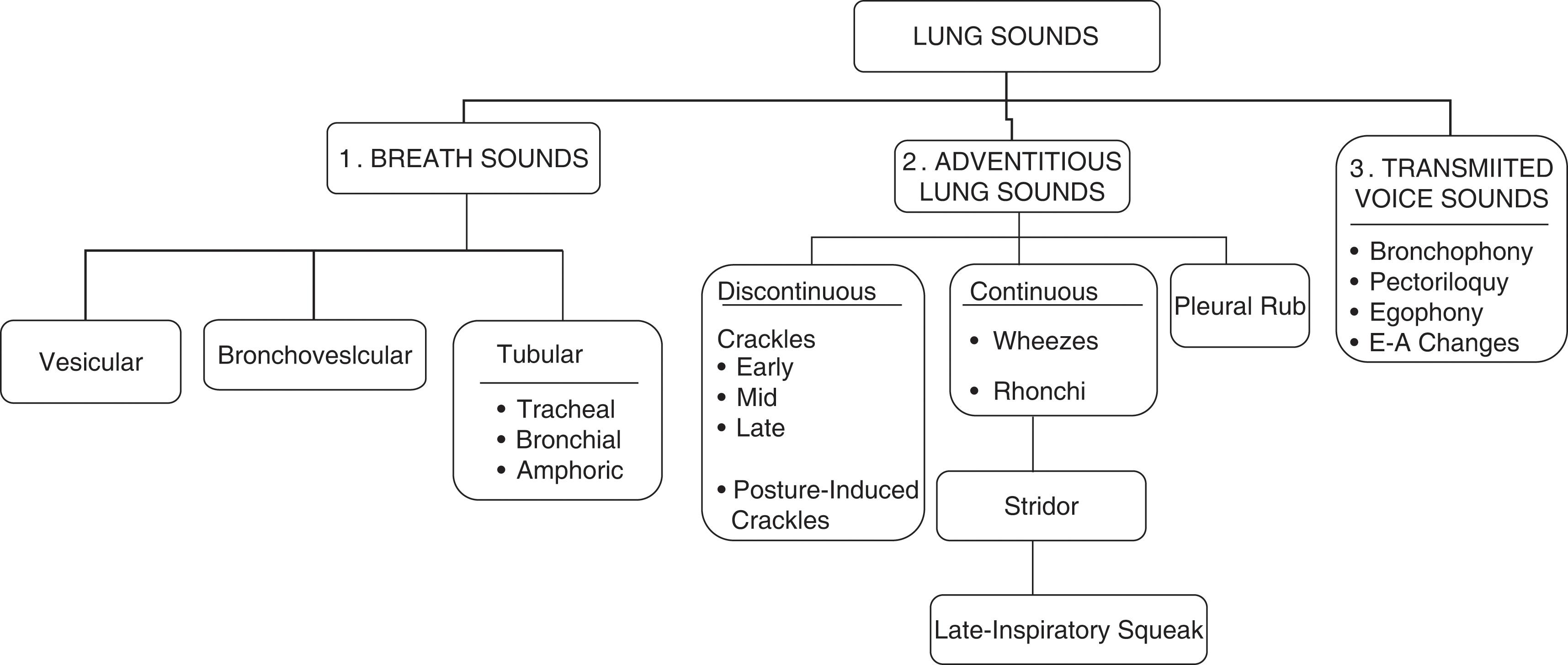
What are the major types of lung sounds (respiratory sounds)?
Basic lung sounds (also called breath sounds )
Adventitious (i.e., extra ) lung sounds
Each of these subgroups contains various other sounds ( Table 12.1 ).
| RESPIRATORY SOUND | MECHANISMS | ORIGIN | ACOUSTICS | RELEVANCE |
|---|---|---|---|---|
| Basic Sounds | ||||
| Normal lung sound ( vesicular ) | Turbulent flow vortices, unknown mechanisms | Central airways (expiration), lobar to segmental airway (inspiration) | Low-pass filtered noise (range <100 to 1000 Hz) | Regional ventilation, airway caliber |
| Normal tracheal sound ( tubular ) | Turbulent flow, flow impinging on airway walls | Pharynx, larynx, trachea, large airways | Noise with resonances (range <100 to >3000 Hz) | Upper airway configuration |
| Adventitious Sounds | ||||
| Wheeze | Airway wall flutter, vortex shedding | Central and lower airways | Sinusoid (range ~100 to >1000 Hz; duration, typically >80 ms) | Airway obstruction, flow limitation |
| Rhonchus | Rupture of fluid films, airway wall vibrations | Large airways | Series of rapidly dampened sinusoids (typically <300 Hz and duration >100 ms) | Secretions, abnormal airway collapsibility |
| Crackle | Airway wall stress – relaxation | Central and lower airways | Rapidly dampened wave deflection (typical duration <20 ms) | Airway closure, secretions |
a This table lists only the major categories of respiratory sounds and does not include other sounds, such as squawks, friction rubs, grunting, snoring, or cough. Current concepts about sound mechanisms and origin are listed, but these concepts may be incomplete and unconfirmed.
How are lung sounds produced?
By two major mechanisms: (1) air movement along the tracheobronchial tree (which is responsible for basic lung sounds, or breath sounds) and (2) vibration of solid tissue (which is responsible for adventitious [or extra] lung sounds).
What are breath sounds?
They are sounds heard over the chest of healthy (and diseased) subjects. They represent the background noise over which adventitious (or extra) sounds are occasionally superimposed.
What are the major types of breath sounds?
Vesicular breath sounds
Bronchial breath sounds (BBS)
Bronchovesicular breath sounds
Tracheal breath sounds
Amphoric (cavernous) breath sounds
This traditional division is based on the sound’s site of production. Conversely, a more recent classification (based on physical characteristics) reports only two major lung sounds: (1) the normal ones (or vesicular breath sounds) and (2) the abnormal ones (or tubular breath sounds). The latter consist of three subvarieties: tracheal, bronchial, and amphoric. As for the bronchovesicular breath sound, it is in a league of its own and one that should probably be abandoned. ( Fig. 12.2 )

What is the air movement responsible for the production of breath sounds?
It depends on the size of the airway. Overall, three types of movement may take place along the tracheobronchial tree ( Fig. 12.3 ). Some are silent and others noisy:
Laminar airflow is characteristic of small peripheral airways. It is so slow that airflow in the alveoli almost comes to an end. Hence, this is a silent movement.
Vorticose airflow is a bit faster and typical of medium-sized branching airways. “Branching” separates airflow in different layers with different velocities, whose interaction generates eddies and vortices – all noisy . This air movement is often called mixed (or transitional) because it resembles both laminar and turbulent airflow.
Turbulent airflow is very rapid, complex, and typical of large central airways (trachea and major bronchi). Air molecules randomly collide against each other and onto the airway walls. This air movement is characteristically noisy .
