Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lower urinary tract dysfunction is common in patients with neurologic disease. The neurogenic bladder can result from lesions affecting any part of the nervous system. Symptoms are often bothersome and may have a significant impact on quality of life. Some patients may also be at risk of developing changes in the upper urinary tract and even renal impairment.
The essential function of the lower urinary tract (bladder and urethra) is storage of urine and its elimination at appropriate times. This function ultimately depends on a local spinal reflex arc which is regulated by supraspinal input to preserve continence until appropriate.
Neurologic control of the key bladder functions of storage and voiding is accomplished by a neural network involving regions of the cortex, brainstem, and spinal cord. The peripheral innervation of the detrusor and sphincter is vital in the execution of this central control. Understanding of the neurologic control of the bladder was derived initially from animal studies, and then refined using functional imaging studies. Experiments on decerebrate cats in the 1920s led to the understanding that the mid-pons played an integral role in micturition. This same region was later stimulated electrically in cats to produce detrusor contractions. Subsequent work demonstrated that stimulation of the medial region of the dorsomedial pontine tegmentum led to relaxation of urethral pressure, silence of the pelvic floor electromyogram, and an increase of detrusor pressures; as a result, this area became known as the pontine micturition center. Later studies established functional continuity of the intermediolateral cell column in the sacral spinal cord to this region.
The development of functional imaging modalities such as positron emission tomography (PET) and functional magnetic resonance imaging (MRI) has allowed further understanding of the central control of micturition. Activation occurs in the region of the medioposterior pons during voiding and in the region of the ventrolateral pontine tegmentum in subjects unable to void in the scanner. In the cortex, PET studies have suggested that the right inferior frontal gyrus and the right anterior cingulate gyrus are activated during voiding along with several other regions including the cerebellum, hypothalamus, thalamus, subthalamic nucleus, and the periaqueductal gray.
The bladder is one of the few visceral organs with voluntary control; it receives innervation from both the autonomic and somatic systems ( Fig. 29-1 ). Parasympathetic fibers arise from neurons in the S2 to S4 segments, activating muscarinic receptors of the detrusor muscle. Sympathetic fibers from the thoracolumbar segments (T11 to L1) pass through the hypogastric nerve and pelvic plexus and activate the β3 receptors of the detrusor muscle and α-adrenergic receptors of the internal urethral sphincter and bladder neck. Somatic fibers pass through the pudendal nerve and activate nicotinic receptors of the external urethral (and anal) sphincter. Sensations of bladder fullness are conveyed to the spinal cord through all these sets of nerves.