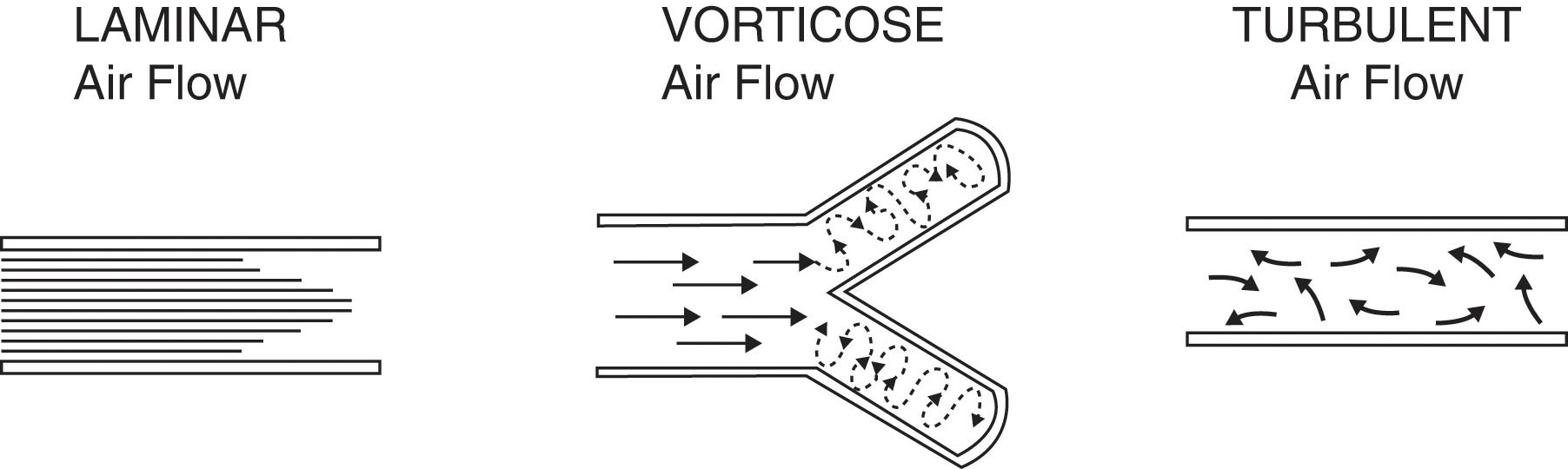
How are breath sounds produced?
Mostly by turbulent and vorticose flow in the trachea and central bronchi (see later, “tubular” breath sounds). Airflow in smaller and more peripheral airways is instead laminar and thus silent.
Can breath sounds be heard over both the chest and mouth?
Yes. Although produced in the central airways, they are transmitted both downward (to the chest) and upward (to the neck and mouth).
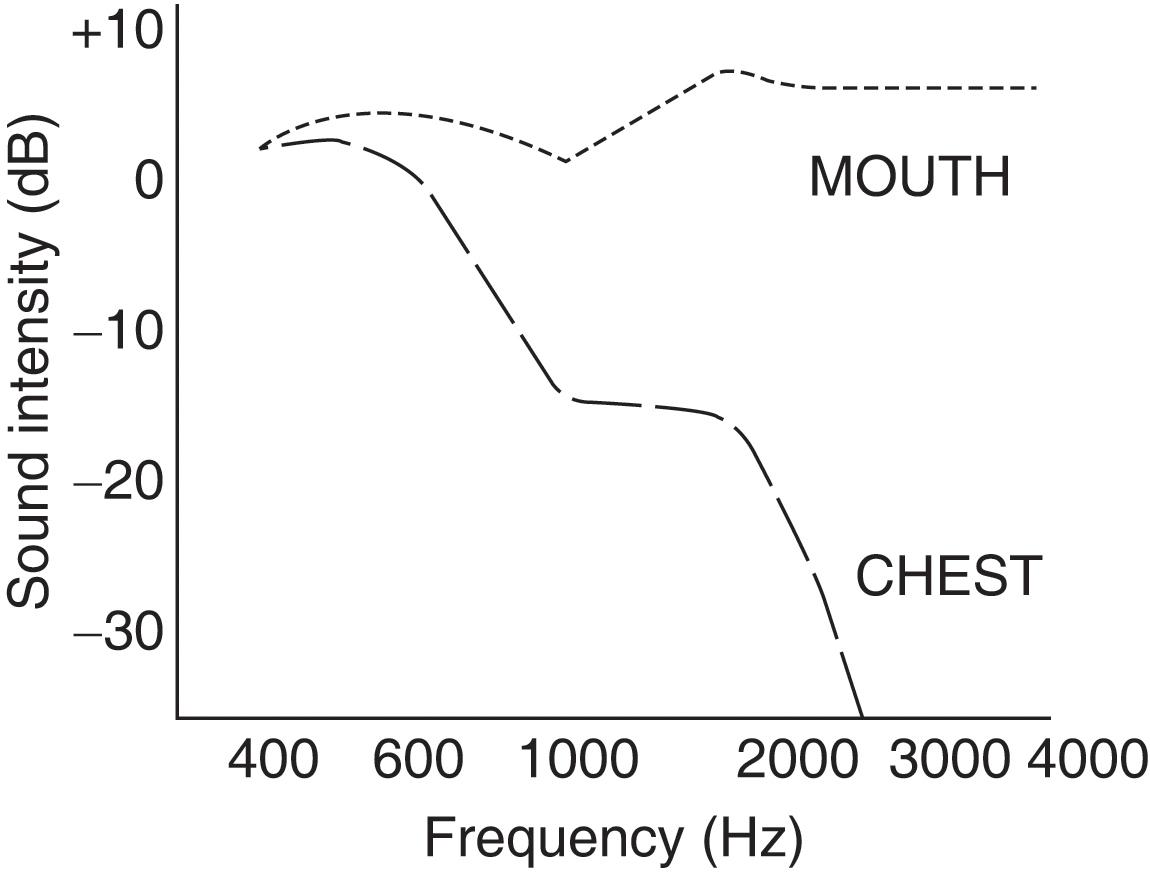
Are breath sounds at the chest different from those at the mouth?
Yes, because sounds’ characteristics depend on the tissues they traverse, especially their filtering properties . Alveolar air, for example, is a high-frequency filter that eliminates components >200 Hz. Since sounds transmitted to the mouth cross very little alveolar air, they will maintain all their original frequencies, including the highest. Sounds transmitted to the chest, however, traverse so much alveolar air to eventually lose most of their high frequencies. Since low frequencies are poorly perceived by the ear, sounds at the chest are typically softer than those at the mouth.
What are the acoustic characteristics of breath sounds at the mouth?
The dominant one is the high pitch (and loudness). Hence, sounds at the mouth have much wider frequencies, ranging between 200 and 2000 Hz – very much like white noise ( Fig. 12.4 ).
What is the value of comparing breath sounds at the mouth with those at the chest?
The value of identifying airflow obstruction. Breath sounds heard at the mouth by the unaided ear have an intensity that is directly related to the spirometric degree of airway obstruction (i.e., the louder the sounds, the greater the obstruction). Conversely, breath sounds over the chest have an intensity that is inversely related to airflow obstruction, with distant sounds reflecting worse flow.
How do breath sounds at the mouth get produced?
By turbulent airflow in proximal bronchi and the trachea. In normal subjects, this flow is slow enough to barely make audible any noise at the mouth. Conversely, in patients with chronic obstructive pulmonary disease (COPD), the turbulence parallels airway narrowing, eventually causing very loud mouth breathing. This increased loudness is purely limited to breath sounds and thus can occur even in the absence of crackles or wheezes. In fact, loudness can become so high that the inspiratory breath sounds of patients with COPD are often heard across the room, even in patients breathing at rest. This concept is not new, since Laënnec himself had noticed an association between noisy mouth breathing and dyspnea, reporting a patient whose breath sounds could be heard at a distance of 20 feet. In fact, he devoted a full page of De l’Auscultation Mediate to this phenomenon, explaining in detail that what he meant by “loud breath sounds at the mouth” was just “loud breathing,” and not the noise of rattles, wheezes, or rhonchi.
Are there differences in intensity of breath sounds between the various types of airflow obstruction?
Yes ( Table 12.2 ). In fact, the intensity of inspiratory breath sounds at the mouth can help differentiate emphysema from chronic bronchitis or asthma, since in only the last two conditions it directly correlates with (1) increased airway resistance; (2) reduced FEV 1 (forced expiratory volume in 1 sec); and (3) reduced peak expiratory flow rate (PEFR). Conversely, in emphysema, inspiratory breath sounds at the mouth are paradoxically quiet and almost silent because emphysema causes no direct narrowing of the bronchi, but only a dynamic expiratory airflow obstruction due to loss of elastic recoil.
Noisy mouth inspiration argues in favor of asthma or chronic bronchitis, where it can predict the degree of airflow obstruction even more reliably than wheezing.
| LUNG DISEASE | BREATH SOUNDS | ADVENTITIOUS LUNG SOUND |
|---|---|---|
| Pneumonia | Bronchial or absent | Inspiratory crackles |
| Atelectasis | Harsh/bronchial | Late inspiratory crackles |
| Pneumothorax | Absent | None |
| Emphysema | Diminished | Early inspiratory crackles |
| Chronic bronchitis | Normal | Wheezes and crackles |
| Pulmonary fibrosis | Harsh | Inspiratory crackles |
| Congestive heart failure | Diminished | Inspiratory crackles |
| Pleural effusion | Diminished | None |
| Asthma | Diminished | Wheezes |
Are there any other unique breath sounds’ characteristics in patients with chronic bronchitis?
The most peculiar is indeed the paradoxical behavior of inspiratory sound intensity: increased at the mouth and softened at the chest. More often, however, patients with chronic bronchitis tend to have noisy chests, not because their breath sounds are indeed louder (in fact, they are actually softer), but because there are so many superimposed adventitious sounds to make the overall intensity appear louder. These extra sounds include early inspiratory crackles and rhonchi (both of which may clear with coughing), but also end-expiratory wheezes.
Should patients with suspected chronic bronchitis be asked to cough during exam?
Yes, since crackles (and rhonchi) of airflow obstruction clear with coughing. This is because they originate from air-fluid interfaces of large to medium airways. Conversely, there are some crackles that may appear after coughing (posttussive crackles), especially over the apical lesions of tuberculosis. Hence, the relationship with cough is a good clue to the sound’s origin and should be routinely elicited. According to an old saying, you can tell a chest specialist from a generalist because the generalist never asks the patient to cough during auscultation, whereas the specialist always does.
What are tubular breath sounds?
They are sounds literally generated by air flowing through a hollow tube. In this regard, all breath sounds are indeed tubular, changing into vesicular only when filtered by alveolar air. To learn more about these sounds, listen to your own neck: tracheal sounds are classically tubular.
What are the main characteristics of tubular breath sounds?
Loudness (graphically represented by thick inspiratory and expiratory lines)
A brief silent pause between inspiration and expiration
A long expiratory phase , usually as long as inspiration (I:E ratio of approximately 1:1) ( Fig. 12.5 )
Which of these characteristics is the most important?
The high frequency. Since they cross very little alveolar air, tracheal sounds (the quintessential tubular breath sounds) will contain higher frequencies than the vesicular ones, thus appearing louder and “hollow.” In fact, they have a range between less than 100 and more than 1500 Hz, with maximal intensity at 300–800. Exhalation also has a slightly higher intensity than inspiration.
What is the clinical significance of tracheal breath sounds?
It is indirect and linked to their resemblance of other (and more clinically relevant) tubular sounds: the bronchial.
What happens to tracheal sounds once they enter the chest?
Once the trachea enters the chest (and branches into bronchi and bronchioli), it gets surrounded by a mantle of alveolar air that acts as a muffler, dampening the intensity (and frequency) of the original tubular sounds and thus transforming them into vesicular sounds. Hence, alveolar air acts as the equalizer of your home stereo equipment, and also as a low-pass filter: it eliminates high-frequency (and thus also louder) components, while preserving low-frequency (and softer) tones.
What are the three characteristics of vesicular breath sounds?
Low frequency. Hence, soft and muffled , graphically represented by thin respiratory lines.
Absence of the silent pause between inspiration and expiration
Short duration of exhalation because most high frequencies are concentrated in the last two-thirds of exhalation and thus totally eliminated by the presence of alveolar air.
Why are these sounds called vesicular?
Because Laënnec believed they were generated by “vesicles” (the alveoli), whereas in reality they are still generated by tubes (i.e., bronchi and bronchioli), albeit filtered (and modified) by alveoli.
What is the clinical significance of vesicular breath sounds?
They are the normal breathing-associated sounds of healthy people. In other words, vesicular breath sounds are the same as normal breath sounds .
Is the ability of the lung to act as a high-frequency filter a constant phenomenon?
No, it can be eliminated by replacing the alveolar air with a medium that transmits sound better – liquid, for example. This can come as pus (pneumonia), serum (pulmonary edema), or blood (alveolar hemorrhage). Alternatively, air can be simply “squeezed out” of the alveoli, as in patients with alveolar collapse. Both mechanisms produce an airless lung (i.e., consolidation), with liquid or solid replacing alveolar air. This allows better transmission of sound, including those high-frequency components that are normally filtered out. Hence, sound will be perceived as louder.
What are the acoustic differences between bronchial and vesicular breath sounds?
BBS have a peculiar quality, similar to that of tracheal breath sounds: hollow, tubular, almost Darth Vader–like. They are also louder than vesicular breath sounds – mostly because they are richer in high frequencies. Hence, their respiratory cycle is graphically represented by thicker lines. Finally, they have a silent pause between inspiration and expiration, with a longer expiratory phase. Because of longer exhalation, BBS also have an inspiratory-to-expiratory (I:E) ratio that is usually 1:1 (instead of 3:1 or 4:1 for vesicular sounds).
What is the most striking physical characteristic of BBS?
Their high frequency. This is because consolidation is associated with an overall increase in lung density, which prevents it from functioning as a low-pass filter (i.e., as an equalizer that eliminates frequencies >200 Hz). Since airless lungs allow the unaltered transmission of high-frequency sounds, BBS will come across as loud. Also, both inspiration and expiration have similar component frequencies (between 100–1200 Hz) with maximal intensity of 300–900 Hz. These frequencies are much higher than those of vesicular breath sounds and are, in fact, similar to those of other tubular breath sounds, such as the tracheal sounds.
What is the clinical significance of tubular breath sounds?