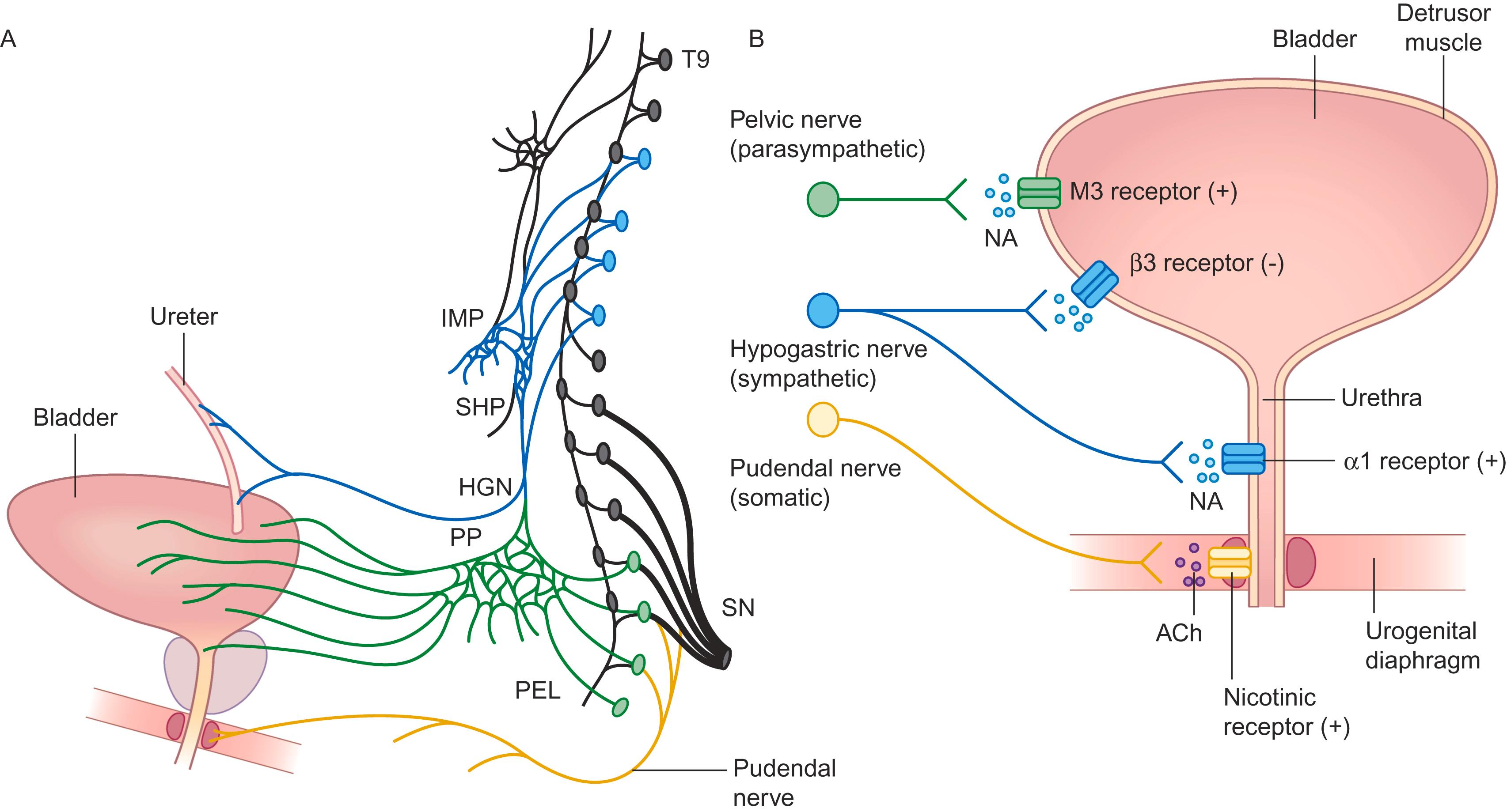
The bladder is in the storage phase 98 percent of the time. During this phase, passive distension of the bladder results in low-level afferent firing. This firing leads to reflex inhibition of parasympathetic efferents and activation of both the sympathetic outflow innervating the internal urethral sphincter and pudendal outflow innervating the external urethral sphincter; these responses promote continence. Ascending afferent signals relay at the periaqueductal gray before reaching cortical centers. Signals from the pontine micturition center are essentially inhibitory and promote storage.
When the bladder is perceived to be full and it is a socially appropriate time and place to void, facilitatory signals from the pontine micturition center result in parasympathetically mediated contractions of the detrusor muscle and inhibition of the sympathetic and pudendal outflow (leading to sphincter relaxation).
The decision to void is based on a combination of factors, including emotional state, an appreciation of the social environment, and sensory signals arising from the bladder. Knowledge of the extent to which one’s bladder content is comfortable and “safe” is central in this process. Thus, voluntary control of the bladder and the urethra has two important aspects: registration of bladder filling sensations and manipulation of the firing of the voiding reflex, both of which are dependent on the periaqueductal gray.
The urothelium (the bladder epithelium) has specialized sensory and signaling properties that allow the bladder to respond to chemical and mechanical stimuli and to engage in reciprocal chemical communication with nerves in the bladder wall. The urothelium expresses nicotinic, muscarinic, tachykinin, adrenergic, bradykinin, and transient-receptor-potential vanilloid receptors. It has the ability to release chemical mediators such as adenosine triphosphate (ATP), acetylcholine (ACh), and nitric oxide, which can regulate the activity of adjacent nerves and trigger local vascular changes or reflex bladder contractions.
The presence of muscarinic and nicotinic receptors in the urothelium has focused attention on the role of ACh as a chemical mediator of neural–urothelial interactions. ACh is released from the urothelium in response to chemical or mechanical stimuli. Thus, the clinical effect of antimuscarinic agents in overactive bladder conditions might not only lead to a motor response, but also influence the afferent pathway.