It depends on location: they are entirely normal over the neck (i.e., originating in the trachea), but pathologic over the chest (originating in the bronchi). In this case, BBS reflect absence of alveolar air and its replacement by media that better transmit high frequencies: liquid or solid (i.e., consolidation). Hence, they indicate patent airways in a setting of airless alveoli.
Where are vesicular breath sounds produced?
In large central airways, with the inspiratory component probably originating more peripherally than the expiratory one. Sounds so produced are then transmitted through the air-filled alveoli surrounding the bronchi. Since the mantle of alveolar air acts as a “low-pass” filter, vesicular sounds only retain low frequencies (100–500 Hz), with a steep cut-off greater than 200 and maximal intensity less than 100.
Are there acoustic differences in vesicular breath sounds between inspiration/expiration?
Expiration has lower pitch and intensity. In fact, its last two-thirds are entirely silent. Hence, expiration is also shorter than inspiration, with an I:E ratio of 3:1 (in tubular sounds, it is 1:1).
Do vesicular breath sounds change with age?
Yes. Those of children (up to age 9) are higher-pitched (and louder) than adults’. In turn, vesicular breath sounds of adults are higher-pitched (and louder) than those of the elderly. Laënnec was the first to notice this phenomenon and called it “puerile respiration.”
Why does this happen?
Probably because of the different resonance of small thoraces, leading to less power in low frequencies for children’s breath sounds (and also because of the progressive emphysema of the elderly, with an increase in “muffling” air). The smaller radius of children’s airways also may be responsible for the higher turbulence and loudness of their vesicular breath sounds.
Are vesicular breath sounds normally heard throughout the chest?
No. Although present over most lung fields of normal patients, vesicular sounds are classically absent in two narrow areas, corresponding anteriorly and posteriorly to the trachea and central bronchi (parascapular areas). Here, the vesicular sounds are replaced by bronchovesicular sounds (see also Questions 58–60). Outside these narrow areas, presence of distinct vesicular sounds remains a sine qua non for a healthy lung ( Fig. 12.6 ).
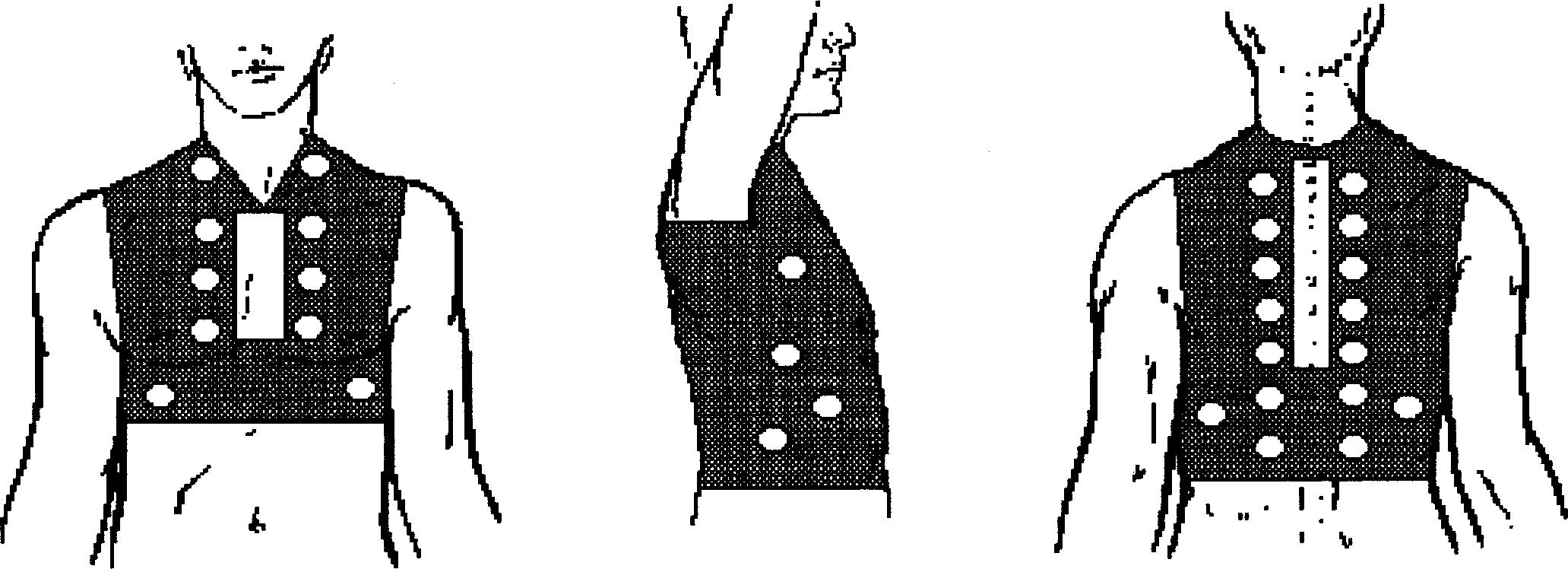
What are the breath sound characteristics of patients with pneumothorax?
The most striking is their softening. In fact, if the pneumothorax is large enough to collapse the entire lung, the corresponding hemithorax will be silent. The distant (or absent) breath sounds of pneumothorax are the result of not only reduced sound production (due to diminished airflow in the collapsed lung) but also reduced sound transmission (due to the cushion of air in the pleural space). The same happens with pleural effusion (of course, in pneumothorax there is an increase in intensity of the percussion note, whereas in pleural effusion there is a softening). Still, air (or fluid) in the pleural cavity forms an acoustic barrier to sound transmission that usually muffles the breath sounds. The only exception is a layer of fluid so thin as to compress only alveoli but not bronchi.
Is the intensity of vesicular breath sounds important?
Yes, since decreased intensity usually indicates reduced airflow – assuming, of course, that (1) chest wall thickness is normal (i.e., no obesity); (2) pleural space is also normal (i.e., no collection of either air or fluid); and (3) respiratory muscles are functioning normally (i.e., no weakness). Hence, distant breath sounds are typical not only of patients whose right main bronchus has been selectively intubated by a misplaced endotracheal tube but also of patients with obstructive lung disease. In turn, vesicular breath sounds of normal intensity virtually exclude a severe reduction in FEV 1 . Finally, higher intensity can also be heard during postexercise hyperventilation.
Is the intensity of breath sounds in airflow obstruction equally diminished at the mouth?
Not at all. In fact, it’s usually increased at the mouth.
What is the best bedside predictor for the presence of chronic obstructive lung disease?
A reduction in breath sound intensity (BSI). A total of 32 findings has been said to indicate COPD, with many arguing strongly for its presence, yet BSI is the single best index of emphysema . Early inspiratory crackles also argue for obstruction (LR, 14.6), but mostly chronic bronchitis . If progressive over time, BSI reduction can help monitor methacholine challenge, even when wheezing is absent. Finally, any two of the following virtually rule in airflow limitation: >70-pack-years of smoking, decreased breath sounds, or history of COPD. Years of cigarette smoking, subjective wheezing, and either objective wheezing or peak expiratory flow rate also predict the likelihood of airflow limitation in males. Although other signs have been linked to obstruction (objective wheezing, barrel chest, positive match test, rhonchi, hyperresonance, and subxiphoid apical impulse), on multivariate analysis only three remain significantly associated with its diagnosis: self-reported history of COPD (LR, 4.4), wheezing (LR, 2.9), and FET >9 seconds (LR, 4.6). Patients with all three have an LR of 33 (ruling in COPD); those with none have an LR of 0.18 (ruling out COPD).
How can one objectively measure BSI at the bedside?
By a scoring system originally devised by Pardee:
Ask the patient to sit up and inspire from residual volume, fast and deep, while at the same time breathing through the mouth. This generates a breath sound as loud as possible.
Auscultate bilaterally over the upper anterior zones, the mid-axillae, and the posterior bases.
Quantify the intensity of the inspiratory component of the vesicular breath sounds as: 0, absent; 1, barely audible; 2, faint but definitely audible; 3, normal; 4, louder than normal.
The sum of sound intensities recorded in each area generates the BSI score. This ranges from 0 to 24 (for, respectively, scores of 0 or 4 in each of the six areas).
To best use this method, you must disregard superimposed adventitious sounds (rhonchi, wheezes, or crackles), which, since they are louder than the underlying breath sound, would overestimate the BSI.
How good is interobsever reliability for BSI determination?
Very good. It had a correlation coefficient of 0.96 in two observers examining independently 20 patients. Hence, the BSI is an accurate indicator of airflow obstruction. In fact, even unscored lung sound intensity correlates closely with FEV 1 (although the BSI correlates even better, both with FEV 1 and FEV 1 /FVC [forced vital capacity]). A BSI <9 argues strongly in favor of obstruction, whereas a BSI >15 argues against it.
In addition to airflow obstruction, is there any other process associated with distant breath sounds?
Pneumonia. A reduced BSI with fever and cough is very suggestive of it.
Can a change in lung sounds’ intensity help monitor patients’ response to airway challenge?
Yes. In patients with a positive methacholine test, a reduced BSI is typical of asthma. In fact, a gradual reduction in BSI may even precede wheezing, thus making it a great tool for monitoring bronchial provocation. Yet, with worsening airflow, the breath sounds’ pitch may paradoxically increase.
What is the mechanism of decreased BSI in COPD?
It is probably more a reduced sound transmission (due to parenchymal destruction, causing greater air-trapping and “muffling”) than a reduced sound production (due to diminished airflow). This is intuitive, considering that airflow limitation in COPD is more expiratory than inspiratory.
How does BSI compare to other bedside findings of airflow obstruction?
Quite well. In a study of COPD patients, the BSI was the best indicator of “obstructive emphysema,” as compared with 14 other physical findings and as judged against spirometry. The reason is its strong correlation with regional distribution of ventilation as measured by radioactive gas.
What is the best bedside predictor for severity of airflow obstruction?
The forced expiratory time (FET). To measure it, instruct patients to perform a forced-expiratory maneuver (i.e., to take a deep breath and blast it out as quickly and forcefully as possible) while you keep the bell of your stethoscope over the patient’s suprasternal notch. Then, clock the duration of audible expiration (FETo) to the nearest half-second. An FETo >6 seconds will correspond to an FEV 1 /FVC <40%. Conversely, a FETo <5 seconds will indicate an FEV 1 /FVC >60%. This simple bedside test is a good and reliable “poor man’s” spirometry. In a study by McAlister of 161 consecutive patients in six different countries, only three elements of the clinical exam were significantly associated with a diagnosis of COPD on multivariate analysis: self-reported history of COPD (adjusted LR, 4.4), wheezing (adjusted LR, 2.9), and FET >9 seconds.
Is there any finding that argues against the presence of COPD?
No single finding rules out airflow obstruction. Yet, a history of having never smoked argues strongly against it (especially if there is no wheezing on either examination or history).
Are BBS ever “physiologic”?
No. Unlike other tubular breath sounds (i.e., the tracheal), BBS are always pathologic because they reflect airless parenchyma in a setting of patent bronchi, thus indicating consolidation . Yet, even this rule has exceptions. In fact, BBS can occur in normal individuals, usually over the posterior right upper chest (where the right lung is contiguous with the trachea through the first thoracic vertebra, thus allowing direct transmission of tracheal sounds). Outside of that area, BBS should be considered pathologic until proven otherwise.
How are BBS produced?
Like all other breath sounds: by air flowing rapidly through large and central airways. Their hollow quality and intensity, however, are due to better sound transmission, usually at the lung periphery.
What is the cause of this improved transmission?
Consolidation (i.e., replacement of alveolar air with a mantle of solidified lung that can better transmit higher frequencies). Consolidation reflects either alveolar collapse or alveolar fluid-filling :
Alveolar collapse (with patent airways) occurs in pleural effusions, whenever the amount of fluid is large enough to compress the alveoli but too small to compress the airways.
Alveolar fluid-filling occurs instead in situations of pneumonia ( pus in the alveoli), alveolar hemorrhage ( blood in the alveoli), or pulmonary edema ( serum in the alveoli). In fact, in patients with cough and fever, the presence of BBS argues strongly in favor of pneumonia. Yet, its absence cannot rule it out, since the finding is specific but poorly sensitive.
BBS also may be heard in situations of pulmonary fibrosis . This mechanism, however, requires severe fibrosis and tends to be less common than simple consolidation.
How deep should the consolidation be in order to generate BBS?
Quite deep . In fact, consolidation and/or fibrosis must extend all the way from the chest surface to 4–5 cm from the hilum (where large airways are located). Only in this way is the range of transmitted frequencies increased enough to make the breath sounds resemble a tracheal sound. This also is the basis for other signs of consolidation, such as egophony, bronchophony, and pectoriloquy.
Can one separate bronchial sounds of fluid-filled alveoli from those of collapsed alveoli?
Yes, by remembering that fluid in the alveoli is often associated with fluid in the interstitium and that this, in turn, is associated with adventitious lung sounds – such as crackles. Hence, BBS without crackles are usually the result of collapsed alveoli (with patent airways), whereas BBS with crackles are more often the result of fluid-filled alveoli (also with patent airways).
What is a common reason for bronchial breath sounds unaccompanied by crackles?
A pleural effusion. Fluid occupying half of the hemithorax presents with (1) vesicular breath sounds in the upper third of the chest (reflecting normal aeration); (2) tubular (or bronchial) breath sounds in the middle third (reflecting collapsed alveoli but patent airways); and (3) respiratory silence at the bottom (reflecting collapsed alveoli and airways, as a result of the gravity-dependent accumulation of fluid).
Other than consolidation, do bronchial breath sounds indicate anything else?
They may help rule out an endobronchial obstruction, thus avoiding unnecessary bronchoscopies. This is because a postobstructive pneumonia is unlikely to occur in patients with densities on chest x-ray but BBS on examination – which is intuitive considering that tubular sounds reflect patent bronchi. Patency can be easily confirmed by imaging, through the presence of air bronchograms (i.e., air-filled airways cast against a surrounding consolidated parenchyma). BBS also may be clues, albeit indirectly, to the presence of tamponade and mitral stenosis.
BBS can indicate tamponade and mitral stenosis?