Various neurotransmitters have been implicated in the central control of the lower urinary tract. Putative excitatory transmitters include glutamate, tachykinins, pituitary-adenylate-cyclase-activating polypeptide, nitric oxide, and ATP. Glutamate seems to be the essential transmitter in spinal and supraspinal reflex pathways that control the bladder and the external urethral sphincter. Inhibitory amino acids (γ-aminobutyric acid and glycine) and opioid peptides (enkephalins) exert a tonic inhibitory control in the pontine micturition center and regulate bladder capacity. These substances also have inhibitory actions in the spinal cord. Drugs used in management of the bladder symptoms have mainly developed in accordance with this neurochemistry. Although antimuscarinics are the mainstay of therapy, other agents have been developed that influence the vanilloid, cannabinoid, and β3 receptors, which are discussed below in the section on treatment.
Lower urinary tract symptoms usually consist of problems with storage or problems with voiding ( Table 29-1 ). The pattern of bladder dysfunction following neurologic disease depends to a large extent upon the level of the lesion. The storage function of the bladder is affected following suprapontine or lesions below the pons but above the sacral spinal cord ( Table 29-2 ) resulting in involuntary, spontaneous, or induced contractions of the detrusor muscle (detrusor overactivity), which can be identified during the filling phase of urodynamic testing. This voiding function can be affected by infrapontine lesions. Following spinal cord damage interrupting the connections between sacral and pontine centers, detrusor–sphincter dyssynergia may also occur, resulting in incomplete bladder emptying and abnormally high bladder pressures. With lesions of the conus medullaris, cauda equina, or peripheral nerves, voiding dysfunction predominates and results in poor-detrusor contraction and nonrelaxing urethral sphincters.
| Storage symptoms | Voiding symptoms |
|---|---|
| UrgencyDaytime frequencyNocturia | HesitancyPoor flowIntermittent flowStrainingIncomplete voiding |
| Suprapontine lesion, e.g., stroke, Parkinson disease | Infrapontine-suprasacral lesion, e.g., spinal cord injury, multiple sclerosis | Infrasacral lesion, e.g., conus medullaris tumor, cauda equina syndrome, peripheral neuropathy | |
|---|---|---|---|
| History/bladder diary | Urgency, frequency, urgency incontinence | Urgency, frequency, urgency incontinence, hesitancy, interrupted stream | Hesitancy, interrupted stream |
| Postvoid residual (PVR) urine | PVR<100 ml | ±Elevated PVR | PVR>100 ml |
| Uroflowmetry | Normal flow | Interrupted flow | Poor/absent flow |
| Urodynamics | Detrusor overactivity | Detrusor overactivity, detrusor–sphincter dyssynergia | Detrusor underactivity, sphincter insufficiency |
Disorders affecting the cortex or subcortical white matter often result in lower urinary tract symptoms. This occurs most commonly with lesions of the anteromedial frontal lobe, including the anterior part of the cingulate gyrus. The clinical picture is one of severe urgency and frequency of micturition with urge incontinence; the patient is usually socially aware and embarrassed by the incontinence. Urinary retention also has been described but is less common; a small number of patients have been described with urinary retention that resolved with treatment of the frontal lobe disorder.
Urinary incontinence may follow stroke, often with more anteriorly placed infarcts. It has not been possible to demonstrate a correlation between any specific lesion site and the urodynamic findings. The most common cystometric finding is that of detrusor overactivity, and voiding is usually well-coordinated. Patients with hemorrhagic stroke are more likely to have detrusor underactivity. Urinary incontinence at 7 days after stroke is predictive of poor survival, disability, and institutionalization independent of the patient’s level of consciousness.
Small-vessel disease of the white matter (leukoaraiosis) has been associated with urgency incontinence as well as falls and cognitive disturbance, especially when it involves the frontal lobes. This may be an important cause of incontinence in functionally independent elderly.
A much less common cause of frontal involvement with urinary incontinence is normal-pressure hydrocephalus, where incontinence is a cardinal feature. Improvement in urodynamic function has been demonstrated in some patients within hours of lumbar puncture or shunting procedures.
The cause of urinary incontinence in patients with dementia is probably multifactorial, but frontal lobe degeneration probably plays a role. In a study of patients with progressive cognitive decline, incontinence was observed to not occur until more advanced stages of Alzheimer disease but occurred earlier in the course of dementia with Lewy bodies.
Lower urinary tract symptoms are reported in 38 to 71 percent of patients with Parkinson disease (PD). Storage symptoms are the most common problem, seen in more than 60 percent of these patients, with considerable impact on quality of life. Nocturia is the most common symptom followed by urinary urgency; these are among the most common nonmotor symptoms of PD. Urodynamic studies typically show detrusor overactivity, probably due to neuronal loss in the substantia nigra disinhibiting the normal effect of the basal ganglia on the micturition reflex. Dopamine receptor stimulation through D1 receptors provides the main inhibitory influence on the micturition reflex normally, although dopaminergic stimulation in PD has little apparent impact.