Yes. Ewart’s sign (posterior dullness to percussion and BBS between the left scapular tip and the vertebral column) is a sign of compressive atelectasis caused by a distended pericardial sac. A similar finding occurs in the Ortner’s syndrome of mitral stenosis, which is due to the raising (and squeezing) of the left main bronchus by an enlarged left atrium. This, in turn, causes (1) compression of the recurrent laryngeal nerve between the aortic arch and the pulmonary artery (with resulting hoarseness and bitonal voice); (2) left-lower lobe atelectasis (with posterior dullness and BBS); and (3) dysphagia (from esophageal impingement).
Who was Ewart?
A British physician (1848–1929). Born in London of a French mother (and reared in England and Paris), Ewart graduated from Cambridge and then worked in a field hospital during the Franco-Prussian War of 1870. After the war, he lived in Berlin for a few years before returning to England, where he practiced at the Brompton and St. George’s hospitals until retiring in 1907. He reported his homonymous finding in 1896. A year later, the Dutch anatomist N. Ortner reported his syndrome.
What are amphoric breath sounds?
They are a variant of tubular breath sounds and thus high-pitched, loud, and resonant. They are typically generated by air flowing into a lung cavity, or very large cysts and blebs. “Amphoric” refers to their metallic timbre, resembling the sound produced by blowing air into a jug ( amphora in Latin). They also are referred to as “cavernous” since they can mimic the sound of wind blowing through a “cave.” They are specific for cavitary disease (often a large tuberculous caverna ), but not sensitive.
What are bronchovesicular breath sounds?
They are “transitional” sounds that may be heard over the parasternal and parascapular areas of normal people (from the third to the sixth intercostal space). They possess characteristics typical of both vesicular and tubular breath sounds. Many authors even dispute their existence.
What are the three acoustic characteristics of bronchovesicular breath sounds?
Like tubular sounds, they have long and well-preserved expiration (with an I:E ratio of 1:1).
Like vesicular sounds, they lack a silent pause between inspiration and expiration.
They are softer/lower-pitched than tubular sounds, but harsher/higher-pitched than vesicular.
These halfway characteristics are all due to the peculiar transmission of these sounds. After being produced by turbulent flow in large and central airways (distal trachea and main bronchi), bronchovesicular sounds cross only a thin mantle of alveolar air before reaching the stethoscope. Hence, in contrast to tracheal breath sounds (which are heard immediately over the neck and thus have no alveolar air to cross), bronchovesicular sounds still have to undergo some physical changes (mostly filtering of high frequencies) before reaching the chest surface. Yet this filtering is not of the same degree as that undergone by vesicular sounds. Hence, their hybrid features.
What is the clinical significance of bronchovesicular breath sounds?
It depends on location. If heard over the parasternal and parascapular areas (both anteriorly and posteriorly), they are normal. Anywhere else, they indicate pathology – usually early consolidation (with enhanced transmission of high frequencies) or a thin pleural effusion , partially compressing the alveoli but not the bronchi. ( Fig. 12.7 )
What are adventitious lung sounds?
They are extra (i.e., adventitious) sounds that are normally absent in a respiratory cycle but become superimposed on the underlying breath sound (vesicular or bronchial) whenever disease occurs. Theirs is a confusing alphabet soup of rubs, squeaks, crackles, wheezes, stridor, and rhonchi. ( Table 12.3 )
| ACOUSTIC CHARACTERISTICS | AMERICAN THORACIC SOCIETY NOMENCLATURE | COMMONLY USED SYNONYMS(OLD TERMINOLOGY) | LAËNNEC EXAMPLE |
|---|---|---|---|
| 1. Discontinuous (<250 msec) | Coarse crackle | Coarse râle | Water gurgling from a bottle |
| Fine crackle | Fine râle crepitation | Crackling of salt on a heated dish | |
| 2. Continuous (>250 msec) | Wheeze (high-pitched) | Sibilant rhonchus | The chirping of little birds |
| Rhonchus (low-pitched) | Sonorous rhonchus | The cooing of a wood pigeon |
How were these sounds first described?
By Laënnec, who through painstaking clinical–pathological correlation reported many of them in his 1819 treatise De l’Auscultation Mediate . He called them “bruits étrangers” (i.e., foreign sounds). He also referred to them as “râles” since many of his patients had tuberculosis, and thus rattling noises ( râles in French) were indeed the sounds he most frequently heard. Yet, realizing that râles were easier to recognize than describe, he tried to teach them by using examples from “daily” life – all actually quite bizarre. For instance, he compared fine crackles to the “crackling of salt on a heated dish,” writing that these “humid râles” (or crepitations) were often present in patients with pneumonia, pulmonary edema, or hemoptysis. He then compared coarse crackles to the “gurgling of water from an upside down bottle,” adding that these “mucous râles” were often heard in patients with abundant secretions of the central airways. Finally, he likened wheezes to the “chirping of little birds” and rhonchi to the “cooing of a wood pigeon” – all bizarre and confusing examples.
When was the classification of adventitious lung sounds revised?
In 1977. The problem, of course, was Laënnec’s terminology and his original “daily life” examples. This was compounded by his inability to even use the term râle at the bedside, since it reminded his patients of the French expression “le râle de la mort” (the death rattle) – that is, the gurgling sound of dying people too ill to clear secretions. To avoid any bedside miscommunication, Laënnec often referred to these sounds as rhonchi , which was the Latin (and less scary) equivalent of rattles. Still, râles and rhonchi meant the same thing to him. This, however, escaped his first English translator, Dr. John Forbes, who decided that rhonchi should only describe long sounds, whereas râles should describe short sounds. Not all translators, however, complied with this rule, thus triggering the beginning of the end for Laënnec’s classification. By the 1970s, the confusion was so bad that Fraser and Pare reported that “every physician seems to have his own classification.” This eventually led to a reclassification by a team of international experts, with recommendations published in 1977, more than 150 years after Laënnec’s original terminology ( Table 12.4 ).
| ACOUSTIC CHARACTERISTICS | WAVEFORM | RECOMMENDED ATS a NOMENCLATURE | TERMS IN SOME TEXTBOOKS | A BRITISH USAGE | LAËNNEC’S ORIGINAL TERM | LAËNNEC’S MODEL |
|---|---|---|---|---|---|---|
| Discontinuous, interrupted explosive sounds |  |
Coarse crackle | Coarse rale | Crackle | Rale muquex ou gargouillement | Escape of water from a bottle held with mouth directly downward |
| Loud, low in pitch | ||||||
| Discontinuous, interrupted explosive sounds |  |
Fine crackle | Fine rale crepitation | Crackle | Rale humide ou crepitation | Crepitation of salts in a heated dish |
| Less loud than above and of shorter duration; higher in pitch than coarse rales or crackles | Noise emitted by healthy lung when compressed in the hand | |||||
| Continuous sounds Longer than 250 ms, high-pitched; dominant frequency of 400 Hz or more, a hissing sound |  |
Wheeze | Sibilant rhonchus | High-pitched wheeze | Rale sibilant sec ou sifflement | Prolonged whisper of various intonations; chirping of birds; sound emitted by suddenly separating two portions of smooth oiled stone. |
| The motion of a small valve | ||||||
| Continuous sounds |  |
Rhonchus | Sonorous rhonchus | Low-pitched wheeze | Rale sec senore ou ronflement | Snoring; bass note of a musical instrument; cooing of a wood pigeon |
| Longer than 250 ms low-pitched; dominant frequency about 200 Hz or less; a snoring sound |
What were the recommendations of the 1977 classification?