Overactive bladder is common in patients with PD, and many patients have nocturnal polyuria. Patients may also report voiding difficulties and bradykinesia of the pelvic floor muscles resulting in pseudo-dyssynergia. Lower urinary tract symptoms may be multifactorial and other non-neurogenic factors such as prostatic enlargement may contribute. Other medical comorbidities such as diabetes mellitus, congestive cardiac failure, medications (such as diuretics), cerebrovascular disease, and cervical spondylosis may also play a role. Sleep disturbances and disturbed circadian rhythm, which are common in PD, may be closely associated with nocturia.
Patients with parkinsonism and early and prominent urogenital complaints may have multiple system atrophy (MSA). Around 40 percent of these patients first present with lower urinary tract symptoms and 97 percent will experience these symptoms during the disease course. Urologic presentations include daytime frequency (about 45%), nocturnal frequency (nearly 70%), urinary urgency (60%), and urge incontinence (66 to 75%).
Bladder symptoms occur much earlier and are more disabling in MSA than PD. Although urgency and frequency occur in both conditions, patients with MSA are more likely than those with PD to have a high (>100 ml) postvoid residual volume, detrusor–sphincter dyssynergia, an open bladder neck at the start of bladder filling on videocystometrogram, and evidence of neurogenic changes on electromyography of the anal sphincter.
Although the pontine micturition center is essential for micturition, the rarity of brainstem lesions in clinical practice makes it less common to encounter bladder dysfunction secondary to brainstem lesions. In cases with brainstem tumors or other mass lesions, the clinical picture is usually dominated by other long tract and ocular features. Due to the proximity of the pontine micturition center to the medial longitudinal fasciculus, internuclear ophthalmoplegia is a common accompanying finding. Voiding difficulty is a rare but well-recognized symptom of a posterior fossa tumor. When urinary symptoms occur with brainstem stroke, lesions are usually situated dorsally. Brainstem involvement in multiple sclerosis is discussed separately.
The spinobulbar reflex arc is crucial in the control of bladder function in health. Following spinal cord lesions, interruption of this reflex, along with loss of supraspinal control, results in a local spinal reflex that drives bladder contractions. The localization of lesions can be guided by the type of bladder dysfunction observed.
The overactive neurogenic (spastic) bladder occurs following lesions that interrupt the connections between the pontine micturition center and sacral cord micturition centers. Commonly these myelopathic conditions cause quadriplegia or paraplegia. Clinically, patients present with detrusor contraction during bladder filling leading to detrusor overactivity, characterized by urinary frequency, urgency, urge incontinence, and inability to initiate micturition voluntarily. Bladder capacity is reduced, although residual urine may be increased. On examination, the bulbocavernosus and anal reflexes are preserved.
Autonomous neurogenic bladder (detrusor areflexia) may occur with complete lesions below the T12 segment that involve the conus medullaris and cauda equina. Common pathologies include sacral myelomeningocele and tumors of the conus medullaris and cauda equina. This type of neurogenic bladder also occurs during the initial shock phase following spinal cord injury; gradually over the course of weeks new reflexes emerge to drive bladder emptying and cause detrusor contractions in response to low filling volumes. The tone of the detrusor muscle is abolished, there is no awareness of fullness, and the clinical presentation is urinary retention. Overflow incontinence and increased residual urine develop later. On examination, associated saddle anesthesia with absence of the bulbocavernosus and superficial anal reflexes are common. Anal sphincter control is often affected similarly.
Motor paralytic bladder results from lesions involving the efferent motor fibers to the detrusor or the detrusor motor neurons in the sacral spinal cord. Common pathologies include lumbar spinal stenosis, lumbosacral meningomyelocele, or following abdominoperineal resection or radical hysterectomy. Clinically painful urinary retention or impaired bladder emptying is the presenting feature, and residual urine is markedly increased. The bulbocavernosus and superficial anal reflexes are usually absent, but sacral and bladder sensation are preserved.