The most striking was the abandonment of Laënnec’s beloved term of “râle” in favor of a nomenclature based on acoustic and physical characteristics. Since priority was given to duration , adventitious lung sounds were divided into discontinuous (if lasting <250 msec) and continuous (if lasting >250 msec). The term crackle became the main descriptor of discontinuous sounds, replacing both the French râle and the British crepitation. Suffixes such as wet and dry were completely abandoned – as well as moist, sticky, atelectatic, close-to-the-ear, metallic, superficial, or consonating. Currently, the only acceptable modifiers for crackles are fine and coarse (in addition to indicators of the crackle’s respiratory timing – such as early, mid, or late).
So how should crackles be described?
Depending on their number , as either scanty or profuse
Depending on their predominant frequency , as high-pitched (fine) or low-pitched (coarse)
Depending on their amplitude of oscillations, as either faint or loud
Finally, depending on their timing during inspiration, as either early, mid, or late inspiratory
How much has this terminology been implemented?
Not much. In common practice, outdated terms such as râles (or crepitations) are still encountered, as shown by several surveys of physicians and respiratory therapists. Even medical journals often rely on some of these old terms. A review of several case reports, for example, has shown the use of as many as 16 different terms to describe similar sounds.
How are adventitious lung sounds produced?
Mostly by vibration of respiratory structures , such as bronchi and pleura. This may occur in four ways:
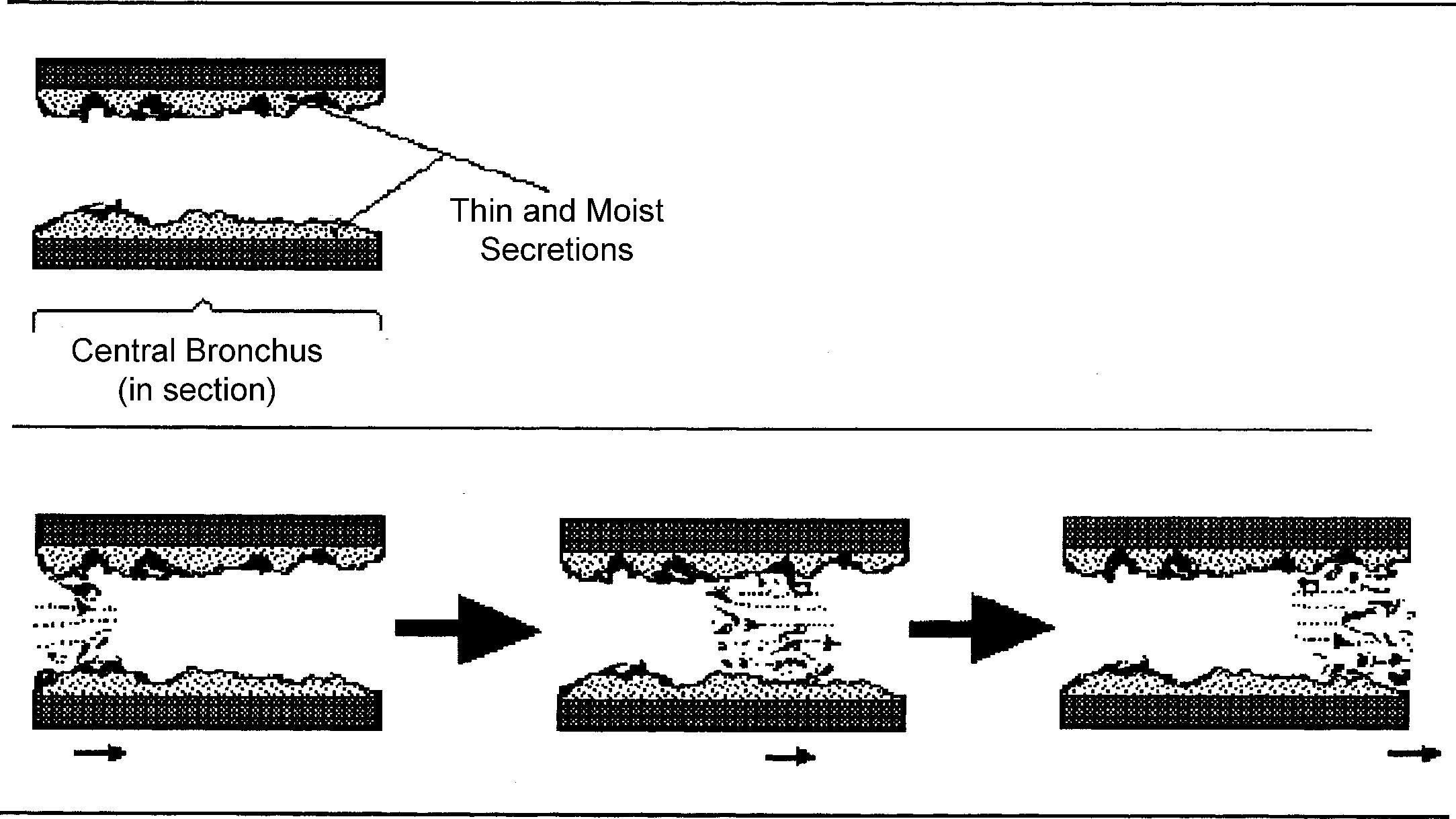
Rupture of fluid films or bubbles: this is responsible for coarse crackles (discontinuous adventitious lung sounds [DALs]) and occurs whenever air flows through large central airways coated with thin secretions. The air–fluid interface causes the rupture of fluid films and bubbles, resulting in crackling noises. These are typical of acute and chronic bronchitis and were called by Laënnec “râles gargouillement,” an expression which included the death rattle . ( Fig. 12.8 )