Sensory paralytic bladder is caused by impairment of the afferent pathways innervating the bladder or by dysfunction of the posterior columns or lateral spinothalamic tract in the spinal cord. Classically, this condition has been described in tabes dorsalis, syringomyelia, and diabetes mellitus. Voluntary initiation of micturition may be retained. On examination, the bulbocavernosus and superficial anal reflexes are variably absent, decreased, or present.
This classification system does not often reflect clinical practice and deviations from these descriptions commonly occur. Following spinal cord injury, the stage of spinal shock is quite variable in presentation. The neurophysiology of recovery from spinal shock has been characterized mainly in cats where, following injury, dormant C fibers emerge as the major afferents, and a spinal segmental reflex is established that results in automatic voiding. The abnormally overactive, small-capacity bladder that characterizes spinal cord disease results in a clinical phenotype characterized by urinary urgency, frequency, and incontinence; however, patients with complete transection of the cord may not complain of urgency.
Lower urinary tract dysfunction can be one of the main features of multiple sclerosis. Urinary incontinence impacts quality of life for patients and is associated with considerable costs. Up to 75 percent of patients with multiple sclerosis have lower urinary tract symptoms, and overactive bladder and dyssynergia are the most common presentations. Urinary tract infections are common; infection is found in 30 percent of those reporting urinary symptoms. The incidence of lower urinary tract symptoms increases with lower extremity weakness from corticospinal tract dysfunction (usually from spinal cord involvement) and longer disease duration. As the neurologic condition progresses, lower urinary tract dysfunction may become more difficult to treat.
The most common urinary symptom in these patients is urgency, and detrusor overactivity is often seen on urodynamic testing. Patients sometimes initially report hesitancy, but those with more severe symptoms may be unable to initiate micturition voluntarily, emptying their bladders only through involuntary overactive contractions followed by interrupted urinary flow. Evidence of incomplete emptying may come from the need to pass urine again within 5 to 10 minutes (double voiding).
Multiple sclerosis is a dynamic disease, and symptoms may appear or worsen during a relapse and then remit or improve with neurologic remissions. Neurologic symptoms may deteriorate acutely when the patient has an infection, including that of the urinary tract. As the disease progresses, recurrent infections may result in the accumulation of deficits.
Peripheral neuropathies may result in autonomic symptoms including bladder dysfunction.
Lower urinary tract symptoms are common, although often asymptomatic, in patients with diabetes. Bladder dysfunction is generally accompanied by other symptoms and signs of a generalized neuropathy. The onset of the bladder dysfunction is insidious, with progressive loss of bladder sensation and impairment of bladder emptying over years, finally leading to chronic low-pressure urinary retention. Urodynamic studies demonstrate impaired detrusor contractility, reduced urine flow, increased postmicturition residual volume, and reduced bladder sensation. It seems likely that both afferent and efferent fibers of the local spinal reflex arc are involved, causing reduced awareness of bladder filling and decreased contractility.
Lower urinary tract symptoms generally appear early in the course of amyloid neuropathy and are present in 50 percent of patients within the first 3 years of the disease. Patients most often complain of difficulty in bladder emptying and incontinence, although bladder dysfunction may be asymptomatic. Urodynamic studies have demonstrated reduced bladder sensations, underactive detrusor, poor-urinary flow, and inappropriate opening of the bladder neck. Bladder wall thickening may be seen on ultrasound. Up to 10 percent of patients with familial amyloid neuropathy type I may proceed to end-stage renal disease, often reporting polyuria as an early symptom. Patients with specific mutations may be treated with liver transplantation, and the presence of postoperative urinary incontinence is associated with a higher post-transplant mortality.
Traditionally immune-mediated neuropathies have not been associated with bladder dysfunction. However, many patients with Guillain–Barré syndrome (as high as 25%) report bladder symptoms; these symptoms are more common with more severe neuropathies, appearing after limb weakness is established. Both detrusor areflexia and bladder overactivity have been described. Long-term complications are unusual, and recovery of lower urinary tract symptoms follows the course of the neuropathy.
Bladder dysfunction is well-recognized in patients with autoimmune autonomic ganglionopathy. The usual presentation is that of the rapid onset of severe autonomic failure, with orthostatic hypotension, gastrointestinal dysmotility, anhidrosis, erectile dysfunction, and sicca symptoms, often with ganglionic acetylcholine receptor (AChR) antibodies. Bladder dysfunction generally manifests as voiding difficulty and incomplete emptying. The severity and distribution of autonomic dysfunction appear to depend upon the level of antibody titers.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here