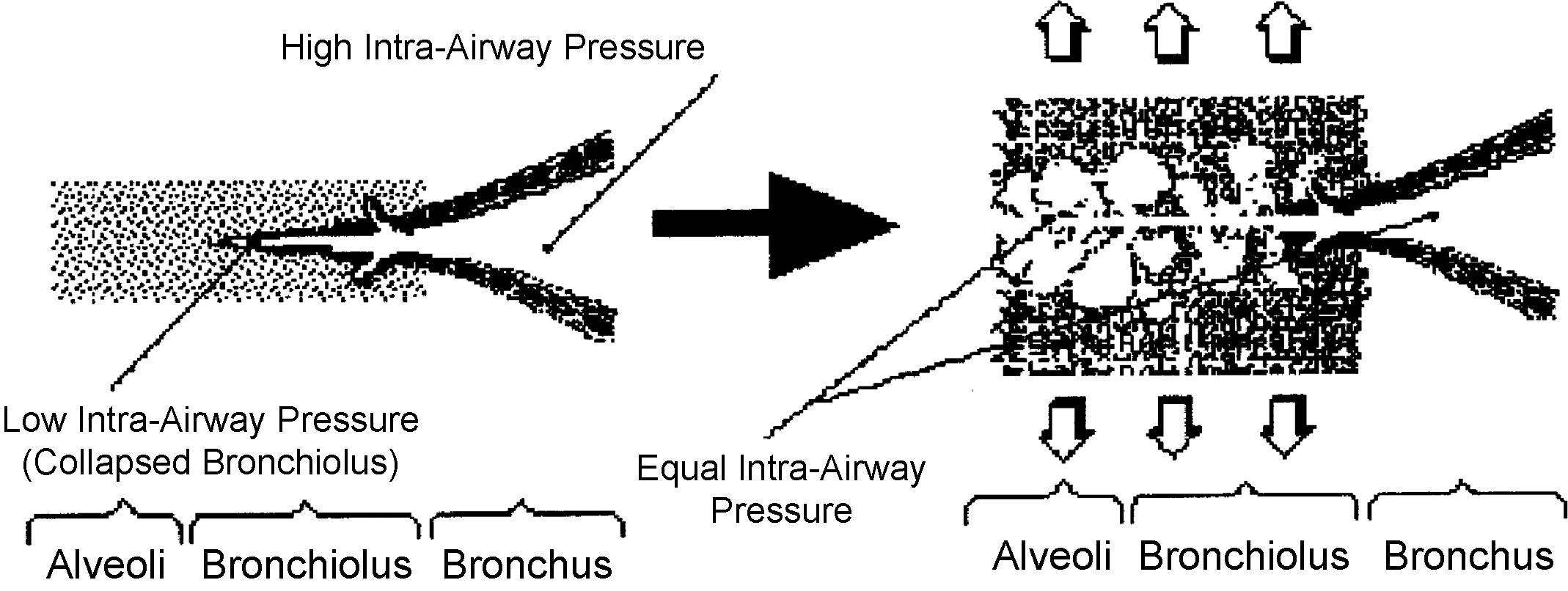
Sudden equalization of intraairway pressure: this is responsible for fine crackles, which are also DALs, but more typical of pneumonia, pulmonary hemorrhage, pulmonary edema, and pulmonary fibrosis. Sudden equalization of intraairway pressure occurs whenever small airways that are partially collapsed suddenly “pop” open in inspiration. The partial collapse of distal airways is due to high interstitial pressure, the result of either scarring (pulmonary fibrosis) or fluid (pus, blood, serum). ( Fig. 12.9 )
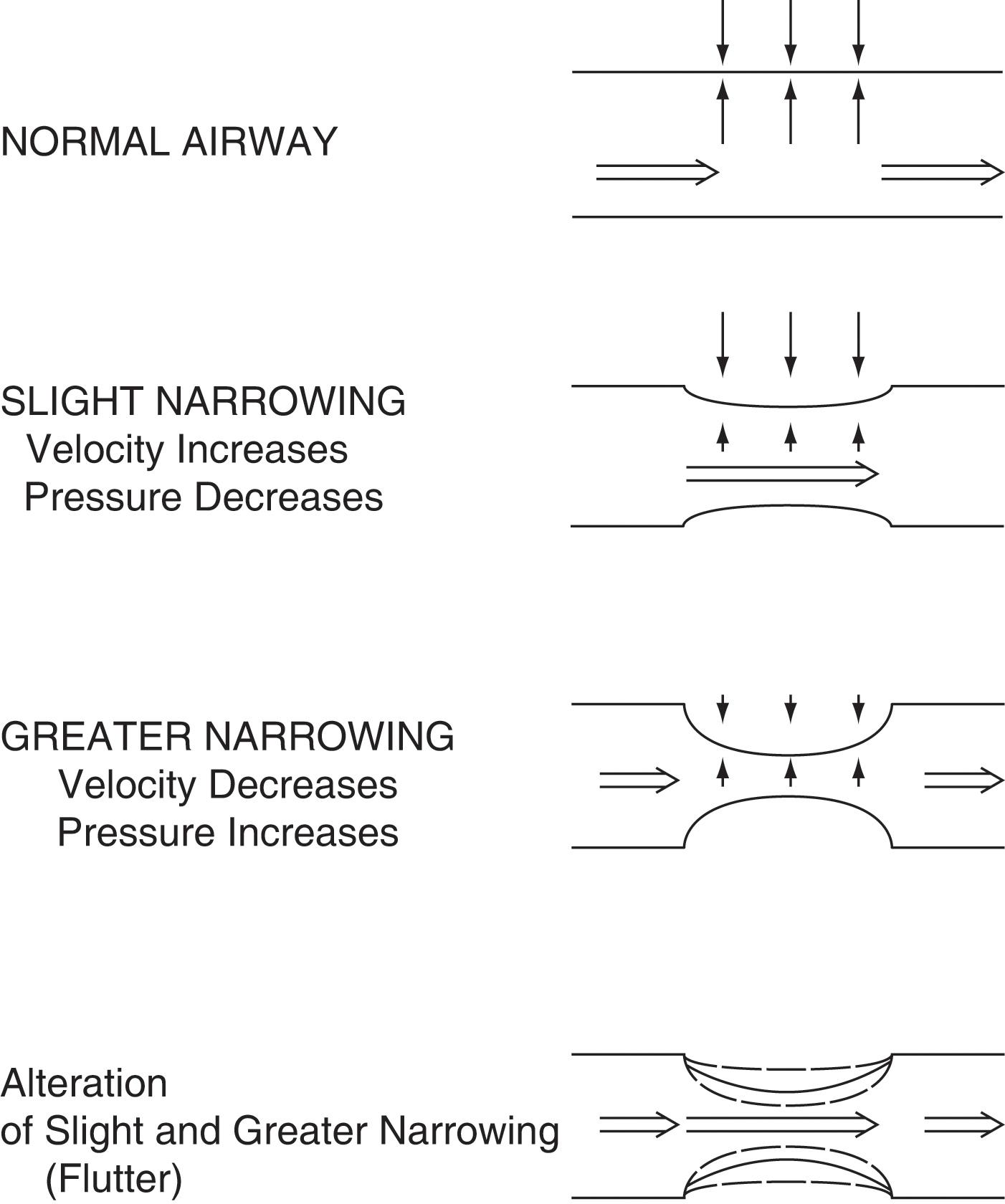
Fluttering of the airway wall: this is responsible for wheezes , which are instead continuous adventitious lung sounds (CALs). Fluttering occurs whenever air flows rapidly through airways that have been narrowed by either bronchospasm or thick secretions/edema. The underlying mechanism is the Bernoulli principle, which also governs the water vacuum pump of many labs. In the case of the pump, it is the water rapidly flowing through a narrow tube that produces a sucking effect (which, in turn, draws in air through a hole in the tube). In the case of wheezes, however, there is no hole in the airway wall. As a result, air flowing rapidly through a narrow bronchus will simply draw-in the airway wall, thus creating a fluttering and a wheeze ( Fig. 12.10 ).
Rubbing of inflamed pleural surfaces: this is responsible for the pleural friction rub. The two pleural layers are roughened by inflammation and covered with fibrin, and they grate against each other during respiration. This produces a leather-like sound that is typically inspiratory and expiratory.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here